Significance
Geographic atrophy is a late stage of age-related macular degeneration (AMD) that causes blindness in millions worldwide characterized by death of the retinal pigmented epithelium (RPE). We previously reported that RPE death is due to a deficiency in the enzyme DICER1, which leads to accumulation of toxic Alu RNA. We also demonstrated that Alu RNA causes RPE death by activating an immune platform called the NLRP3 inflammasome. However, the precise mechanisms of RPE death in this disease remained unresolved. The present study indicates that Alu RNA induces RPE death by activating the enzyme Caspase-8 downstream of inflammasome activation and that blocking Caspase-8 rescues RPE degeneration. This implicates apoptosis as the cell death pathway responsible for Alu RNA cytotoxicity, and these findings provide new potential therapeutic targets for this disease.
Keywords: macular degeneration, inflammasome, caspase
Abstract
Geographic atrophy, an advanced form of age-related macular degeneration (AMD) characterized by death of the retinal pigmented epithelium (RPE), causes untreatable blindness in millions worldwide. The RPE of human eyes with geographic atrophy accumulates toxic Alu RNA in response to a deficit in the enzyme DICER1, which in turn leads to activation of the NLRP3 inflammasome and elaboration of IL-18. Despite these recent insights, it is still unclear how RPE cells die during the course of the disease. In this study, we implicate the involvement of Caspase-8 as a critical mediator of RPE degeneration. Here we show that DICER1 deficiency, Alu RNA accumulation, and IL-18 up-regulation lead to RPE cell death via activation of Caspase-8 through a Fas ligand-dependent mechanism. Coupled with our observation of increased Caspase-8 expression in the RPE of human eyes with geographic atrophy, our findings provide a rationale for targeting this apoptotic pathway in this disease.
DICER1, encoded by the DICER1 gene in humans, is a type III ribonuclease (RNase) best known for its role in processing precursor microRNAs (premiRNAs) into mature miRNAs that are involved in posttranscriptional gene regulation (1, 2). Our recent work highlighted another important role for DICER1 in healthy cell function: to process primary Alu RNA transcripts and prevent their cytotoxic accumulation in the retinal pigmented epithelium (RPE) (3–5). In humans with geographic atrophy, a form of untreatable age-related macular degeneration (AMD) that results in irreversible blindness, DICER1 protein levels in the RPE are reduced (3). DICER1 deficiency induces accumulation of cytotoxic Alu RNA, which in turn induces TLR-independent activation of the NLRP3 inflammasome (4).
NLRP3, a member of the NLR (nucleotide-binding domain, leucine-rich repeat containing, or NOD-like receptor) subfamily, is involved in the induction of the innate immune response and becomes activated when the cell is exposed to a variety of different agents, including pathogens, adenosine triphosphate, toxins, reactive oxygen species (ROS), and nucleic acids (6). Caspase-1 activation through cleavage of the Pro-Caspase-1 precursor is a known product of NLRP3 inflammasome activation (7) and typically is required for processing of pro-IL-1β and pro-IL-18 to their active forms (8). This signaling pathway is an essential mediator of Alu RNA-induced RPE toxicity (4).
Although the role of Caspase-1 with respect to NLRP3 inflammasome activation is established, the identity of downstream signaling molecules mediating cell death remains largely unknown. Among these candidates is Caspase-8, which can mediate Caspase-1 activation (9) and pro-IL-1β processing (10). The NLRP3 inflammasome is most notably associated with cell death via pyroptosis, but previous work by our laboratory indicated that Alu RNA accumulation does not induce pyroptosis (4). Caspase-8 has been shown to play varying roles in different experimental systems; in systems deficient in Caspase-1, Caspase-8 is involved in inflammasome-mediated IL-1β processing (9–11), whereas in other systems, Caspase-8 operates downstream of inflammasome activation and is instead important with respect to apoptosis (12). Because Caspase-8 can operate either upstream or downstream of inflammasome activation, we sought to test whether Alu RNA required Caspase-8 for cytokine processing or RPE cell death for its toxic effects. Here we present evidence that Caspase-8 is associated with RPE degeneration in human eyes with geographic atrophy and that it operates downstream of Alu RNA-induced Caspase-1 activation to mediate nonpyroptotic cell death following NLRP3 inflammasome activation.
Results
Caspase-8 Is Activated in Human RPE Cells.
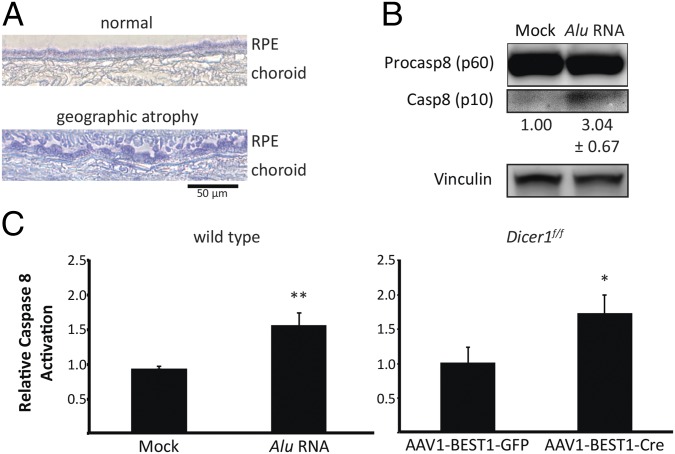
We observed a marked increase in the abundance of total Caspase-8 protein in the RPE of human eyes with geographic atrophy compared with healthy, age-matched eyes (Fig. 1A). Further, primary human RPE cells treated with Alu RNA exhibited activated Caspase-8 protein (p10), which was undetectable in mock treated cells (Fig. 1B). We next interrogated whether conditional ablation of the Dicer1 gene in the RPE of mice, which leads to Alu RNA accumulation, could induce Caspase-8 activation. Indeed, Dicer1 floxed mice exposed to a subretinal injection of recombinant adeno-associated virus (rAAV) that encodes an RPE-specific Cre recombinase exhibited significantly greater Caspase-8 activation compared with controls (Fig. 1C). Activated Caspase-8 protein levels were also increased in the RPE/choroid of Alu RNA-treated wild-type mice (Fig. 1C).
Fig. 1.
Caspase-8 is up-regulated in the RPE of human eyes with geographic atrophy and mouse eyes with DICER1/Alu RNA dysmetabolism. (A) Caspase-8 (blue) abundance is increased in the RPE of human eyes with geographic atrophy compared with normal age-matched eyes. (B) Immunoblotting shows that Alu RNA activates Caspase-8 in cultured human RPE cells 24 h after treatment. Caspase-8 active p10 fragment is 3.04 ± 0.67 fold higher in abundance in Alu RNA-treated cells. Fold change in active Caspase-8 levels compared with Mock treatment, normalized to Vinculin, as determined by densitometry (mean ± SEM), are reported below their respective bands. (C) Caspase-8 activity assays of RPE/choroid lysates of AAV1-BEST1-Cre-treated Dicer1f/f mice and Alu RNA-treated wild type mice reveal that Caspase-8 is activated by Dicer1 knockdown or Alu RNA delivery (n = 6 independent experiments, *P = 0.0004 and **P < 0.0001 by two-tailed Student t test). Images are representative of at least three independent experiments (A).
Caspase-8 Blockade Protects the RPE Against Alu RNA Toxicity.
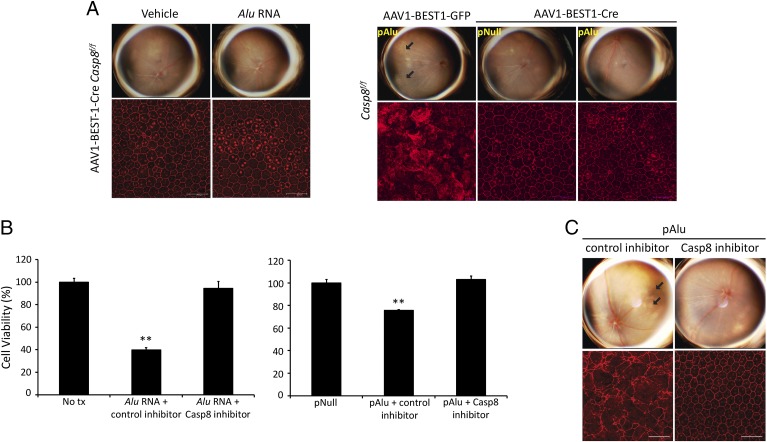
DICER1 deficiency leads to an accumulation of Alu RNA (3, 4) that results in a loss of retinal function as measured by electroretinography (SI Appendix, Fig. S1), and we found there was greater Caspase-8 activation in wild-type mice treated with Alu RNA via subretinal injection (Fig. 1C). To determine whether Caspase-8 is necessary for Alu RNA-mediated RPE degeneration, we assessed whether delivery of Alu-expression vector (pAlu) or Alu RNA via subretinal injection induced RPE degeneration in Caspase-8 deficient systems. Casp8f/f mice treated with subretinal injection of rAAV1-BEST1-Cre, which induced efficient, nontoxic Cre recombinase expression and Caspase-8 knockdown in the RPE, were protected from Alu RNA- and pAlu-induced RPE degeneration (Fig. 2A and SI Appendix, Figs. S2–S4). Additionally, exposure of human RPE cells to a Caspase-8 peptide inhibitor prevented Alu RNA- and pAlu-induced cell death (Fig. 2 B and C). Corroborating these findings, intravitreous administration of the Caspase-8 peptide inhibitor prevented pAlu-induced degeneration in wild-type mice (Fig. 2C). Supporting the hypothesis that Alu RNA-induced cell death requires Caspase-8, retinal morphology (SI Appendix, Fig. S5) and electrical function (SI Appendix, Fig. S6) were preserved in mice treated with Caspase-8 peptide inhibitor and genetic ablation, respectively. Taken together, these data demonstrate that Caspase-8 is necessary for Alu RNA-induced RPE degeneration.
Fig. 2.
Caspase-8 is required for Alu RNA-mediated RPE degeneration in mice. (A) Fundus photographs and ZO-1–stained (red) flat mounts show that AAV-BEST1-Cre–treated Casp8f/f mice are protected against Alu RNA- and pAlu-induced RPE degeneration. (B) Administration of a Caspase-8 inhibitor peptide (Z-IETD-FMK), but not control peptide (Z-FA-FMK), prevents loss of cell viability in cultured human RPE cells either treated with synthetic Alu RNA or transfected with Alu RNA-expressing plasmid (pAlu). **P < 0.001 by two-tailed Student t test. (C) Fundus photographs and ZO-1–stained (red) flat mounts show that Caspase-8 inhibitor peptide, but not control peptide, protects wild-type mice from Alu RNA-mediated RPE degeneration. n = 10, P = 0.008 by Fisher exact test. Representative images shown (A and C).
Caspase-8 Blockade Protects Against IL-18–Mediated Toxicity.
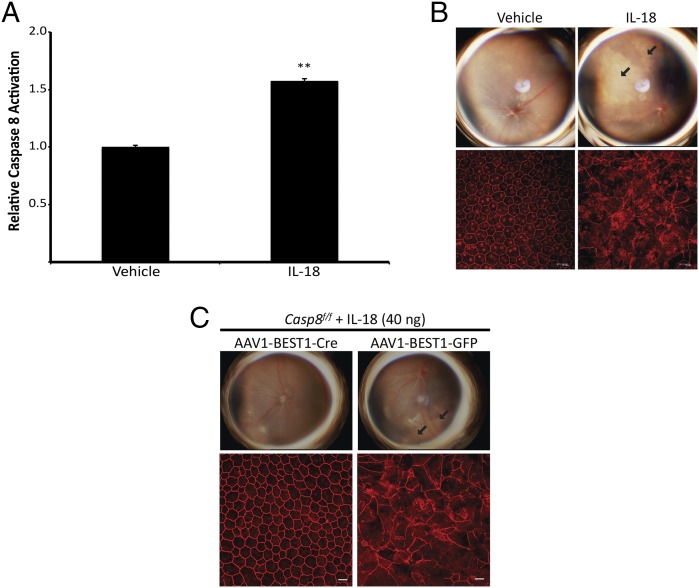
Recently we demonstrated that Alu RNA-induced RPE degeneration in geographic atrophy is mediated through the activation of NLRP3 inflammasome and ensuing IL-18–driven signaling (4, 13). We next sought to determine whether Caspase-8 executes the IL-18–mediated RPE degeneration by injecting recombinant mature IL-18 into wild-type and Caspase-8 deficient systems. Subretinal injection of recombinant IL-18 increases Caspase-8 activation (Fig. 3A) and induces RPE degeneration in wild-type mice (Fig. 3B). Casp8f/f mice treated with rAAV-BEST1-Cre are protected against IL-18–induced RPE degeneration (Fig. 3C). Collectively, these data indicate that Caspase-8 is a critical mediator of IL-18–induced RPE degeneration.
Fig. 3.
Caspase-8 is required for IL-18–induced RPE degeneration in mice. (A) Caspase-8 activity assays of RPE/choroid lysates of mature IL-18 treated wild-type mouse eyes reveal that Caspase-8 is activated 3 d postinjection (n = 3 independent experiments, *P < 0.0001 by two-tailed Student t test). (B) Fundus photographs and flat mounts stained with ZO-1 antibody (in red) show that subretinal administration of recombinant mature IL-18 induces RPE degeneration in wild-type mice. n = 12, P = 0.002 by Fisher exact test. (C) Fundus photographs and ZO-1–stained (red) flat mounts show that AAV1-BEST1-Cre–treated Casp8f/f mice are protected against IL-18–induced RPE degeneration. n = 8, P = 0.03 by Fisher exact test (C). Images are representative of at least three independent experiments (B and C).
Fas and FasL Are Required for Alu RNA- and IL-18–Mediated RPE Toxicity.
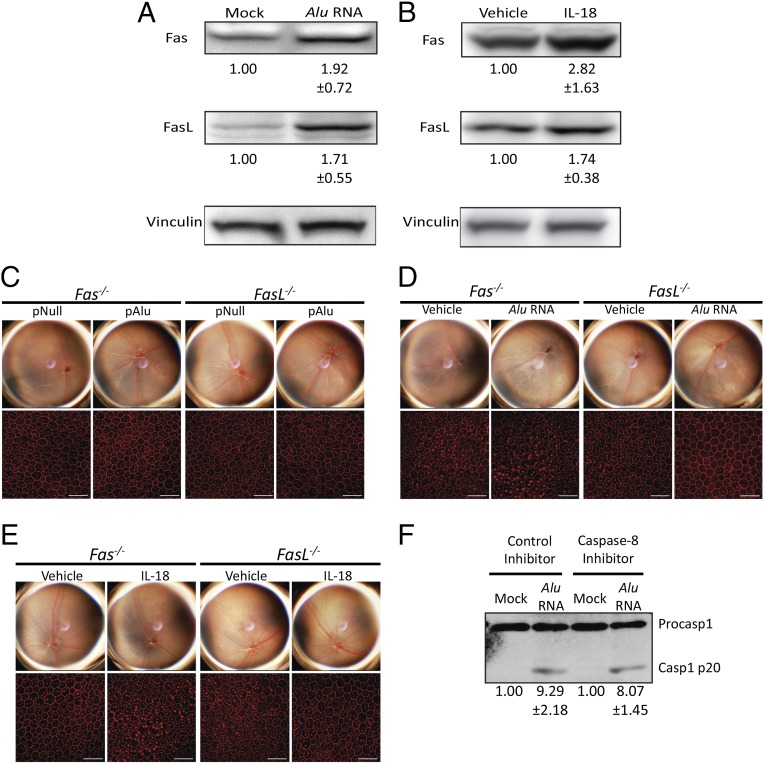
Caspase-8 activation requires upstream Fas ligand (FasL) binding to Fas receptor for cleavage of pro-Caspase-8 (14). Subretinal injection of Alu RNA causes up-regulation of both Fas and FasL (Fig. 4A), and intravitreous injection of recombinant mature IL-18 up-regulates both Fas and FasL in the RPE/choroid of wild-type mice (Fig. 4B). To determine whether increased Fas and FasL expression were involved in Alu RNA- and IL-18–induced RPE toxicity, we examined the susceptibility of Fas−/− and FasL−/− mice to RPE degeneration induced by Alu RNA or IL-18. Administration of Alu RNA, through subretinal injection of either Alu RNA or pAlu, did not induce RPE degeneration in either Fas−/− or FasL−/− mice (Fig. 4 C and D). Similarly, IL-18–mediated RPE degeneration was blocked in Fas−/− and FasL−/− mice (Fig. 4E). We found that Caspase-1 activation in Alu RNA-treated human RPE cells was not reduced by exposure to the Caspase-8 inhibitor peptide (Fig. 4F), implying that Caspase-8 acted downstream of Caspase-1.
Fig. 4.
Caspase-8 induces cell death via Fas and FasL. (A) Fas and FasL protein abundance in the RPE/choroid increases 3 d after subretinal injection of synthetic Alu RNA in wild-type mice as monitored by immunoblotting. Fas and FasL abundance is 1.92 ± 0.72 fold higher and 1.71 ± 0.55 higher in abundance in Alu RNA-injected mice, respectively. Fold change in Fas and FasL levels, compared with Mock treatment, normalized to Vinculin, as determined by densitometry (mean ± SEM), are reported below their respective bands. (B) Immunoblotting reveals that Fas and FasL protein abundance in the RPE/choroid increases 2 d after subretinal injection of recombinant IL-18 in wild-type mice. Fas and FasL abundance is 2.82 ± 1.63 fold higher and 1.74 ± 0.38 higher in abundance in IL-18–injected mice, respectively. Fold change in Fas and FasL levels, compared with vehicle treatment, normalized to Vinculin, as determined by densitometry (mean ± SEM), are reported below their respective bands. (C) Fundus photography and flat mounts stained with ZO-1 antibody (in red), show that Fas−/− and FasL−/− mice are protected against pAlu- (C) and Alu RNA-induced RPE degeneration (D). (E) Fundus photography and ZO-1–stained (red) flat mounts show that Fas−/− and FasL−/− mice are protected against IL-18–induced RPE degeneration. (F) The Caspase-8 inhibitory peptide does not impair Alu RNA-induced Caspase-1 activation in human RPE cells. Caspase-1 active p20 fragment is 9.29 ± 2.18 fold higher in abundance in control peptide inhibitor-treated cells and is 8.07 ± 1.45 higher in abundance in Caspase-8 inhibitor peptide-treated cells. Fold change in active Caspase-1 levels, compared with Mock treatment, normalized to Vinculin, as determined by densitometry (mean ± SEM) are reported below their respective bands. n = 8, P = 0.03 by Fisher exact test. Representative images shown (C–F).
Alu RNA Induces RPE Cell Death via Apoptosis.
Previously, we showed that Alu RNA induced activation of Caspase-3 in human RPE cells (3), a critical executioner in apoptotic cell death. We now show that Alu RNA induces Caspase-3 activation in vivo in the RPE of wild-type mice and that Caspase-3 activation is blocked by a Caspase-8 inhibitor peptide (SI Appendix, Fig. S7). Taken together, these data suggest that Caspase-8 lies between Caspase-1 and Caspase-3 in the mechanistic stream of Alu RNA-induced cell death. In addition, we found that Necrostatin-1, an inhibitor of an alternate cell death pathway termed necroptosis, did not confer protection against Alu RNA-induced RPE degeneration (SI Appendix, Fig. S8). Overall our data suggest that Alu RNA/IL-18–induced RPE degeneration in geographic atrophy is mediated via activation of Caspase-8–induced apoptosis in a manner dependent on Fas and FasL signaling.
Discussion
Human eyes with geographic atrophy exhibit decreased DICER1 expression and greater Alu RNA accumulation in their RPE (3, 5), and Alu RNA accumulation triggers RPE cell death via the NLRP3 inflammasome (4, 13). NLRP3 inflammasome activity requires two distinct events for downstream cell death pathways: priming (the up-regulation of inflammasome components) and activation (assembly of active NLRP3 inflammasome complexes) (15). Alu RNA induces priming of the NLRP3 inflammasome through mitochondrial ROS production and NF-κB signaling (4, 13). NF-κB signaling is triggered independently of TLR activation (4, 13), possibly through the action of a currently unidentified RNA sensor in the RPE. It is unclear how Alu RNA activates the NLRP3 inflammasome, but it is known that Caspase-1 is activated via a P2X7-dependent pathway (13). Although Caspase-1 is required for Alu RNA-induced toxicity, it is not required for IL-18–induced RPE cell toxicity, suggesting that RPE cells can undergo inflammasome-triggered cell death independent of pyroptosis (4). The precise death signaling pathways invoked by DICER1/Alu RNA dysmetabolism had been ill-defined.
Because we found that Alu RNA toxicity was not dependent on pyroptosis for cell death, this finding suggested that another cellular pathway may mediate RPE cell death in geographic atrophy. IL-18 induces MyD88-dependent signaling (16) by extracellular binding of IL-18 to IL-18 receptor (17), and the resultant signaling can up-regulate both Fas and FasL (18) to enhance Fas signaling of apoptosis (19).
We and others have shown that a synthetic, high-molecular weight, fully complementary double-stranded RNA known as poly I:C induces RPE degeneration (20, 21). A recent report showed that poly I:C can trigger necroptosis in the RPE (22), which can be unleashed in the absence of Caspase-8 (23). However, we found that an inhibitor of necroptosis, which blocked poly I:C-induced RPE degeneration (22), did not prevent Alu RNA-induced RPE degeneration when used at the same dose. This difference between Alu RNA, an AMD-associated endogenous transcript with a complex RNA structure, and synthetic poly I:C could be due to the fact that the RNA sensor MAVS/IPS-1, which is required for necroptosis (23), is activated by poly I:C (24) but not by Alu RNA (4).
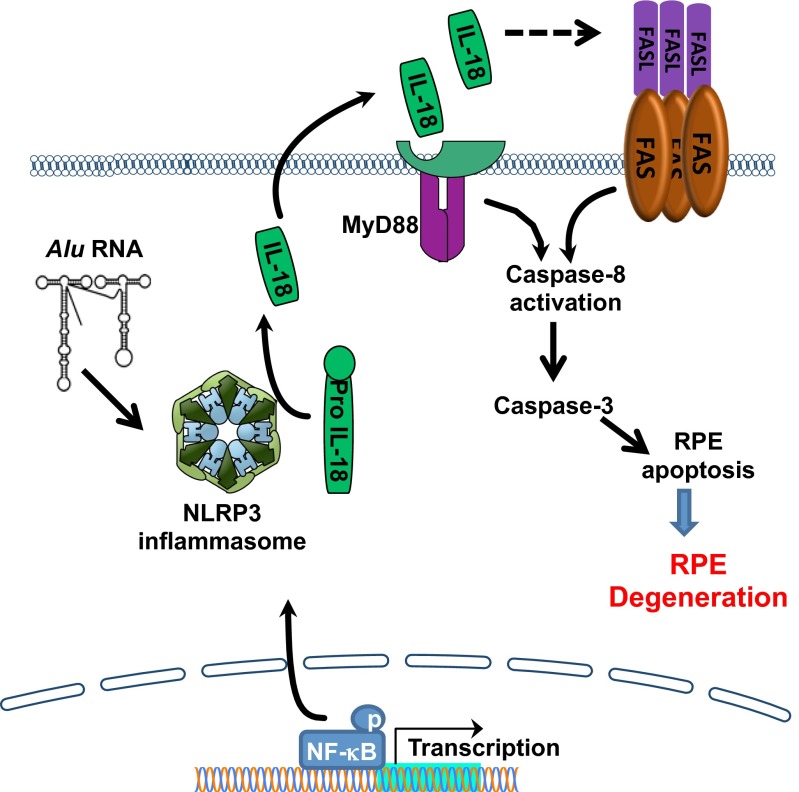
Caspase-8 modulates innate immune responses in a variety of ways: Caspase-8 can be involved in IL-1β and IL-18 processing independent of Caspase-1 activation by both AIM2-ASC (12) and MALT1-ASC (11) inflammasomes. Also, Caspase-8 can suppress NLRP3 inflammasome-dependent activation of IL-1β and IL-18 (25). Because we have previously shown that Alu RNA induces IL-18 processing in a Caspase-1–dependent manner (4) and we demonstrate here that Caspase-1 activation occurs despite Caspase-8 inhibition (Fig. 4F), we conclude that Caspase-8 mediates Alu RNA toxicity downstream of IL-18. A summary of our findings concerning Caspase-8 in RPE cell death in the context of the known Alu RNA toxicity mechanistic pathway is shown in Fig. 5.
Fig. 5.
Alu RNA induces Caspase-8–mediated cell death via the NLRP3 inflammasome. Alu RNA accumulation induces NF-κB–induced priming of the NLRP3 inflammasome, which then activates to cleave Pro-Caspase-1 to its mature, active form, Caspase-1. Caspase-1 in turn processes Pro-IL-18 to its mature, active form, which is then secreted by the RPE. IL-18 signaling induces up-regulation of Fas and FasL via IL-18 receptor and MyD88 to activate Caspase-8–mediated RPE cell death via Caspase-3.
Recently, it was reported that IL-18 neutralization augments choroidal neovascularization (CNV) in a mouse laser injury model (26). However, a conglomeration of five laboratories determined that this finding was in fact due to glycerol, a proangiogenic excipient in the IL-18 neutralizing antibody preparation (27). Subsequently, it was reported that recombinant murine IL-18 reduced choroidal neovascularization in a laser injury model (28). However, a multicentered study failed to reproduce those data, finding instead that IL-18 had no effect on CNV over 5-log dose range, and instead that IL-18 induced RPE degeneration (27), which has been independently corroborated (29).
Given the findings of this study, Fas, FasL, and Caspase-8 emerge as potential therapeutic targets for atrophic AMD. There are currently no treatment strategies that are capable of slowing the irreversible loss of vision in atrophic AMD despite increased understanding of the role the innate immune system plays in its pathology and progression (30, 31). Apoptosis of various cell types in CNV has been associated with Fas and FasL expression (32). Future investigation of the role Caspase-8 plays in AMD pathogenesis could aid in the improvement of our understanding of a complex disease that affects an increasing proportion of the population as it ages and in the development of therapies that focus on either Caspase-8 itself, its activators, or downstream pathway components. Any therapies that target Fas, FasL, or Caspase-8 would require rigorous testing for safety and efficacy, however, given the widespread importance of these proteins in cellular pathways in tissues not affected by disease.
Materials and Methods
Detailed descriptions of the following procedures are available in SI Appendix: human tissue, immunoblotting, subretinal injection, RPE flat mounts, Caspase-8 inhibitor, protein isolation, and protein quantification.
Immunohistochemistry.
Donor eyes or ocular tissues from age-matched patients with geographic atrophy (GA) due to age-related macular degeneration (AMD) or patients without AMD were obtained from various eye banks upon securing informed consent from donors or their families. These diagnoses were confirmed by dilated ophthalmic examination before acquisition of the tissues or eyes or upon examination of the eye globes post mortem. The study followed the guidelines of the Declaration of Helsinki. Studies of these de-identified cadaver specimens were exempt from human subjects research approval requirements. All diseased eyes had central GA involving the fovea whereas all of the control eyes had no visible features of AMD.
Ocular tissue was obtained from age-matched patients with or without geographic atrophy. Human Caspase-8 protein was stained with antibody from Cell Signaling.
Cell Culture and Transient Transfection.
Primary human fetal RPE cells were cultured in DMEM media (Cellgro) supplemented with 10% FBS at 37 °C.
Cell Viability.
Cell viability was quantified using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) assays (CellTiter 96 AQueous One Solution Cell Proliferation Assay) according to the manufacturer’s instructions (Promega).
Animals.
All animal studies were approved by University of Kentucky Institutional Animal Care and Use Committee (IACUC) and performed according to their guidelines. C57BL/6J (wild type), Dicer1f/f, Fas−/−, and FasL−/− mice were purchased from The Jackson Laboratory. Casp8f/f mice have been previously described (33). Ablation of Dicer1 and Casp8 in those mice was performed using adeno-associated virus (AAV1) vector coding for Cre recombinase under the control of an RPE-specific promoter (BEST1).
Subretinal Injection.
Plasmids (1 µL volume), 2 µg Caspase-8 inhibitor peptide, and 40 ng recombinant IL-18 were delivered to the subretinal space in mouse eyes using an Ito microsyringe (Ito Corporation).
In Vitro Transcription of Alu RNA.
Synthetic Alu RNA was created using the AmpliScribe T7-Flash Transcription Kit (Epicentre) using the manufacturer’s protocol.
Supplementary Material
Acknowledgments
We thank L. Toll, G. R. Pattison, R. King, L. Xu, M. McConnell, C. Payne, D. Robertson, G. Botzet, K. Ambati, and A. Uittenbogaard for technical assistance. J.A. was supported by National Institutes of Health (NIH) Grants DP1GM114862, R01EY018350, R01EY018836, R01EY020672, R01EY022238, and R01EY024068 and Doris Duke Distinguished Clinical Scientist Award, Burroughs Wellcome Fund Clinical Scientist Award in Translational Research, Ellison Medical Foundation Senior Scholar in Aging Award, Dr. E. Vernon Smith and Eloise C. Smith Macular Degeneration Endowed Chair, Foundation Fighting Blindness Individual Investigator Research Award, Carl Reeves Foundation, and Harrington Discovery Institute Scholar-Innovator Award. Y.H. was supported by an Alcon Japan Research award; T.Y. was supported by a Fight for Sight postdoctoral award; B.J.F. was supported by NIH Grants T32HL091812 and UL1RR033173; A.B.-C. was supported by the Programme for Advanced Medical Education (sponsored by Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal) and Bayer Global Ophthalmology Research Award; N.K. was supported by Beckman Initiative for Macular Research and NIH Grant K99EY024336; B.D.G. was supported by American Heart Association and International Retinal Research Foundation (IRRF); B.K.A. was supported by NIH Grants R01EY017182 and R01EY017950, VA Merit Award, and Department of Defense; D.R.H. was supported by NIH Grants P30EY003040 and R01EY001545 and Arnold and Mabel Beckman Foundation; W.W.H. was supported by NIH Grants P30EY021721 and R01EY17549 and Macular Vision Research Foundation, Overstreet Fund and Research to Prevent Blindness; and C.W. was supported by The Loris and David Rich Postdoctoral Scholar Award (IRRF).
Footnotes
Conflict of interest statement: W.W.H. and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403814111/-/DCSupplemental.
References
- 1.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 2.Ketting RF, et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15(20):2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneko H, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarallo V, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149(4):847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dridi S, et al. ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci USA. 2012;109(34):13781–13786. doi: 10.1073/pnas.1206494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haneklaus M, O’Neill LA, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: Recent developments. Curr Opin Immunol. 2013;25(1):40–45. doi: 10.1016/j.coi.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurung P, et al. FADD and Caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192(4):1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. J Immunol. 2013;191(9):4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13(3):246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 12.Pierini R, et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19(10):1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerur N, et al. TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54(12):7395–7401. doi: 10.1167/iovs.13-12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012;19(1):42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: An integrated view. Immunol Rev. 2011;243(1):136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi H, et al. TRAM is involved in IL-18 signaling and functions as a sorting adaptor for MyD88. PLoS ONE. 2012;7(6):e38423. doi: 10.1371/journal.pone.0038423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother. 2002;25(Suppl 1):S4–S11. doi: 10.1097/00002371-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, et al. IL-18 mediates proapoptotic signaling in renal tubular cells through a Fas ligand-dependent mechanism. Am J Physiol Renal Physiol. 2011;301(1):F171–F178. doi: 10.1152/ajprenal.00339.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsuki T, et al. Interleukin 18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res. 1997;17(5A):3253–3258. [PubMed] [Google Scholar]
- 20.Yang Z, et al. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359(14):1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiose S, et al. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011;286(17):15543–15555. doi: 10.1074/jbc.M111.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami Y, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21(2):270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou J, et al. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 2013;38(4):717–728. doi: 10.1016/j.immuni.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Doyle SL, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano Y, et al. IL18 is not therapeutic for neovascular age-related macular degeneration. Nat Med 2014 doi: 10.1038/nm.3671. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle SL, et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med. 2014;6(230):230ra244. doi: 10.1126/scitranslmed.3007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ijima R, et al. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Invest Ophthalmol Vis Sci. September 18, 2014 doi: 10.1167/iovs.14-15367. [DOI] [PubMed] [Google Scholar]
- 30.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinton DR, He S, Lopez PF. Apoptosis in surgically excised choroidal neovascular membranes in age-related macular degeneration. Arch Ophthalmol. 1998;116(2):203–209. doi: 10.1001/archopht.116.2.203. [DOI] [PubMed] [Google Scholar]
- 33.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17(7):883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.