Significance
This study addresses a fundamentally important and widely debated issue in the field of inflammation, which is why inflammation can be simultaneously deleterious after injury and yet is essential for tissue repair. Recently, an important new paradigm has emerged in the macrophage field: Organs are replete with resident macrophages of embryonic origin, distinct from monocyte-derived macrophages. In this article, we use a new model of cardiac injury and show that distinct macrophage populations derived from embryonic and adult lineages are important determinants of tissue repair and inflammation, respectively. Our data suggest that therapeutics, which inhibit monocyte-derived macrophages and/or selectively harness the function of embryonic-derived macrophages, may serve as novel treatments for heart failure.
Keywords: cardiac repair, macrophages, inflammation
Abstract
The mechanistic basis for why inflammation is simultaneously both deleterious and essential for tissue repair is not fully understood. Recently, a new paradigm has emerged: Organs are replete with resident macrophages of embryonic origin distinct from monocyte-derived macrophages. This added complexity raises the question of whether distinct immune cells drive inflammatory and reparative activities after injury. Previous work has demonstrated that the neonatal heart has a remarkable capacity for tissue repair compared with the adult heart, offering an ideal context to examine these concepts. We hypothesized that unrecognized differences in macrophage composition is a key determinant of cardiac tissue repair. Using a genetic model of cardiomyocyte ablation, we demonstrated that neonatal mice expand a population of embryonic-derived resident cardiac macrophages, which generate minimal inflammation and promote cardiac recovery through cardiomyocyte proliferation and angiogenesis. During homeostasis, the adult heart contains embryonic-derived macrophages with similar properties. However, after injury, these cells were replaced by monocyte-derived macrophages that are proinflammatory and lacked reparative activities. Inhibition of monocyte recruitment to the adult heart preserved embryonic-derived macrophage subsets, reduced inflammation, and enhanced tissue repair. These findings indicate that embryonic-derived macrophages are key mediators of cardiac recovery and suggest that therapeutics targeting distinct macrophage lineages may serve as novel treatments for heart failure.
There are numerous examples of seemingly contradictory reports claiming that inflammation is both harmful after injury and essential for tissue repair (1). This paradox is well established in models of cardiac injury. Macrophages within the infarcted heart not only drive robust inflammatory responses and pathological left ventricular (LV) remodeling but also are required for the resolution of inflammation and reparative activities, including angiogenesis (2). One proposed explanation for these findings suggests that distinct macrophage populations may mediate inflammatory (M1) and reparative (M2) macrophage behaviors (3). However, the exact identities of these proposed macrophages remain undefined.
Paradigm shifting studies have revealed that tissue macrophages represent a heterogeneous population of cells derived from distinct developmental origins (4), including embryonic-derived and adult monocyte-derived subsets (5–8). Consistent with these observations, we have recently reported that the adult heart also contains embryonic-derived and monocyte-derived macrophages (9) with differing recruitment dynamics and gene expression profiles. Although details describing the tissue distribution of embryonic-derived macrophages and their relationship to traditional monocyte-derived macrophages are beginning to emerge, the critical issue of whether macrophages of distinct origin have unique inflammatory, reparative, and immunologic functions remains largely unexplored.
Previous work has demonstrated that the neonatal heart has a remarkable capacity for tissue repair compared with the adult, offering an ideal context in which to examine these concepts. After surgical apical resection, cryoablation, or myocardial infarction, neonatal mice efficiently regenerate myocardium (10–13), a process that is dependent on macrophages (14). The precise identity of neonatal macrophages and their relationship to macrophages found in the adult heart is unknown. On the basis of these findings, we hypothesized that distinct macrophage lineages are present in the neonatal and adult heart and explain the repeated observation that the neonatal heart recovers LV structure and function after tissue injury, whereas the adult heart undergoes pathologic remodeling and does not fully recover function.
To test these hypotheses, we established an in vivo mouse model of cardiomyocyte injury, using a diphtheria toxin receptor (DTR)-based system. This model provides a simple approach to delivering precise and titratable cardiomyocyte cell death at varying stages of development and also avoids unintended consequences of surgical thoracotomy, including systemic inflammation and cardiac fibrosis (15). Through a combination of flow cytometry, genetic lineage tracing techniques, and depletion studies, we demonstrate that neonatal mice contain a resident macrophage lineage that is derived from the embryo, produces minimal inflammation, and is required for cardiac repair through the promotion of coronary angiogenesis and myocardial proliferation. During homeostasis, the adult heart contained embryonic-derived macrophages with similar properties. However, after injury, these cells were replaced by proinflammatory monocytes and monocyte-derived macrophages, which have a limited capacity to promote cardiac repair and, instead, generate inflammation and oxidative stress. We further demonstrate that manipulation of distinct macrophage lineages improves the adult heart’s intrinsic capacity for cardiac repair.
Results and Discussion
DT Cardiomyocyte Injury Model.
To gain insights into immune mechanisms that govern cardiac recovery, we developed an in vivo cardiomyocyte cell ablation model. To selectively target ventricular cardiomyocytes, we crossed myosin light chain 2v (Mlc2v)-Cre with Rosa26-DTR mice (16, 17) (Fig. 1A). Resultant progeny express the DTR specifically in ventricular cardiomyocytes and are susceptible to DT. To induce cardiomyocyte cell death, we subjected mice to daily DT (20 ng/kg i.p.) injections. Despite uniform DTR expression, TUNEL staining revealed that cardiomyocyte cell death occurred in aggregates reminiscent of small infarcts, which expanded in number and size with increasing DT injections. Evan’s blue staining revealed that cardiomyocyte cell death occurred through necrosis as well as apoptosis. DT treatment resulted in a dose-dependent reduction in LV systolic function, indicating physiologically relevant extents of cardiac injury (SI Appendix, Fig. S1).
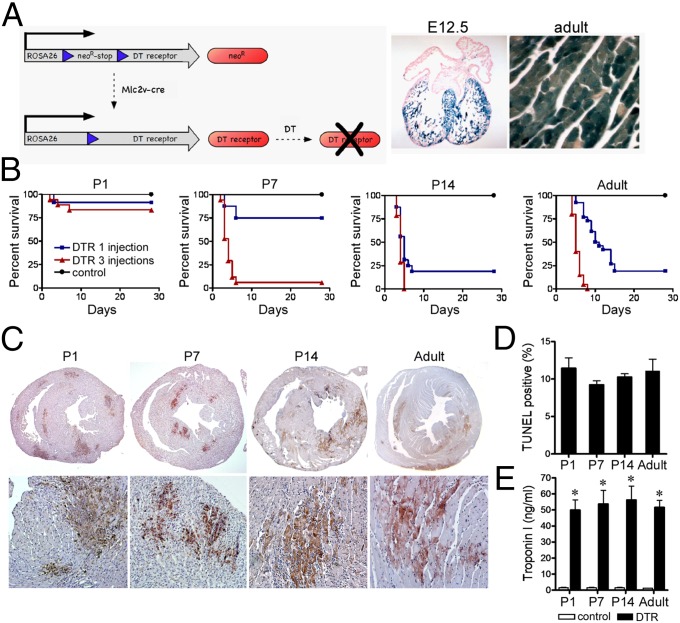
Fig. 1.
Age-dependent susceptibility to DT-induced cardiac injury. (A) Schematic of the DT cardiomyocyte injury model (Left). β-Galactosidase staining of E12.5 (Middle) and adult (Right) Rosa26R-LacZMlc2v-cre hearts. (B) Kaplan-Meier graphs of P1, P7, P14, and adult animals subjected to DT-induced cardiac injury. Black, control; blue, one DT injection; red, three DT injections. (C) TUNEL staining of P1, P7, P14, and adult animals after three DT injections. (D) Quantification of the percentage TUNEL-positive area in injured P1, P7, P14, and adult animals. (E) Troponin I serum levels immediately after three DT injections. *P < 0.05 compared with control.
Consistent with previous studies reporting age-related reduction in the ability to regenerate myocardium after apical resection or coronary artery ligation (10, 11, 13), postnatal day 1 (P1) mice demonstrated minimal mortality after either one or three DT injections. In contrast, P14 and adult mice displayed significantly higher dose-dependent mortality. P7 mice demonstrated an intermediate mortality profile (Fig. 1B). TUNEL staining after three DT injections revealed similar extents of cardiomyocyte death in P1, P7, P14, and adult animals (Fig. 1 C and D). Measurement of serum troponin I concentration immediately after three DT injections confirmed similar extents of cardiomyocyte injury between groups (Fig. 1E).
Distinct Macrophage Subsets Populate the Neonatal and Adult Heart.
We have previously shown that the adult heart contains distinct resident macrophage subsets, with differing recruitment dynamics and ontogeny. Among these subsets, chemokine (C–C motif) receptor 2 (CCR2) expression identified macrophages recently derived from circulating monocytes (9). To define monocyte and macrophage subsets that populate the neonatal and adult heart, we performed flow cytometry (SI Appendix, Fig. S2) in combination with genetic lineage tracing. Although the total number of macrophages and monocytes increased in the neonatal and adult heart immediately after injury (P3 for the neonate), we observed marked differences in macrophage composition (Fig. 2 A and B). At baseline, the adult heart contained two resident macrophage (MHC-IIlowCCR2−, MHC−IIhighCCR2−) subsets and one monocyte-derived macrophage (MHC-IIhighCCR2+) and one monocyte (MHC-IIlowCCR2+) subset. The neonatal heart contained one macrophage (MHC-IIlowCCR2−) and one monocyte (MHC-IIlowCCR2+) subset. In response to injury, the neonatal heart selectively expanded the number of MHC-IIlowCCR2− macrophages and did not recruit additional CCR2+ monocytes. In contrast, the injured adult heart selectively recruited monocytes and MHC-IIhighCCR2+ monocyte-derived macrophages. MertK and CD64 staining confirmed that MHC-IIlowCCR2−, MHC-IIhighCCR2−, and MHC-IIhighCCR2+ cells represented macrophages, whereas MHC-IIlowCCR2+ cells were monocytes both in the neonatal and adult heart (Fig. 2C) (18). Immunostaining revealed a rapid expansion of macrophages in the neonatal heart that resolved within 5 d after injury. In contrast, monocytes and macrophages progressively infiltrated the adult heart after injury (SI Appendix, Fig. S3). Together, these data indicate that in response to injury, the neonatal heart expands a population of resident CCR2− cardiac macrophages, whereas the adult heart recruits CCR2+ monocytes and monocyte-derived macrophages.
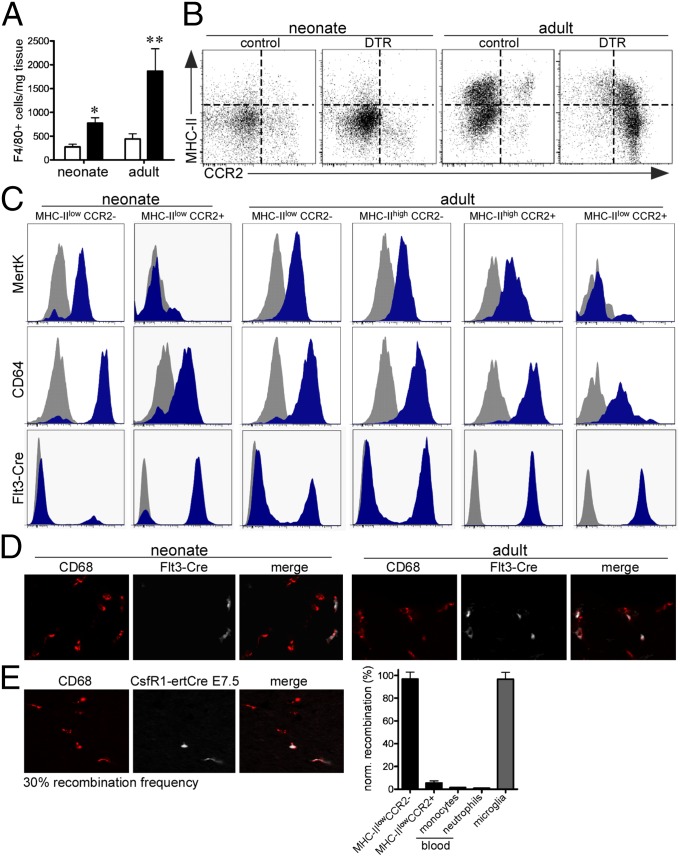
Fig. 2.
Distinct macrophage and monocyte subsets populate the neonatal and adult heart. (A) Quantification of F4/80+ cells in the injured neonatal and adult heart. (B) FACS analysis of monocyte and macrophage populations in the injured neonatal and adult heart. (C) MertK and CD64 staining demonstrating that MHC-IIlowCCR2−, MHC-IIhighCCR2−, and MHC-IIhighCCR2+ subsets are macrophages, whereas the MHC-IIlowCCR2+ subset are monocytes. Flt3-Cre lineage tracing of neonatal and adult macrophage populations. Gray, isotype control; blue, antibody or Rosa26td. (D) CD68 immunostaining of Flt3-Cre Rosa26td positive and negative macrophages and monocytes. 40× objective. (E) CD68 immunostaining of CSF1R-MerCre Rosa26td macrophages in the neonatal heart (Left). 40× objective. FACS analysis demonstrating that the vast majority of MHC-IIlowCCR2− macrophages in the neonatal heart are embryonic-derived (Right).
To define the origin of MHC-IIlowCCR2− macrophages in the neonatal heart, we performed genetic lineage tracing with fms-related tyrosine kinase 3 (Flt3)-Cre, a marker of self-renewing adult definitive hematopoietic cells (19). By crossing Flt3-cre mice to Rosa26-td reporter mice, we were able to differentiate definitive hematopoietic cell-derived macrophages from embryonic-derived macrophages. Flow cytometry analysis of Flt3-Cre Rosa26td neonatal hearts revealed that MHC-IIlowCCR2− macrophages were not derived from definitive hematopoiesis (i.e., embryonic-derived), whereas MHC-IIlowCCR2+ monocytes were derived from definitive hematopoiesis. In the adult heart, MHC-IIlowCCR2− and MHC-IIhighCCR2− macrophages represented a mixed population of embryonic-derived and definitive hematopoietic-derived cells, whereas MHC-IIhighCCR2+ macrophages and MHC-IIlowCCR2+ monocytes were exclusively derived from definitive hematopoiesis (Fig. 2C). CD68 immunostaining confirmed that Flt3-Cre Rosa26td positive cells were monocytes and macrophages (Fig. 2D). To directly label embryonic-derived macrophages in the neonatal heart, we crossed colony stimulating factor 1 receptor (CSF1R)-MerCre mice with the Rosa26td reporter and treated pregnant mothers with a single dose of tamoxifen at embryonic day 7.5. This strategy led to selective labeling of embryonic-derived macrophages that are established at the time of yolk sac hematopoiesis with a recombination frequency of 30% (based on brain microglia) (9). Immunostaining and flow cytometry showed that ∼30% of MHC-IIlowCCR2− macrophages in the neonatal heart were labeled, indicating that MHC-IIlowCCR2− macrophages represented a relatively pure embryonic-derived macrophage population (Fig. 2E).
Robust Inflammatory Response to Injury in the Adult Heart.
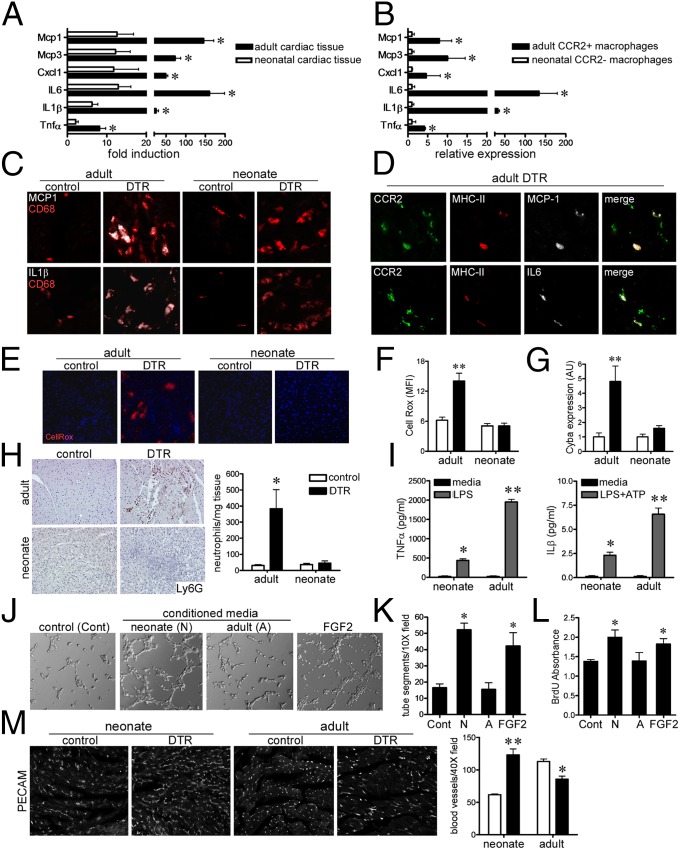
To determine whether the presence of distinct macrophage subsets in the injured adult and neonatal heart was associated with alterations in the inflammatory response to injury, we measured the expression of classic chemokines and cytokines. Quantitative RT-PCR demonstrated marked increases in the expression of Mcp1, Mcp3, Cxcl1, IL6, IL1β, and Tnfα mRNA in the injured adult heart compared with in age-matched controls. In contrast, only minimal increases in Mcp1, Mcp3, IL6, and IL1β mRNA were detected in injured neonatal hearts (Fig. 3A). Consistent with these findings, CCR2+ macrophages sorted from injured adult hearts expressed high levels of Mcp1, Mcp3, Cxcl1, IL6, IL1β, and Tnfα mRNA compared with CCR2− macrophages sorted from injured neonatal hearts (Fig. 3B). Immunostaining for monocyte chemotactic protein 1 (MCP1), IL1β, IL6, and TNFα demonstrated that these factors were specifically expressed in CD68+ monocytes and macrophages in the adult heart with minimal expression in the neonatal heart (Fig. 3C and SI Appendix, Fig. S4A). To more specifically examine which macrophage subsets expressed MCP1 and IL6 in the injured adult heart, we stained sections with antibodies against MCP1, IL6, CCR2, and MHC-II. These studies demonstrated that MCP1 and IL6 were expressed specifically in MHC-IIhighCCR2+ macrophages (Fig. 3D). IL1β and TNFα were expressed in all CD68+ monocytes and macrophages in the injured adult heart. CellRox staining demonstrated a robust induction of oxidative activity in the adult heart after injury and no significant increase in CellRox staining in the injured neonatal heart (Fig. 3 E and F). Consistent with elevations in oxidative activity in the injured adult heart, increased mRNA expression of the essential NADPH oxidase enzyme Cyba1 was only detected in the injured adult heart (Fig. 3G). Ly6G immunostaining revealed neutrophil influx only in the injured adult heart (Fig. 3H).
Fig. 3.
Distinct inflammatory responses after neonatal and adult cardiac injury. (A) Quantitative RT-PCR assays of proinflammatory chemokine and cytokines in the injured neonatal and adult heart. (B) Proinflammatory chemokine and cytokine expression in sorted neonatal CCR2− and adult CCR2+ macrophages subsets isolated from injured hearts. (C) MCP-1 (white), IL1β (white), and CD68 (red) immunostaining in the injured neonatal adult heart. (D) MCP-1 (white), IL6 (white), MHC-II+ (red), and CCR2+ (green) immunostaining in injured adult hearts. (E and F) CellRox (red) and DAPI (blue) staining, indicating increased oxidative stress in the injured adult heart. (G) Cyba1 mRNA expression after neonatal adult cardiac injury. (H) Ly6G immunostaining for neutrophils in the injured neonatal and adult heart. (I) Neonatal CCR2− macrophages produce less TNFα and IL1β in response to LPS compared with adult CCR2+ macrophages. (J and K) Matrigel endothelial tube assay showing that only conditioned media obtained from neonatal CCR2− macrophages possess proangiogenic activity. (L) BrdU ELISA demonstrating that only neonatal CCR2− macrophage conditioned media stimulated neonatal rat cardiomyocyte proliferation. (M) platelet endothelial cell adhesion molecule-1 immunostaining of neonatal and adult hearts after injury. (C–E) 40× objective. (H and M) 20× objective. *P < 0.05 compared with control; **P < 0.05 compared with all other groups.
Neonatal Resident Cardiac Macrophages Are Reparative and Produce Minimal Inflammation.
To further characterize macrophage subsets in the injured neonatal and adult heart, we sorted and cultured relevant populations (SI Appendix, Fig. S5). Consistent with the above findings, treatment of CCR2+ macrophages isolated from the injured adult heart with LPS and LPS+ATP resulted in robust production and release of TNFα and IL1β, respectively. In contrast, CCR2− macrophages isolated from the injured neonatal heart produced significantly lower levels of TNFα and IL1β (Fig. 3I).
To determine whether macrophage subsets that populate the injured neonatal and adult heart have differing reparative activates, we harvested conditioned media from each macrophage subset and assayed for the ability to stimulate angiogenesis and cardiomyocyte proliferation. Conditioned media harvested from CCR2− neonatal macrophages efficiently stimulated endothelial cell tube formation, whereas conditioned media obtained from CCR2+ adult macrophages was unable to stimulate endothelial cell tube formation in vitro (Fig. 3 J and K). Similarly, only CCR2− neonatal macrophage conditioned media were sufficient to promote neonatal rat cardiomyocyte proliferation in vitro (Fig. 3L and SI Appendix, Fig. S6). Consistent with these findings, CD31 and BrdU immunostaining demonstrated that the neonatal heart undergoes coronary angiogenesis and cardiomyocyte proliferation after injury, whereas the injured adult heart displays minimal increases in coronary density and negligible cardiomyocyte proliferation (Fig. 3M and SI Appendix, Fig. S7). Given the inherent limitations of studying macrophages in vitro and Matrigel angiogenesis assays, these data suggest that neonatal CCR2− macrophages are reparative and not inflammatory, while CCR2+ macrophages that are recruited to the injured adult heart have only inflammatory activity.
Neonatal Mice Recover Cardiac Function and Undergo Minimal Adverse Remodeling After Cardiac Injury.
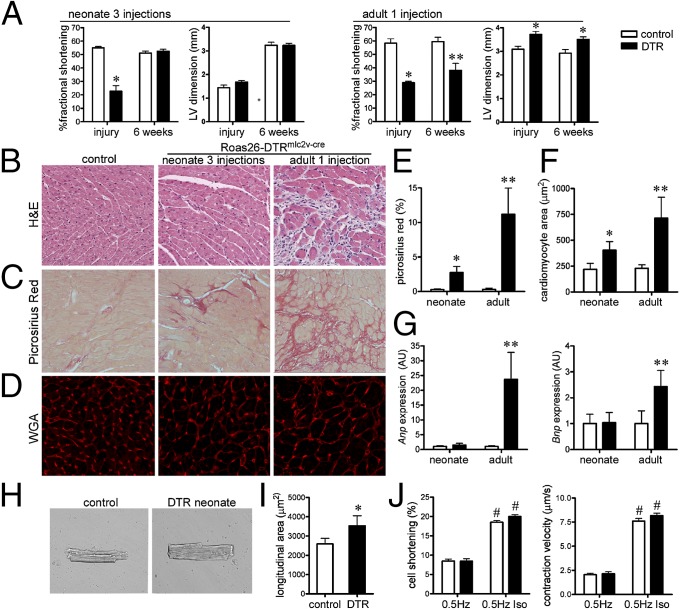
To determine whether differences in inflammatory and reparative potential are functionally important, we investigated whether injured neonatal and adult hearts recover after injury. To minimize confounding effects arising from subtle differences in the extent of cardiomyocyte injury, we compared adults who received one DT injection with neonates who received three DT injections. Immediately after DT treatment, both neonatal and adult hearts had marked reductions in LV fractional shortening. In contrast to adult animals, which displayed LV dilation and increased LV mass index acutely, neonatal mice did not demonstrate evidence of early LV remodeling. Serial echocardiography performed 6 wk after neonatal injury demonstrated that LV fractional shortening returned to baseline values, and LV diastolic dimension and LV mass index remained indistinguishable from controls. In contrast, 6 wk after adult injury, mice displayed persistent LV systolic dysfunction, LV dilation, and increased LV mass index (Fig. 4A and SI Appendix, Fig. S4B). Mice that survived neonatal cardiac injury demonstrated normal tissue architecture and minimal cardiomyocyte hypertrophy or myocardial fibrosis. Mice that survived adult cardiac injury displayed marked cardiomyocyte hypertrophy, interstitial fibrosis, and increased Anp and Bnp expression consistent with pathological LV remodeling (Fig. 4 B–G).
Fig. 4.
The neonatal heart functionally recovers after DT-induced cardiac injury. (A) Echocardiographic analysis of neonatal (three DT injections) and adult (one DT injection) hearts immediately after and 6 wk after injury. (B) H&E staining of surviving control and Rosa26-DTRmlc2v-cre hearts 6 wk after DT-induced cardiac injury. (C) Picrosirius red staining (fibrosis) 6 wk after neonatal and adult cardiac injury. (D) Wheat germ agglutinin (WGA) staining showing a cardiomyocyte cross-sectional area after cardiac injury. (E and F) Quantification of Picrosirius red staining (E) and cardiomyocyte cross-sectional area (F). (G) Anp and Bnp mRNA expression 6 wk after cardiac injury. (H and I) Longitudinal area of cardiomyocytes isolated from control and surviving Rosa26-DTRmlc2v-cre mice 6 wk after neonatal cardiac injury. (J) Cardiomyocyte cell shortening and contraction velocity at baseline and after isoproterenol treatment. (B and C) 20× objective. (D) 40× objective. *P < 0.05 compared with control; **P < 0.05 compared with all other groups; #P < 0.05 compared with cardiomyocytes not treated with isoproterenol.
To examine whether mice that survived neonatal cardiac injury had normal cardiomyocyte function, we isolated cardiomyocytes from mice that underwent neonatal cardiac injury 6–8 wk earlier. Consistent with in situ measurements of a cardiomyocyte cross-sectional area, cardiomyocytes isolated from mice that survived neonatal cardiac injury had increased longitudinal area compared with controls (Fig. 4H). Analysis of cardiomyocyte contractility using live cell optical imaging revealed that cardiomyocytes isolated from mice that survived neonatal cardiac injury were indistinguishable from controls with respect to fractional cell shortening and contraction velocity at baseline and after isoproterenol treatment, indicating that cardiomyocytes obtained from mice that recovered from neonatal cardiac injury have intact function and contractile reserve in vitro (Fig. 4 I and J).
Neonatal Macrophages Are Essential Mediators of Cardiac Recovery After Injury.
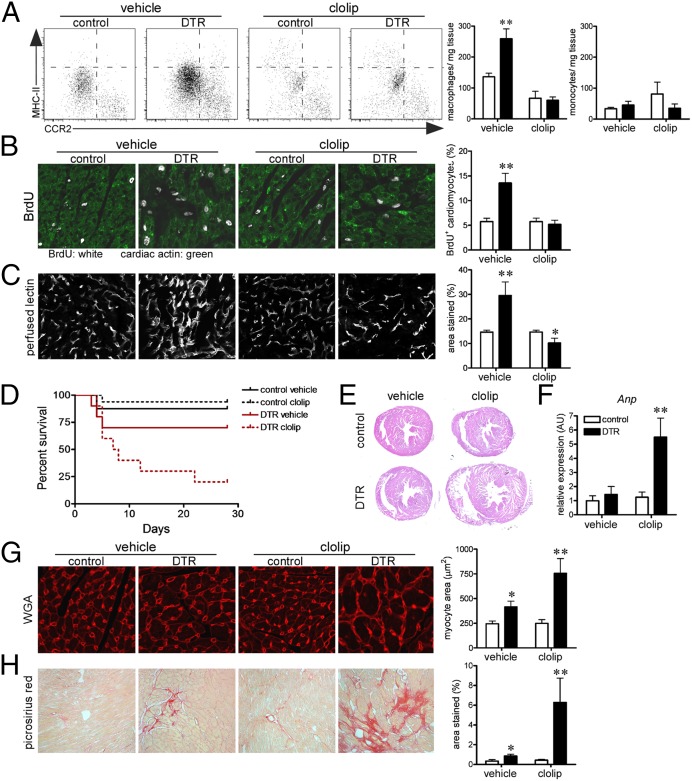
To determine whether neonatal macrophages are required for recovery from cardiac injury, we treated neonatal control and Rosa26-DTRmlc2v-cre mice with vehicle or liposomal clodronate and subsequently subjected animals to DT-induced cardiac injury. Flow cytometry demonstrated that liposomal clodronate efficiently blunted the accumulation of MHC-IIlowCCR2− embryonic-derived macrophages in the injured neonatal heart and did not significantly affect MHC-IIlowCCR2+ monocyte numbers (Fig. 5A). Consistent with a role in cardiac repair, BrdU immunostaining and lectin perfusion imaging performed immediately after cardiac injury demonstrated that macrophage depletion resulted in marked reductions in cardiomyocyte proliferation, vascular density, and endothelial proliferation (Fig. 5 B and C and SI Appendix, Fig. S8). As a consequence, depletion of neonatal macrophages resulted in a significant increase in mortality after neonatal cardiac injury (Fig. 5D). Consistent with the development of adverse remodeling, only mice that underwent neonatal cardiac injury and macrophage depletion displayed evidence of cardiac chamber dilation, marked increased Anp mRNA expression, cardiomyocyte hypertrophy, and interstitial fibrosis (Fig. 5 E–H). These data indicate that neonatal macrophages are essential for recovery from cardiac injury and are required for reparative activities, including cardiomyocyte proliferation and coronary angiogenesis. Inhibition of monocyte recruitment after neonatal cardiac injury did not alter macrophage accumulation, angiogenesis, or cardiomyocyte proliferation (SI Appendix, Fig. S9). Although we cannot exclude a contribution from macrophages residing in other tissues, these results are consistent with a role for resident neonatal cardiac macrophages and not recruited monocyte-derived macrophages in tissue repair.
Fig. 5.
Macrophages are essential for cardiac recovery after neonatal cardiac injury. (A) Flow cytometry revealing that liposomal clodronate specifically inhibits the accumulation of MHC-IIlowCCR2− macrophages after neonatal cardiac injury. (B) BrdU (white) immunostaining showing that macrophages are essential for cardiomyocyte (green) proliferation after neonatal cardiac injury. (C) Perfused lectin staining revealing that macrophages mediate coronary angiogenesis after neonatal cardiac injury. (D) Kaplan-Meier plot demonstrating that macrophage depletion results in increased mortality after neonatal cardiac injury. (E and F) Low-magnification images of H&E stained sections (E) and Anp mRNA expression (F) from hearts of adult mice that underwent neonatal cardiac injury and macrophage depletion. (G and H) Increased cardiomyocyte size (WGA; G) and interstitial fibrosis (Picrosirius red; H) in adult mice that underwent macrophage depletion and neonatal cardiac injury. (B, C, G) 40× objective. (H) 20× objective. *P < 0.05 compared with control; **P < 0.05 compared with all other groups.
Inhibition of Monocyte Recruitment Preserves Embryonic-Derived Macrophage Subsets and Improves Adult Cardiac Repair.
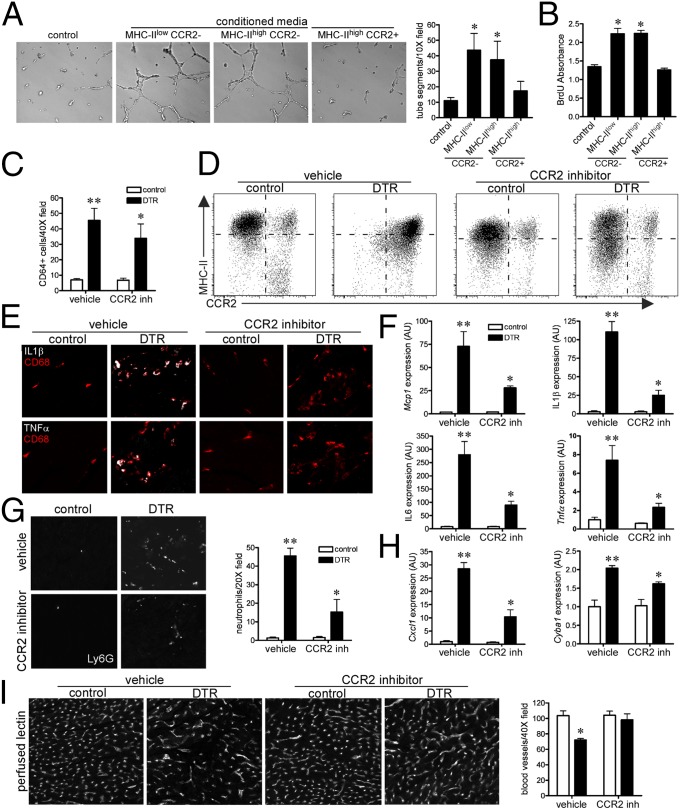
Adult resident macrophage subsets that contained embryonic-derived lineages (MHC-IIlowCCR2− and MHC-IIhighCCR2−) demonstrated robust proangiogenic and mitogenic properties ex vivo (Fig. 6 A and B), which is consistent with the observation that the adult heart contains embryonic-derived macrophage populations with the capacity for tissue repair. Remarkably, although these subsets are present in the resting adult heart, CCR2− resident macrophages are lost after adult cardiac injury and are instead replaced by inflammatory CCR2+ monocyte-derived macrophages (Fig. 2B). On the basis of these findings, we sought to determine whether inhibition of monocyte recruitment in the injured adult heart would preserve CCR2− resident macrophage subsets, decrease inflammation, and enhance cardiac repair.
Fig. 6.
Inhibition of monocyte recruitment preserves embryonic-derived macrophage subsets and improves adult cardiac repair (A and B). Conditioned media obtained from adult MHC-IIlowCCR2− and MHC-IIhighCCR2− macrophages stimulates endothelial tube formation (A) and neonatal rat cardiomyocyte proliferation (B). (C) Quantification of CD68+ monocyte/macrophages after cardiac injury and CCR2 inhibition. (D) Flow cytometry revealing that CCR2 inhibition blocked CCR2+ monocyte and macrophage recruitment to the injured heart and preserved CCR2− resident macrophage subsets. (E and F) Immunostaining (E) and quantitative RT-PCR (F) assays measuring cardiac expression of IL1β, IL6, MCP-1, and TNFα after cardiac injury and CCR2 inhibition. (G and H) Ly6G immunostaining (G) and quantitative RT-PCR (H) showing that CCR2 inhibition reduced neutrophil influx, Cxcl1, and Cyba1 mRNA expression after cardiac injury. (I) Perfused lectin staining showing that CCR2 inhibition preserves coronary microvascular integrity after cardiac injury. *P < 0.05 compared with control; **P < 0.05 compared with all other groups.
To inhibit monocyte influx into the injured adult heart, we administered either vehicle or a selective CCR2 inhibitor to mice after cardiac injury. Although the absolute number of F4/80+ monocytes and macrophages was only modestly affected, CCR2 inhibition resulted in marked alterations in monocyte and macrophage subsets (Fig. 6 C and D). CCR2 inhibition blocked monocyte recruitment into the injured adult heart and preserved MHC-IIlowCCR2− and MHC-IIhighCCR2− subsets largely through suppression of cell death (SI Appendix, Fig. S10). As a consequence, injured mice that received the CCR2 inhibitor displayed decreased MCP-1, IL1β, IL6, and TNFα mRNA and protein expression (Fig. 6 E and F). Moreover, CCR2 inhibition blunted neutrophil influx, Cxcl1, and Cyba1 mRNA expression after adult cardiac injury (Fig. 6 G and H). Consistent with enhanced reparative activity, CCR2 inhibition preserved coronary microvascular density after adult cardiac injury (Fig. 6I). Together, these data demonstrate that blockade of monocyte recruitment to the injured heart preserved CCR2− resident macrophage subsets, decreased inflammation, and enhanced coronary angiogenesis.
In this study, we developed an in vivo cardiomyocyte cell ablation system and show for the first time to our knowledge that differences in macrophage composition and function contribute to the disparate patterns of LV recovery and remodeling observed after neonatal and adult cardiac injury. Consistent with previous reports, we demonstrate that neonatal mice have a remarkable ability to recover cardiac function, whereas adult mice have a significantly limited capacity (10–14). The precise macrophage characterization and lineage tracing studies performed herein show that the injured neonatal heart contains an embryonic-derived lineage of cardiac macrophages that promote cardiomyocyte proliferation and coronary angiogenesis, but produce minimal inflammation. In contrast, the injured adult heart recruits monocytes and monocyte-derived macrophages that have a robust proinflammatory phenotype and a diminished capacity to promote cardiomyocyte proliferation or angiogenesis. The more robust proinflammatory phenotype of monocyte-derived macrophages in the adult heart leads to enhanced monocyte and neutrophil influx, collateral tissue damage, and loss of resident macrophages, limiting the amount of LV recovery that is possible in the adult heart. Consistent with the concept that distinct macrophage lineages drive tissue repair and inflammation, we demonstrate that inhibition of monocyte recruitment after adult cardiac injury is sufficient to preserve resident macrophage subsets that contain embryonic-derived lineages, suppress inflammation, and stimulate some aspects of cardiac repair.
A large resident macrophage population has previously been described in the adult mouse heart (9, 20). Here, we extend these observations and underscore the emerging concept that distinct macrophage lineages exist within tissues and have different biological roles in maintaining homeostasis, consistent with Ilya Metchnikoff’s empirically derived concept of “physiologic inflammation” originally proposed in 1901 (21). The concept that distinct macrophage subsets and lineages populate the injured neonatal and adult heart provides a mechanistic basis to explain why the neonatal and adult heart demonstrate different responses to injury and is consistent with and expands on the findings of Aurora and colleagues (14), who demonstrated that cardiac regeneration in the neonatal heart after myocardial infarction was dependent on macrophages. Furthermore, Aurora and colleagues demonstrate that macrophages isolated from infarcted neonatal hearts express a M2 gene expression profile, whereas macrophages isolated from infarcted P14 hearts display a M1 gene expression profile (14). One potential explanation for these intriguing observations is that distinct macrophage subsets and lineages are found in the infarcted neonatal and P14 heart. Consistent with this, CCR2+ monocyte-derived macrophages enter the heart at P14 and are not found in P1 or P7 hearts (SI Appendix, Fig. S11).
The observations that macrophage depletion results in delayed cardiac repair and worsened outcomes after MI (3), whereas inhibition of monocyte recruitment to the heart improves outcomes after MI (22), have long been viewed as contradictory. In light of our recent understanding of macrophages ontology, it may now be possible to reconcile these seemingly contradictory findings. The observation that the adult heart contains embryonic-derived resident macrophages that have reparative function would explain why macrophage depletion results in adverse tissue remodeling and adverse outcomes, whereas the observation that the heart recruits monocyte-derived macrophages that are proinflammatory but lack reparative function explains why inhibition of monocyte recruitment leads to favorable tissue remodeling and improved outcomes.
The results of the present study raise the important question of whether inhibition of monocyte and monocyte-derived macrophage recruitment and/or preservation of embryonic-derived lineages in the injured or failing heart would be therapeutically beneficial. This strategy has the potential to limit excessive inflammation and collateral myocardial damage and preserve embryonic-derived macrophage lineages that may maximize endogenous reparative activities. Although the adult myocardium certainly has a reduced capacity for cardiac repair compared with the neonatal myocardium, it is likely that some aspects of repair such as coronary angiogenesis and possible effects on resident progenitor cells continue to exist. In support of these concepts, we demonstrated that inhibition of monocyte recruitment to the injured adult heart preserved embryonic-derived macrophage subsets, reduced inflammation, and enhanced coronary angiogenesis. Further characterization of the signals that regulate monocyte-derived and embryonic-derived macrophage recruitment and activation has the potential to provide novel therapeutic avenues to recover the injured and/or failing heart. Further studies will be necessary to address these interesting and important questions.
Methods
Mouse strains used included Mlc2v-Cre, Rosa26-DTR, Rosa26-td, Flt3-Cre, CSF1R-MerCre, and CCR2GFP (9, 16, 17, 19, 23, 24). To ablate cardiomyocytes in vivo, Rosa26-DTRMlc2v-Cre animals were injected i.p. with 20 ng/kg of DT (Sigma). To deplete macrophages in the neonatal heart, 15 μL liposomal clodronate (18 mg/mL) (25) was injected i.p. on P1 and P5. CCR2 inhibitor (Tocris RS 504393) was administered at 2 mg/kg twice daily by oral gavage. Detailed methods are available in SI Appendix: Detailed Methods.
Supplementary Material
Acknowledgments
K.J.L. was supported by NIH Grants T32 HL007081 and K08 HL123519 and the Oliver Langenberg Physician-Scientist Training Program. This project was made possible by funding from the NIH [Grant R01 HL111094 (to D.L.M.) and Grant R01 HL105732 (to D.M.O.)] and the Washington University Center for the Investigation of Membrane Excitability Diseases Live Cell Imaging Facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406508111/-/DCSupplemental.
References
- 1.Glaros T, Larsen M, Li L. Macrophages and fibroblasts during inflammation, tissue damage and organ injury. Front Biosci. 2009;14:3988–3993. doi: 10.2741/3506. [DOI] [PubMed] [Google Scholar]
- 2.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nahrendorf M, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haubner BJ, et al. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging. 2012;4:966–977. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc. 2014;9:305–311. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porrello ER, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA. 2013;110:187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurora AB, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124:1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewald O, et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, et al. Selective requirement of myosin light chain 2v in embryonic heart function. J Biol Chem. 1998;273:1252–1256. doi: 10.1074/jbc.273.2.1252. [DOI] [PubMed] [Google Scholar]
- 17.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 18.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto AR, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS ONE. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Biol. 2003;4:897–901. doi: 10.1038/nrm1244. [DOI] [PubMed] [Google Scholar]
- 22.Kaikita K, et al. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seiler P, et al. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–2633. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.