Significance
The human eye with its prominent white sclera is thought to facilitate social and cooperative interactions among humans. While there is evidence for brain mechanisms that allow for the unconscious detection of eye cues in adults, it is not known whether this ability of the human brain emerges early in ontogeny and can therefore be considered a key feature of human social functioning. The current study provides neural evidence for the unconscious detection of emotion and gaze cues from the sclera in 7-mo-old infants. Our findings demonstrate the existence of fast, efficient, and reliable social cue detection mechanisms in the human infant brain that likely provide a vital foundation for the development of social interactive skills.
Keywords: infancy, social perception, human eye, subliminal processing, emotion processing
Abstract
Human eyes serve two key functions in face-to-face social interactions: they provide cues about a person’s emotional state and attentional focus (gaze direction). Both functions critically rely on the morphologically unique human sclera and have been shown to operate even in the absence of conscious awareness in adults. However, it is not known whether the ability to respond to social cues from scleral information without conscious awareness exists early in human ontogeny and can therefore be considered a foundational feature of human social functioning. In the current study, we used event-related brain potentials (ERPs) to show that 7-mo-old infants discriminate between fearful and nonfearful eyes (experiment 1) and between direct and averted gaze (experiment 2), even when presented below the perceptual threshold. These effects were specific to the human sclera and not seen in response to polarity-inverted eyes. Our results suggest that early in ontogeny the human brain detects social cues from scleral information even in the absence of conscious awareness. The current findings support the view that the human eye with its prominent sclera serves critical communicative functions during human social interactions.
Eyes play a key role in human social encounters, as they are critically involved in perceiving others as having minds (1), in attributing mental states to others (2), and in social coordination during face-to-face interactions (3). The presence of eyes has also been shown to increase cooperative behavior in laboratory and in real-world contexts (2, 4–7). The human eye is unique in that it is characterized by a prominent white sclera several times larger than that of other primates (8, 9), which allows for the efficient communication and detection of social information. It is thought that the human sclera is adapted to facilitate interpersonal communication and cooperative interactions among humans (10). When humans observe others’ faces, eyes are typically the first features that are scanned for information (11), and, compared with other primates, humans show a stronger focus on the eye region than on other parts of the face when scanning faces (12, 13). Conversely, failure to devote special attention to the eye region during face perception has been linked to severe social deficits that can, for instance, be observed in autism spectrum disorder (14).
One reason why human eyes have such prime importance is that emotion perception, as a vital part of any social interaction, heavily relies on information from the eye region (14). This is especially important for the detection of fear in others, as one of the most basic forms of identifying threatening situations. Fear detection has been observed in response to eyes alone (15, 16). This mechanism operates exceptionally fast (17) and occurs irrespective of conscious awareness (15). On a neural level, the processing of fearful eyes critically depends on the amygdala. Depending on the context (18, 19), fearful eyes can elicit an enhanced activation of the amygdala (20), even if not perceived consciously (15). Patients with bilateral amygdala lesions show deficits in recognizing fear, which disappear when they are instructed to focus on the eye region (21). Furthermore, there is recent evidence to show that the amygdala is involved in reflexively directing attention to the eyes and in predicting gaze to fearful eyes (22, 23).
Another important social cue conveyed by the eye is the direction of gaze. Eye gaze can inform us about another person’s attentional focus, thereby providing clues about future behavior (24). Critically, eye gaze and emotion perception have been shown to powerfully interact. For example, fearful eyes elicit stronger behavioral and neural responses when averted from than when directed at an observer (25, 26). This presumably relates to the fact that averted fearful eyes inform an observer about a potential danger in the environment (clear threat), whereas directed fearful eyes signal fear of the observer (ambiguous threat). At the brain level, this also relies on the amygdala, as reflected in a differential activation for direct compared with averted gaze (25, 27). Furthermore, behavioral studies suggest that similar to emotion processing, eye gaze discrimination operates even in the absence of conscious awareness (28).
Attending and responding to eyes is thought to play a vital role in the early development of social skills (29). From birth, infants respond sensitively to human eyes: Newborns prefer direct gaze faces over averted gaze faces (30) and even show a rudimentary form of gaze following (31). Newborns’ sensitivity to eyes has been shown to be specific to the human sclera, as behavioral preferences disappear when the contrast polarity of the eye is reversed (32). Nevertheless, the ability to attend to the eyes and follow gaze improves considerably over the course of the first year of life and is viewed as an important marker of healthy social development (33, 34). Indeed, orienting to the eyes is present early in infancy but declines between 2 and 6 mo in infants later diagnosed with autism (29). With respect to responding to emotional information, newborns also show a basic sensitivity to (familiar) emotional facial expressions for which they may also use eye cues (30, 35, 36). However, it is not until the age of 7 mo that they show a robust attentional bias to fear, as reflected in their neural and behavioral responses (37–41). The developmental emergence of this fear bias has been linked to the maturation of frontolimbic circuits (42–45) and occurs at a point in development when infants begin to first experience fear themselves (46, 47). Despite our growing understanding of the developmental origins of emotion and gaze processing in humans, some fundamental questions concerning the exact nature of this ability remain unanswered.
In the present study we therefore addressed two key questions, which are essential to understanding the mechanisms that underpin sensitive responding to human eyes in infants. First, we asked whether infants’ detection of social cues such as fear and gaze from eyes occurs in the absence of conscious awareness. Second, we examined whether the detection of these social cues can be seen in response to scleral information alone. To address these questions, we conducted two experiments, based on an established paradigm from adult literature (15), which investigated whether the infant brain can discriminate between fearful and nonfearful eyes (experiment 1) and between direct and averted fearful eyes (experiment 2), even if the stimuli are not perceived consciously. We hypothesized that if the eyes indeed serve a critical function in human social communication, then the unconscious detection of social cues from scleral information should be evident early in ontogeny. Specifically, according to our hypothesis, using event-related brain potentials (ERPs), we expected infants to show evidence for neural discrimination between fearful and nonfearful human eyes (experiment 1) and direct and averted gaze (experiment 2).
Experiment 1: Emotion Processing
In experiment 1, we examined ERPs in a group of 7-mo-old infants in response to fearful and nonfearful (happy) sclerae as well as polarity-inverted versions of these stimuli (Fig. 1). Stimuli were presented for only 50 ms, which is well below the perceptual threshold established for this age group (48, 49). Each stimulus was followed by a mask consisting of a neutral facial expression performed by a different actor, which was presented for 750 ms. Our analysis was focused on early visual processes at occipital electrodes (P1, a typical event-related response indicating early visual processing) and later attentional processes at frontal electrodes (Pb and Nc, two event-related responses commonly observed in attentional orienting in infants). We assessed the mean amplitude and peak latency of these ERP components during three time windows: 150–250 ms after stimulus onset at occipital electrodes (P1), and 300–400 (Pb) as well as 400–800 ms (Nc) after stimulus onset at frontal electrodes. Visual inspection indicated a later occipital effect (following the P1) corresponding to the infant P400; however, statistical analysis did not reveal any significant effects for this component. For each time window, we computed two repeated measure ANOVAs with the factors emotion (fearful, nonfearful), polarity (original, inverted), and hemisphere (left, right), one investigating effects on the mean amplitude and one investigating effects on the peak latency.
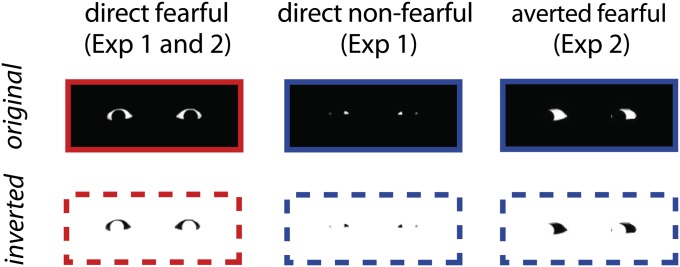
Fig. 1.
Stimuli. Top Row shows the stimulus material for experiments 1 and 2 is shown in the original condition. Bottom Row shows the corresponding polarity-inverted version. Direct fearful eyes (first column) were used in both experiments, direct nonfearful eyes (second column) were shown only in experiment 1, and averted fearful eyes (third column) only in experiment 2.
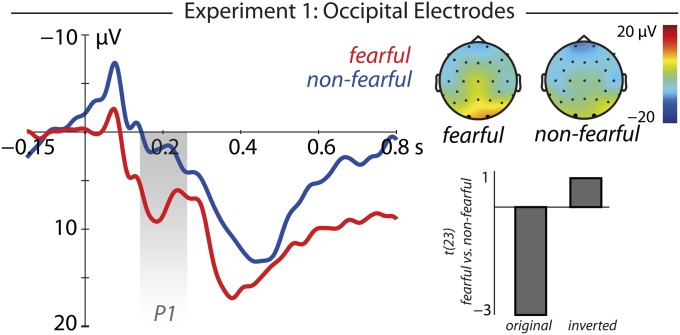
We observed an interaction between the factors emotion and polarity (F(1,23) = 5.88, P = 0.024, η2 = 0.20) at occipital electrodes in the time window of 150–250 ms after stimulus onset. Further analysis revealed that for original stimuli, fearful sclerae elicited a larger amplitude than nonfearful ones [t(23) = −3.00, P = 0.006, r = 0.53, nonfearful 1.93 ± 2.59 μV (mean ± SEM), fearful 7.69 ± 2.57 μV; Fig. 2]. Critically, no difference was observed for polarity-inverted stimuli (see Fig. S1 for ERP results of the polarity-inverted control conditions), indicating that the observed ERP differences are unlikely to be explained by basic perceptual differences.
Fig. 2.
Results at occipital electrodes in experiment 1. At occipital electrodes, original fearful eyes elicited a larger P1 amplitude than original nonfearful eyes. No such difference was observed for polarity-inverted stimuli, as shown by the contrast between fearful and nonfearful eyes (t values).
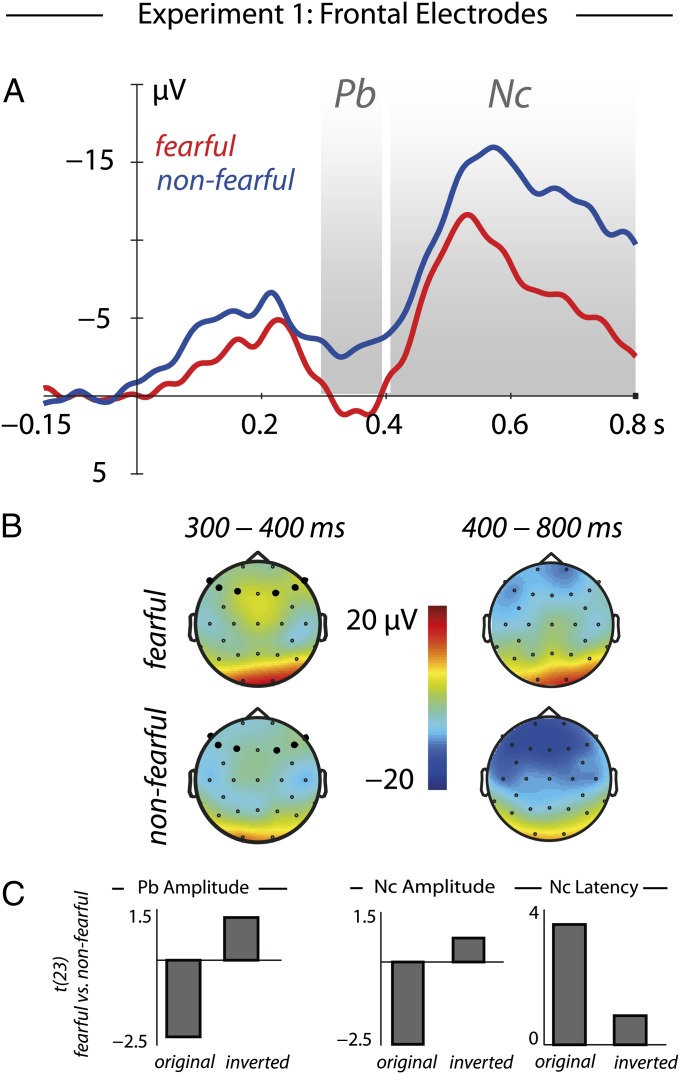
Our analysis further revealed similar effects at frontal electrodes during later time windows (Fig. 3). Specifically, we observed an interaction between emotion and polarity [300–400 ms: F(1,23) = 5.97, P = 0.023, η2 = 0.20, 400–800 ms: F(1,23) = 4.28, P = 0.050, η2 = 0.16], showing a more negative amplitude in response to nonfearful compared with fearful human eyes [300–400 ms: t(23) = −2.24, P = 0.035, r = 0.42, nonfearful −3.13 ± 1.41 μV, fearful 0.63 ± 1.58 μV; 400–800 ms: t(23) = −2.49, P = 0.02, r = 0.46, nonfearful −11.77 ± 1.27 μV, fearful −6.67 ± 1.83 μV]. No difference was found in response to polarity-inverted stimuli. Furthermore, an interaction between emotion and polarity [F(1,23) = 7.32, P = 0.013, η2 = 0.24] revealed that fearful eyes resulted in an earlier peak of the Nc compared with nonfearful eyes [t(23) = 3.55, P = 0.0017, r = 0.56, nonfearful 609 ± 18 ms, fearful 546 ± 14.2 ms]. These frontal effects demonstrate that in addition to early visual processes (P1) at occipital electrodes, the detection of fearful eyes impacts later processes associated with prefrontal brain regions involved in attentional resource allocation.
Fig. 3.
Results at frontal electrodes in experiment 1. (A) For original eyes, the responses to fearful and nonfearful eyes clearly differed at frontal electrodes between 300 and 400 ms (Pb) in amplitude as well as between 400 and 800 ms (Nc) in amplitude and latency. (B) Topographical distributions during these two time windows for fearful and nonfearful eyes. Whereas these effects were observed for original stimuli, no differences were found for the polarity-inverted control condition, as shown by the contrast between fearful and nonfearful eyes (t values) (C), for Pb amplitude, Nc amplitude, and Nc latency (from Left to Right).
The results presented in experiment 1 demonstrate that in 7-mo-old infants the neural discrimination between fearful and nonfearful eyes is limited to human sclera and occurs in the absence of conscious perception. This had previously only been shown in adults (15). In experiment 2, we examined whether in addition to emotion cues infants can also detect more subtle cues from human sclera important for the discrimination of gaze direction.
Experiment 2: Gaze Processing
In experiment 2, we examined ERPs in response to gaze cues in another group of 7-mo-old infants. Infants viewed fearful direct gaze and fearful averted gaze sclerae as well as a contrast-inverted version of these stimuli (Fig. 1); otherwise, the experimental protocol and analysis were identical to experiment 1.
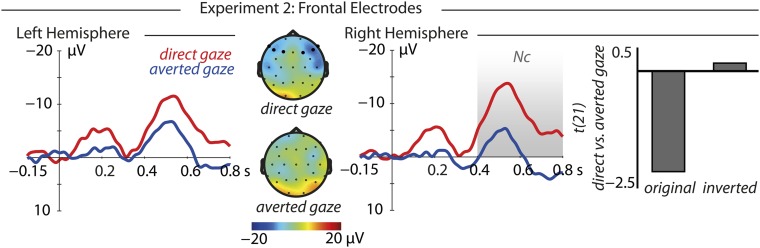
Contrary to experiment 1, our analysis did not reveal any significant effects at occipital electrodes (all P > 0.1). However, similar to experiment 1, we observed an interaction between gaze direction, polarity, and hemisphere at frontal electrodes [F(1,21) = 4.3, P = 0.05, η2 = 0.17] (Fig. 4). Only in the right hemisphere and only for original stimuli did the Nc differ between gaze directions [F(1,21) = 4.4, P = 0.048, η2 = 0.17]; direct gaze elicited greater Nc than averted gaze [t(21) = −2.14, P = 0.044, r = 0.42, direct −8.27 ± 2.95 μV, averted −0.25 ± 3.65 μV]. Note that although this effect was only significant in the right hemisphere, which is sometimes observed in studies of face processing (50, 51), the ERPs showed a similar modulation in the left hemisphere and the overall topography of the effect was similar to experiment 1 (Fig. 4; see Fig. S2 for ERP results of the polarity-inverted control condition).
Fig. 4.
Results at frontal electrodes in experiment 2. At right-hemispheric electrodes, direct gaze eyes elicited larger Nc amplitudes than averted gaze eyes (400–800 ms). The contrast between fearful and nonfearful stimuli (t values) shows that this pattern was only observed in response to original but not polarity-inverted stimuli.
The results in experiment 2 show that, whereas gaze direction does not affect early visual processing, it does impact later frontal brain processes associated with attentional orienting in 7-mo-old infants. Extending the findings of experiment 1, experiment 2 demonstrates that the neural discrimination between direct and averted gaze is limited to human sclera and occurs in the absence of conscious perception. Thus, the data from these two experiments indicate that unconscious fear and gaze processing is tuned to the human-specific sclera.
Discussion
The current findings demonstrate that 7-mo-old infants’ brains detect social cues from human-specific scleral information without conscious awareness. Our results show that infants are able to unconsciously distinguish between fearful and nonfearful sclerae (experiment 1) and direct and averted gaze (experiment 2). The finding that in both experiments ERP effects were only observed for original but not for polarity-inverted stimuli suggests that responding to these cues is limited to human-specific scleral information and is unlikely to be accounted for by basic perceptual differences (see also 32, 52). Our results indicate that the human brain is tuned to social cues conveyed by the eyes from early in ontogeny. This early emergence of responding to unconscious scleral information is in line with evolutionary accounts that have stipulated that the human eye (and sclera) is adapted to serve human-unique communicative functions and is critically involved in promoting prosocial behavior and cooperation (4–10, 53).
With respect to the neural processes elicited by subliminally viewing eye whites, the present data indicate that the detection of fear and gaze cues is reflected in the Nc evoked over frontal brain regions. This is in line with work that has implicated the Nc in differential attention allocation in response to supraliminally presented emotion and gaze stimuli (41, 54–57). It suggests that the brain processes associated with the Nc function regardless of whether or not emotional (fear) or gaze information is consciously perceived. The existence of detection mechanisms that operate independently of conscious perception in early ontogeny is evidence for how effectively the human brain is tuned to pick up on evolutionarily relevant social signals. The finding that these detection processes can be observed in response to cues provided by the human sclera alone is in agreement with the view that selective attention to the human eye emerges during infancy and is a key feature of healthy social functioning (29).
Moreover, the finding that in both experiments ERP effects were observed for the Nc at frontal electrodes is interesting with regard to the underlying brain mechanisms. Specifically, prior work has localized the sources of the Nc in anterior cingulate and prefrontal cortex (58), which are brain regions that in adults and young children are strongly interconnected with the amygdala (59) and are viewed as key components of frontolimibic circuits in the human brain. Whereas our results point to a role of the amygdala in the processes observed in the current experiments and are thus principally in support of the view that the amygdala is important for social processing early in development (42–44), there is no direct evidence for amygdala involvement in our ERP data. Nonetheless, our findings are consistent with accounts that assign a major role to amygdala–prefrontal cortex circuits in the unconscious processing of fear as well as gaze in adults (15, 25) and are also in line with developmental models according to which amygdala–prefrontal circuits become functional at around 7 mo of age (43).
Interestingly, in adults, enhanced amygdala activity has also been observed in response to surprised eyes, which, similar to fearful eyes, are characterized by large visible sclerae (60–62). It is thus not clear whether the current results are specific to fear or can be attributed to a more general sensitivity to expressions that signal uncertainty (63). Moreover, with respect to the observed discrimination between fearful and nonfearful (happy) eyes it is important to mention that the effects might be related to the fact that infants are likely more familiar with happy than fearful faces (64). However, in prior behavioral work with infants of the same age as tested in the current study, it has been shown that infants look longer at and are slower to disengage attention from fearful but not equally unfamiliar facial expressions (65), suggesting that the fear bias observed in this age group cannot easily be explained by the familiar versus novel expression distinction. Furthermore, in prior infant ERP work, it has also been shown that different negative emotions that are similarly unfamiliar to the infant evoke distinct responses in the infant brain, which further supports the notion that negative expressions are not simply processed as novel (unfamiliar) by infants of that age (55, 66). Nevertheless, although unlikely, we cannot completely rule out the possibility that the emotion effects observed in the present study might partly be driven by a familiarity versus novelty distinction.
Regarding the exact patterns of brain responses observed in the current study, our results revealed that nonfearful eyes evoked a larger Nc than fearful eyes (experiment 1). This is in contrast to prior ERP work with infants using face stimuli presented above the perceptual threshold (for at least 500 ms) that found a larger Nc for fearful than for happy faces (38, 39). This discrepancy might be related to methodological differences across studies concerning the duration of presentation (subliminal, supraliminal), the nature of the stimulus (face, eyes), or a combination of the two. The results of experiment 1 further showed that viewing fearful eyes evoked a greater Pb (positivity before the Nc) at frontal electrodes and resulted in an earlier peak of the Nc than nonfearful eyes. This suggests that viewing fearful eyes elicits brain processes that allow for the rapid discrimination between fearful eyes and other eye stimuli (17). Furthermore, a frontal positivity similar to the Pb observed here in infants is involved in the detection of fearful faces in adults (67, 68), pointing to shared brain processes implicated in fear detection across development. As this effect (and the occipital effect discussed hereafter) was absent in experiment 2, this may suggest that the discrimination of emotion cues from eyes occurs more rapidly than the detection of eye gaze cues.
In experiment 2, fearful eyes with direct gaze evoked a larger Nc than fearful eyes with averted gaze, which is in contrast to adult models of fear detection that show greater sensitivity to fearful faces in the context of averted gaze (25, 26). This increased neural sensitivity to eyes showing direct gaze might be explained by the fact that especially during infancy, direct gaze (eye contact) serves as such a powerful signal in directing attention and learning (eye contact effect, ref. 69) and only with further development, mature (adult-like) responding to averted gaze fear is achieved. Regardless of the exact interpretations, the current results clearly demonstrate that brain mechanisms exist, which allow infants to readily respond to information from eyes, even in the absence of conscious awareness.
In addition, only in experiment 1, we observed ERP effects during early visual processing. Specifically, in 7-mo-old infants, fearful eyes were detected during the earliest stages of visual processing as reflected in an enhanced P1. This is similar to what is known from adults (17, 70) and points to an efficient detection of visual information that indexes threat. The findings that this effect is absent in experiment 2 may be explained by the fact that the contrast between fearful and nonfearful eyes in experiment 1 is greater than the gaze manipulation used in experiment 2. This is the case both at a physical stimulus level, where the difference in scleral size between fearful and nonfearful eyes is larger compared with the difference between direct and averted gazes, but also on a more conceptual level, where the former potentially leads to a distinction between two different emotions and the latter is related to the detection of subtler differences for the same emotion depending on gaze. Therefore, gaze manipulations may not be salient enough to influence processing at this early sensory stage. This is supported by findings with adults reporting emotion effects during early visual processing but an interaction with gaze only during later processing stages (71).
Conclusion
The current study, to our knowledge, is the first to demonstrate that fear and gaze detection in infants occurs in the absence of conscious perception and is based on human-specific scleral information. This shows that two essential functions of human eyes during communication, namely emotion and gaze cueing, emerge early in ontogeny, emphasizing their pivotal role in human social functioning. From an evolutionary point of view, our data further support the hypothesis that the human eye and particularly its white sclera plays a special role in social communication (10) and cooperative behavior among humans (4–7). Our findings provide evidence for the existence of fast, efficient, and reliable social cue detection mechanisms in the human infant brain that likely provide a vital foundation for the development of social interactive skills.
Methods
Participants.
All infants were born full term and had a birthweight of at least 2,800 g (experiment 1: n = 24, mean age: 216 d, range: 209–225 d, 13 female; experiment 2: n = 22, mean age: 211 d, range: 194–223 d, 9 female). An additional six infants (three for each experiment) were tested but not included in the final sample because of continued crying (n = 1) or failure to contribute at least 10 artifact-free trials per condition (n = 5). The infants in experiment 1 contributed on average 31.6 (SD = 14.3) trials per condition, and the infants in experiment 2 contributed on average 28.3 (SD = 10.6) trials per condition. The number of trials did not differ between conditions for either experiment. The parents gave written informed consent and the study was approved by the ethics committee of the University of Leipzig and conducted according to the declaration of Helsinki.
Stimuli.
The stimulus material used in experiment 1 was based on Whalen et al. (15). It consisted of fearful and happy (i.e., nonfearful) photographs from eight individuals (5 female, ref. 72), from which all facial information was removed except for the sclera. The remainder of the photograph was colored in black. Furthermore, polarity-inverted versions of these stimuli were created, showing a black “sclera” on a white background. Grayscale neutral expressions of the same individuals thresholded to contain only black and white were used as masks. Both stimuli and masks were presented against a gray background [red, green, and blue (RGB) values of 128] to achieve an equal distance in luminance from the original (white sclera on black background) and the inverted picture (black sclera on white background). The stimuli had a width of 9 cm and a height of 15 cm. For experiment 2, instead of happy photographs, we manipulated the fearful stimuli by shifting the pupil to the left or the right using Adobe Photoshop. As in experiment 1, polarity-inverted versions of these stimuli were created.
Design.
Each experiment contained four conditions, resulting in a 2 × 2 design with the factors polarity (original, inverted) and emotion (fearful, nonfearful) for experiment 1 and polarity (original, inverted) and gaze direction (direct, averted) for experiment 2 (for the averted gaze condition left and right gaze occurred with the same probability of 50%). In each condition, 80 trials were shown (10 per actor), adding up to a total of 320 trials. Each participant received an individual randomization, consisting of 10 blocks with 32 trials (8 per condition) that were presented consecutively without interruption. We ascertained that the same condition did not occur more than twice in a row.
Each trial started with the presentation of a black fixation star for 500 ms, followed by the actual stimulus for 50 ms. After the stimulus, a neutral face of a different actor was presented as a mask for 750 ms. The mask was followed by an intertrial interval, during which a gray screen was shown for a randomly varying duration of 800–1200 ms. The presentation of the images was synchronized to the vertical refresh rate of the monitor.
Procedure.
Upon arrival in the laboratory, parents and infants were familiarized with the environment, and parents were informed about the study and signed a consent form. The EEG recording was prepared while the infant was sitting on his/her caregiver’s lap. For the recording, an elastic cap was used, in which 27 AgAgCl electrodes were mounted according to the 10–20 system. The Cz electrode was used as a reference during recording. We attached an additional electrode below the infant’s right eye to compute the electrooculogram. A PORTI-32/MREFA (Twente Medical Systems) amplifier was used to record the EEG signal with a sampling rate of 500 Hz.
The experiment took place in a sound-shielded chamber in which the caregiver was seated with the infant on his/her lap. The caregiver was instructed not to interact with the child during the experiment. Stimuli were presented on a monitor (resolution: 1,024 × 768, refresh rate: 60 Hz) positioned ∼90 cm in front of the infant. Above the monitor, a camera was mounted to record the infant’s looking behavior during the experiment. If the infant did not look at the screen during the experiment, short video clips with colorful moving shapes accompanied by ring tones were presented to redirect the infant’s attention to the screen. The experiment ended when the infant had seen the maximum number of trials or became too fussy.
EEG Analysis.
Data were rereferenced to the mean of TP9 and TP10, and bandpass filtered between 0.2 and 20 Hz. Trials were segmented into 1-s epochs from 200 ms before stimulus onset until 800 ms after stimulus onset. In several participants (four in experiment 1 and six in experiment 2), one electrode was noisy and was therefore interpolated using spherical spline interpolation (73). To detect trials contaminated by artifacts, the SD was computed in a sliding window of 200 ms within these epochs. If the SD exceeded 80 μV at any electrode, the entire trial was rejected from further analysis. Furthermore, all data were inspected visually to screen for any remaining artifacts. In addition, the video recording of the infant’s looking behavior was checked and trials in which the infant did not attend to the screen were excluded from the analysis.
Data were averaged for each condition and a baseline correction was performed using an interval of 150 ms before stimulus onset as baseline. We analyzed ERPs in two regions of interest (ROI), a frontal ROI including F9, F7, F3, F4, F8, and F10 and an occipital ROI including O1 and O2. For the frontal ROI, time windows of 300–400 as well as 400–800 ms after stimulus onset were analyzed and in the occipital ROI a time window of 150–250 ms was analyzed. This was done to examine effects on infant Pb, Nc, and P1, respectively. For each time window, the mean amplitudes across the respective electrodes were computed and entered into a repeated measures ANOVA with the factors emotion (fearful, nonfearful) (experiment 1) or gaze direction (direct, averted) (experiment 2), polarity (original, inverted), and hemisphere (left, right). In addition, we computed the same analysis investigating the peak latency by using either the maximum (Pb, P1) or the minimum (Nc) in the same time windows as mentioned above. Student t tests were computed to further analyze significant interaction effects.
Supplementary Material
Acknowledgments
We thank Paul J. Whalen for providing us with the stimulus material and the families for participating in the study. This work was supported by funding awarded by the Max Planck Society (T.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411333111/-/DCSupplemental.
References
- 1.Looser CE, Wheatley T. The tipping point of animacy. How, when, and where we perceive life in a face. Psychol Sci. 2010;21(12):1854–1862. doi: 10.1177/0956797610388044. [DOI] [PubMed] [Google Scholar]
- 2.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- 3.Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neurosci Biobehav Rev. 2009;33(6):843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateson M, Nettle D, Roberts G. Cues of being watched enhance cooperation in a real-world setting. Biol Lett. 2006;2(3):412–414. doi: 10.1098/rsbl.2006.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernest-Jones M, Nettle D, Bateson M. Effect of eye images on everyday cooperative behavior: A field experiment. Evol Hum Behav. 2011;32:172–178. [Google Scholar]
- 6.Nettle D, Nott K, Bateson M. ‘Cycle thieves, we are watching you’: Impact of a simple signage intervention against bicycle theft. PLoS ONE. 2012;7(12):e51738. doi: 10.1371/journal.pone.0051738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnham TC, Hare B. Engineering human cooperation. Does involuntary neural activation increase public goods contributions? Hum Nat. 2007;18:88–108. doi: 10.1007/s12110-007-9012-2. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Kohshima S. Unique morphology of the human eye. Nature. 1997;387(6635):767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: Comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40(5):419–435. doi: 10.1006/jhev.2001.0468. [DOI] [PubMed] [Google Scholar]
- 10.Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: The cooperative eye hypothesis. J Hum Evol. 2007;52(3):314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Bindemann M, Scheepers C, Burton AM. Viewpoint and center of gravity affect eye movements to human faces. J Vis. 2009;9(2):7.1–16. doi: 10.1167/9.2.7. [DOI] [PubMed] [Google Scholar]
- 12.Kano F, Call J, Tomonaga M. Face and eye scanning in gorillas (Gorilla gorilla), orangutans (Pongo abelii), and humans (Homo sapiens): Unique eye-viewing patterns in humans among hominids. J Comp Psychol. 2012;126(4):388–398. doi: 10.1037/a0029615. [DOI] [PubMed] [Google Scholar]
- 13.Kano F, Tomonaga M. Face scanning in chimpanzees and humans: Continuity and discontinuity. Anim Behav. 2010;79:227–235. [Google Scholar]
- 14.Baron-Cohen S. Mindblindness. An Essay on Autism and Theory of Mind. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- 15.Whalen PJ, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- 16.Fox E, Damjanovic L. The eyes are sufficient to produce a threat superiority effect. Emotion. 2006;6(3):534–539. doi: 10.1037/1528-3542.6.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W, et al. Human brain responsivity to different intensities of masked fearful eye whites: An ERP study. Brain Res. 2009;1286:147–154. doi: 10.1016/j.brainres.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 18.Straube T, Dietrich C, Mothes-Lasch M, Mentzel HJ, Miltner WH. The volatility of the amygdala response to masked fearful eyes. Hum Brain Mapp. 2010;31(10):1601–1608. doi: 10.1002/hbm.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MJ, et al. Behind the mask: The influence of mask-type on amygdala response to fearful faces. Soc Cogn Affect Neurosci. 2010;5(4):363–368. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JS, deBonis M, Dolan RJ. Human amygdala responses to fearful eyes. Neuroimage. 2002;17(1):214–222. doi: 10.1006/nimg.2002.1220. [DOI] [PubMed] [Google Scholar]
- 21.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 22.Gamer M, Büchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29(28):9123–9126. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamer M, Schmitz AK, Tittgemeyer M, Schilbach L. The human amygdala drives reflexive orienting towards facial features. Curr Biol. 2013;23(20):R917–R918. doi: 10.1016/j.cub.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Carlin JD, Calder AJ. The neural basis of eye gaze processing. Curr Opin Neurobiol. 2013;23(3):450–455. doi: 10.1016/j.conb.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Adams RBA, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300(5625):1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- 26.Adams RBA, Jr, Kleck RE. Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5(1):3–11. doi: 10.1037/1528-3542.5.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Straube T, Langohr B, Schmidt S, Mentzel HJ, Miltner WH. Increased amygdala activation to averted versus direct gaze in humans is independent of valence of facial expression. Neuroimage. 2010;49(3):2680–2686. doi: 10.1016/j.neuroimage.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 28.Sato W, Okada T, Toichi M. Attentional shift by gaze is triggered without awareness. Exp Brain Res. 2007;183(1):87–94. doi: 10.1007/s00221-007-1025-x. [DOI] [PubMed] [Google Scholar]
- 29.Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proc Natl Acad Sci USA. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farroni T, Massaccesi S, Pividori D, Johnson MH. Gaze following in newborns. Infancy. 2004;5(1):39–60. [Google Scholar]
- 32.Farroni T, et al. Newborns’ preference for face-relevant stimuli: Effects of contrast polarity. Proc Natl Acad Sci USA. 2005;102(47):17245–17250. doi: 10.1073/pnas.0502205102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farroni T, Johnson MH, Csibra G. Mechanisms of eye gaze perception during infancy. J Cogn Neurosci. 2004;16(8):1320–1326. doi: 10.1162/0898929042304787. [DOI] [PubMed] [Google Scholar]
- 34.Gliga T, Csibra G. Seeing the face through the eyes: A developmental perspective on face expertise. Prog Brain Res. 2007;164:323–339. doi: 10.1016/S0079-6123(07)64018-7. [DOI] [PubMed] [Google Scholar]
- 35.Farroni T, Menon E, Rigato S, Johnson MH. The perception of facial expressions in newborns. Eur J Dev Psychol. 2007;4(1):2–13. doi: 10.1080/17405620601046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rigato S, Menon E, Johnson MH, Farroni T. The interaction between gaze direction and facial expressions in newborns. Eur J Dev Psychol. 2011;8(5):624–636. doi: 10.1080/17405620601046832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson CA, De Haan M. Neural correlates of infants’ visual responsiveness to facial expressions of emotion. Dev Psychobiol. 1996;29(7):577–595. doi: 10.1002/(SICI)1098-2302(199611)29:7<577::AID-DEV3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Leppänen JM, Moulson MC, Vogel-Farley VK, Nelson CA. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grossmann T, et al. Genetic and neural dissociation of individual responses to emotional expressions in human infants. Dev Cogn Neurosci. 2011;1(1):57–66. doi: 10.1016/j.dcn.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltola MJ, Hietanen JK, Forssman L, Leppänen JM. The emergence and stability of the attentional bias to fearful faces in infancy. Infancy. 2013;18(6):905–926. [Google Scholar]
- 41.Peltola MJ, Leppänen JM, Mäki S, Hietanen JK. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc Cogn Affect Neurosci. 2009;4(2):134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 43.Leppänen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nat Rev Neurosci. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci. 2010;11(10):697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- 45.Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braungart-Rieker JM, Hill-Soderlund AL, Karrass J. Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Dev Psychol. 2010;46(4):791–804. doi: 10.1037/a0019673. [DOI] [PubMed] [Google Scholar]
- 47.Campos JJ, et al. Travel broadens the mind. Infancy. 2000;1(2):149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- 48.Gelskov SV, Kouider S. Psychophysical thresholds of face visibility during infancy. Cognition. 2010;114(2):285–292. doi: 10.1016/j.cognition.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Kouider S, et al. A neural marker of perceptual consciousness in infants. Science. 2013;340(6130):376–380. doi: 10.1126/science.1232509. [DOI] [PubMed] [Google Scholar]
- 50.de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Dev Psychol. 1999;35(4):1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- 51.de Haan M, Belsky J, Reid V, Volein A, Johnson MH. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. J Child Psychol Psychiatry. 2004;45(7):1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 52.Ichikawa H, Otsuka Y, Kanazawa S, Yamaguchi MK, Kakigi R. Contrast reversal of the eyes impairs infants’ face processing: A near-infrared spectroscopic study. Neuropsychologia. 2013;51(13):2556–2561. doi: 10.1016/j.neuropsychologia.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Haley KJ, Fessler DM. Nobody's watching? Subtle cues affect generosity in an anonymous economic game. Evol Hum Behav. 2005;26:245–256. [Google Scholar]
- 54.Grossmann T, Striano T, Friederici AD. Developmental changes in infants’ processing of happy and angry facial expressions: A neurobehavioral study. Brain Cogn. 2007;64(1):30–41. doi: 10.1016/j.bandc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Kobiella A, Grossmann T, Reid VM, Striano T. The discrimination of angry and fearful facial expressions in 7-month-old infants: An event-related potential study. Cogn Emotion. 2008;22(1):134–146. [Google Scholar]
- 56.Hoehl S, Striano T. The development of emotional face and eye gaze processing. Dev Sci. 2010;13(6):813–825. doi: 10.1111/j.1467-7687.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 57.Hoehl S, Striano T. Neural processing of eye gaze and threat-related emotional facial expressions in infancy. Child Dev. 2008;79(6):1752–1760. doi: 10.1111/j.1467-8624.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Dev Psychol. 2005;41(4):598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gee DG, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14(18):2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 61.Kim H, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 62.Vrticka P, Lordier L, Bediou B, Sander D. Human amygdala response to dynamic facial expressions of positive and negative surprise. Emotion. 2014;14(1):161–169. doi: 10.1037/a0034619. [DOI] [PubMed] [Google Scholar]
- 63.Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Vaish A, Grossmann T, Woodward A. Not all emotions are created equal: The negativity bias in social-emotional development. Psychol Bull. 2008;134(3):383–403. doi: 10.1037/0033-2909.134.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peltola MJ, Leppänen JM, Palokangas T, Hietanen JK. Fearful faces modulate looking duration and attention disengagement in 7-month-old infants. Dev Sci. 2008;11(1):60–68. doi: 10.1111/j.1467-7687.2007.00659.x. [DOI] [PubMed] [Google Scholar]
- 66.Missana M, Grigutsch M, Grossmann T. Developmental and individual differences in the neural processing of dynamic expressions of pain and anger. PLoS ONE. 2014;9(4):e93728. doi: 10.1371/journal.pone.0093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiss M, Eimer M. ERPs reveal subliminal processing of fearful faces. Psychophysiology. 2008;45(2):318–326. doi: 10.1111/j.1469-8986.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith ML. Rapid processing of emotional expressions without conscious awareness. Cereb Cortex. 2012;22(8):1748–1760. doi: 10.1093/cercor/bhr250. [DOI] [PubMed] [Google Scholar]
- 69.Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends Cogn Sci. 2009;13(3):127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Pourtois G, Dan ES, Grandjean D, Sander D, Vuilleumier P. Enhanced extrastriate visual response to bandpass spatial frequency filtered fearful faces: Time course and topographic evoked-potentials mapping. Hum Brain Mapp. 2005;26(1):65–79. doi: 10.1002/hbm.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nomi JS, Frances C, Nguyen MT, Bastidas S, Troup LJ. Interaction of threat expressions and eye gaze: An event-related potential study. Neuroreport. 2013;24(14):813–817. doi: 10.1097/WNR.0b013e3283647682. [DOI] [PubMed] [Google Scholar]
- 72.Ekman P, Friesen V. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- 73.Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.