Significance
In nature, nearly all plants have only a single layer of endodermis. The number of cortex cell layers often varies between species and between roots on the same plant. Here we show that the expression of conserved SHORT-ROOT (SHR) protein, in the context of the root of Arabidopsis thaliana, is responsible for determining the number of cortex cell layers and that the number of cell layers is a function of the extent of SHR movement. These results provide a plausible model for regulating the number of cortex cell layers in plant roots that relies upon controlled intercellular movement of the SHR transcription factor.
Keywords: SHORT-ROOT, root development, cellular patterning, SCARECROW, rice
Abstract
Formation of specialized cells and tissues at defined times and in specific positions is essential for the development of multicellular organisms. Often this developmental precision is achieved through intercellular signaling networks, which establish patterns of differential gene expression and ultimately the specification of distinct cell fates. Here we address the question of how the SHORT-ROOT (SHR) proteins from Arabidopsis thaliana (AtSHR), Brachypodium distachyon (BdSHR), and Oryza sativa (OsSHR1 and OsSHR2) function in patterning the root ground tissue. We find that all of the SHR proteins function as mobile signals in A. thaliana and all of the SHR homologs physically interact with the AtSHR binding protein, SCARECOW (SCR). Unlike AtSHR, movement of the SHR homologs was not limited to the endodermis. Instead, the SHR proteins moved multiple cell layers and determined the number of cortex, not endodermal, cell layers formed in the root. Our results in A. thaliana are consistent with a mechanism by which the regulated movement of the SHR transcription factor determines the number of cortex cell layers produced in the roots of B. distachyon and O. sativa. These data also provide a new model for ground tissue patterning in A. thaliana in which the ability to form a functional endodermis is spatially limited independently of SHR.
The root of Arabidopsis thaliana is composed of distinct single cell layers that are concentrically arranged around a central core of largely vascular tissues, which, along with the pericycle, forms the stele. The ground tissue layers, endodermis plus cortex, directly surround the stele. The ground tissue of most, if not all, roots contains a single layer of endodermis, which forms a water-impermeable barrier that protects the vascular tissues. To the outside of the endodermis is the cortex. The number of cortex cell layers differs between roots of different species, and in some cases, between roots (e.g., primary versus lateral) on the same plant. In A. thaliana, where root development has been extensively examined, the number of ground tissue layers in both primary and secondary roots is two, with a single endodermal and a single cortical cell layer (1). In contrast, the roots of both Brachypodium distachyon and Oryza sativa (rice) have a single layer of endodermis but multiple layers of cortex, with the number of cortex cell layers varying between root types (2, 3). For example, in the primary root of rice, there are five to six layers of cortex, but in some of the thin lateral roots, there may be only one layer of cortex. In all roots, it is thought that the number of endodermal cell layers is controlled through the conserved function and regulation of the SHR transcriptional network (4); however, it is not known how the number of cortex cell layers is adjusted. Here we show that controlled movement of SHORT-ROOT (SHR) in A. thaliana regulates the number of cortex cell layers independent of the number of endodermal cell layers. SHR movement may therefore represent a tunable mechanism for controlling the number of cortex cell layers in roots.
In A. thaliana, as in other dicots, the endodermis and cortex are clonally related cell layers that arise from the asymmetric periclinal cell divisions of a cortical endodermal daughter (CED) cell located at the tip of the root adjacent to the stele. Division of the CED is under the control of the SHR and the SCARECROW (SCR) transcription factors. The SHR protein is expressed in the stele and moves into the neighboring endodermis and CED cells where it activates the expression of the downstream transcription factor, SCR. In the CED, SHR and SCR together activate expression of a D-type cyclin (CYCD6;1), which triggers periclinal division of the CED (5−8). The CED cells of roots that lack SHR or SCR fail to divide periclinally and therefore form only a single layer of ground tissue. Roots that lack SHR fail to specify an endodermis, so the single ground tissue layer in shr-2 null mutants has cortex cell identity. In contrast, roots that lack SCR form a chimeric ground tissue layer with aspects of both cortical and endodermal cell fates. These results show that SHR, independent of cell division, is required for endodermal cell fate.
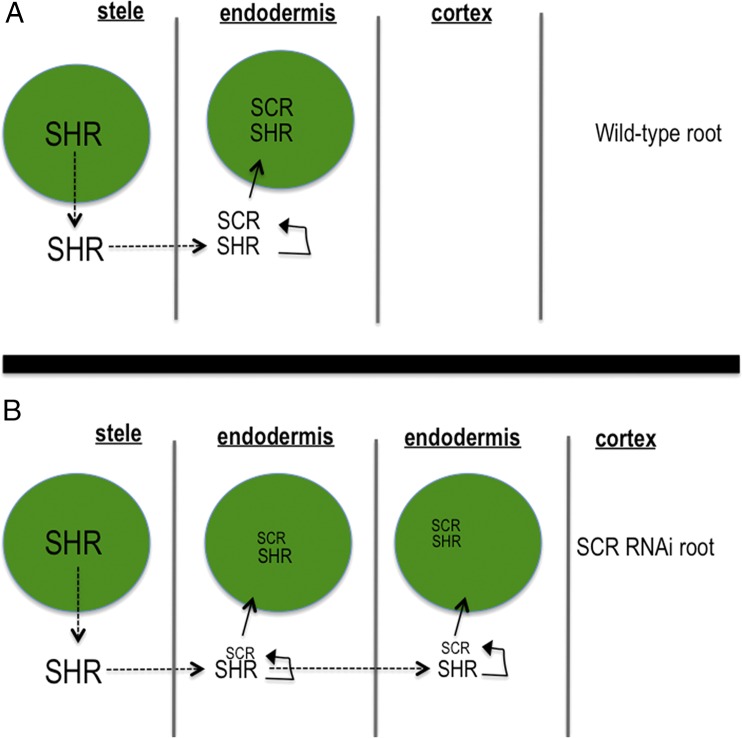
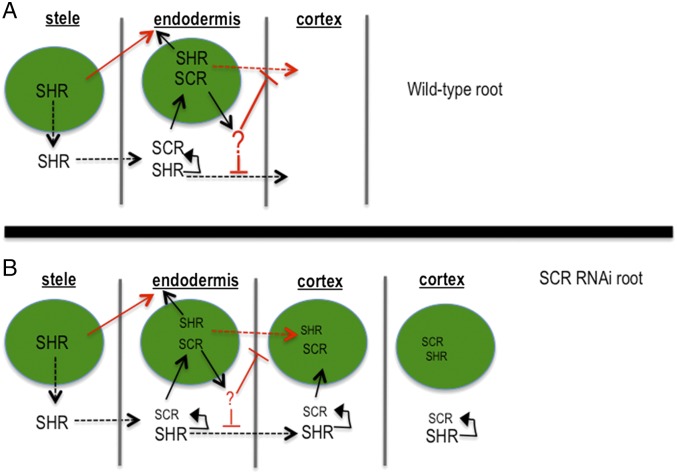
The prevailing model for how a single layer of endodermis is formed in A. thaliana is that once SHR turns on the expression of SCR in the endodermis, SCR physically interacts with SHR and sequesters SHR in the nuclei of endodermal cells, preventing further movement into the cortex cell file (Fig. 1) (4). In addition to SCR, the JACKDAW (JKD) and MAGPIE (MGP) transcription factors may also play a role in limiting SHR movement, again through direct binding to the SHR protein (9). Because, outside of the stele, SHR is thought to be both necessary and sufficient for endodermal cell specification, the physical interaction between SHR and SCR prevents the formation of additional endodermal cell layers by limiting SHR movement. This model is supported by the finding that the domain of SHR movement is increased in SCR knockdown lines (SCR RNAi; Fig. 1) and that increases in the extent of SHR movement lead to the formation of additional SCR-expressing cell layers, with SCR expression being used as a marker of endodermis (4). Also in support of this model is the finding that ectopic expression of SHR can cause cells to produce suberin, a waxy substance produced by the endodermis. Although JKD and MGP also affect the movement of SHR, they appear to affect the position of the endodermis but not the number of endodermal cell layers (9).
Fig. 1.
Cartoon summarizing the prevailing model for ground tissue patterning proposed by Cui et al. (4). The green circles are nuclei. Dotted lines indicate protein movement. The size of the font is meant to indicate the amount of the protein. (A) The pathway for the formation of a normal root and (B) one in which SCR is reduced via RNAi.
Because all plants have a single layer of endodermis in the mature root, and most plants contain homologs of both SHR and SCR, Cui et al. (4) tested whether the physical interaction between SHR and SCR is conserved in rice. They found that OsSHR1 interacted with both OsSCR1 and AtSCR in yeast. Based upon these findings, they proposed an elegant model in which the interaction between SHR and SCR represents an evolutionarily conserved mechanism that delimits SHR movement and thereby defines a single layer of endodermis (4). Although this mechanism for the specification of a single endodermis is often cited in the literature, the hypothesis has not been fully tested. In addition, this model provides no mechanism for the formation of extra cortex cell layers in roots like those of rice.
Here, using the SHR protein from A. thaliana and the homologous SHR proteins from two species of grasses, Brachypodium distachyon and Oryza sativa, we examined the function of SHR in the root of A. thaliana. All of the SHR homologs were able to move out of the stele into the ground tissue. The movement of the SHR proteins was dependent upon plasmodesmata (intercellular channels that connect plant cells) and conserved sequences within the GRAS domain of the SHR proteins. Interestingly, we found that although all of the SHR homologs interacted strongly with AtSCR (as well as AtMGP and AtJKD), their movement was not limited to a single layer of ground tissue, indicating that physical association between SHR and SCR is insufficient to restrict SHR movement within the endodermis. In addition, although all of the SHR proteins had the ability to restore endodermal specification in the shr-2 mutants, none of them (including AtSHR) had the ability to specify more than one layer of functional endodermis. Instead, all extra cell layers produced by the expression of the SHR proteins adopted cortex cell characteristics. In contrast with previous findings (4), our results show that the ability of SHR to specify endodermis is spatially limited to the cell layer immediately outside of the stele. Therefore, our data significantly revise the previous established model for the regulation of ground tissue patterning (Fig. 1) and suggest a credible mechanism by which the regulated movement of SHR controls the number of cortex cell layers produced in roots of different species.
Results
The SHR Homologs Are Mobile and Move Beyond the Endodermis.
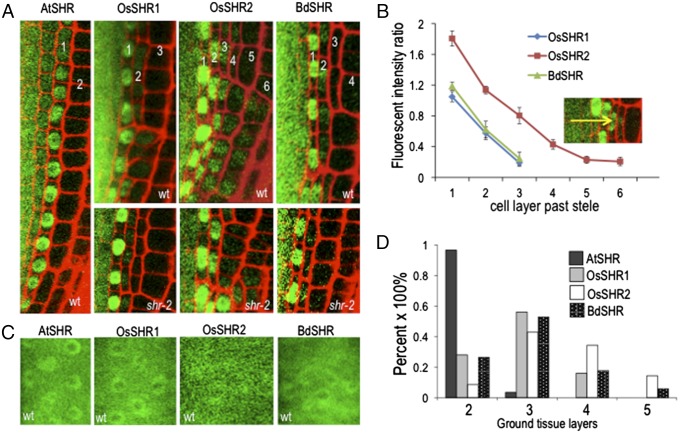
Although a single endodermis seems to be the rule in normal root development, multiple layers of cortex are common in many plant species, including rice and B. distachyon (Fig. S1) (2, 3). When expressed as a YFP fusion under the AtSHR promoter in either wild-type or in shr-2 mutant roots, OsSHR1 (LOC_Os7g39820), OsSHR2 (LOC_Os3g31880), and BdSHR (LOC100830802) were detected as YFP fusions in multiple cell layers beyond the stele forming a protein gradient with the highest levels directly outside of the stele (Fig. 2 A and B) These results indicate that all of the SHR proteins are mobile. Similar to AtSHR, the YFP-tagged SHR homologs all showed both nuclear and cytoplasmic localization in the stele (Fig. 2 A and C); outside of the stele the protein localization was restricted to the nucleus (Fig. 2A), indicating that similar mechanisms control the subcellular localization of the A. thaliana and the grass SHR proteins.
Fig. 2.
The monocot SHR proteins move beyond the endodermis in A thaliana roots. (A) Confocal images of the SHR homologs expressed as YFP fusions under the SHR promoter in both wild-type (A, as labeled, B−D) and in shr-2 (A, as labeled) roots. The numbers represent the ground tissue layers relative to the stele, which are quantified in D. (B) The SHR homologs as YFP fusions formed a protein gradient, with the highest levels in the cell layer directly outside of the stele. The Inset is a representative image from OsSHR2-YFP, and the arrow shows the direction of the gradient. The relative fluorescence intensity was quantified using the fluorescence intensity of each ground tissue layer against that in stele (n = 8 roots, 96 cells for each SHR homolog). (C) Confocal image showing the YFP-tagged SHR homologs localized to both the nucleus and cytoplasm of stele cell. (D) Quantification of additional ground tissue layers caused by the expression of SHR homologs (n = 3 replicates, 36 roots for each SHR homolog).
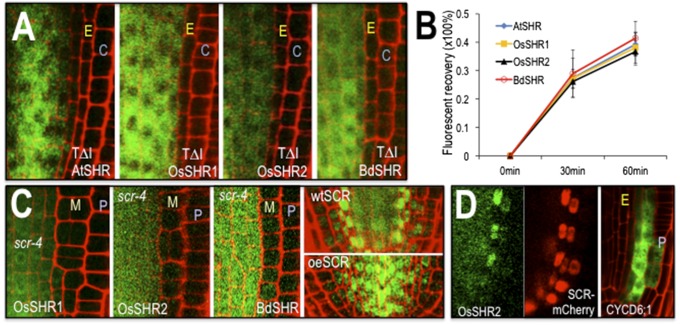
To determine whether the mechanisms promoting the intercellular movement of OsSHR1, OsSHR2, BdSHR, and AtSHR are similar, we examined the role of a conserved threonine (T289) in protein transport. In AtSHR, mutation of T289 to alanine (A), in the VHIID domain of the protein, inhibits nuclear localization and intercellular movement of AtSHR (4). We converted the conserved threonine to alanine in OsSHR1, OsSHR2, and BdSHR and found that as in AtSHR, the conserved threonine is required for nuclear localization and movement of all three of the SHR homologs (Fig. 3A and Fig. S2). To determine whether the kinetics of protein movement are similar for the SHR proteins, we measured the fluorescent recovery of the BdSHR-YFP, OsSHR1-YFP, and OsSHR2-YFP proteins after photobleaching of the YFP signal in the inner ground tissue layers. The kinetics of recovery were similar for all of the SHR proteins (Fig. 3B and Fig. S3), indicating that these proteins traffic by a similar, if not the same, pathway.
Fig. 3.
The SHR proteins have similar requirements for localization and movement. (A) Mutation of a conserved threonine (residue 289 in AtSHR, 345 in OsSHR1, 352 in OsSHR2, and 354 in BdSHR) in the VHIID domains of the SHR proteins inhibits nuclear localization and intercellular movement. C, cortex; E, endodermis. Full root tip images are in Fig. S2. (B) Quantification of fluorescence recovery after photobleaching (FRAP) showed that the kinetics of protein movement between the stele and the first ground tissue is comparable among all SHR homologs [n = 3 replicates, 9 roots and 35–132 cells for each SHR homolog; assays were done in wild type (WT)]. (C) The scr-4 mutation is epistatic to the effects of expression of the SHR homologs. M, mutant cell layer; P, epidermis. The first three panels show transgene expression from the SHR promoter in the scr-4 (null) background. The last two panels show OsSHR2-YFP in a WT root and a root overexpressing (oe) AtSCR-mTFP from the SHR promoter. (D) Expression of OsSHR2 from the SHR promoter (Left and Middle) drives expression of the SCR:mCherry marker and expression of CYCD6;1:erGFP (Right), which is visualized without the OsSHR2-YFP signal.
Previously, we showed that the intercellular movement of AtSHR occurs via plasmodesmata (PD) (10). PD-dependent movement of AtSHR was demonstrated using a semidominant inducible allele of callose synthase (icals3m) that deposits callose around the PD in response to estradiol treatment. The accumulation of callose around the PD decreases the PD aperture and movement of the SHR protein from the stele into the endodermis. Induction of the icals3m transgene (expressed in the stele from the WOODENLEG promoter) reliably blocked movement of the OsSHR1 and OsSHR2 proteins from the stele into the ground tissue layers (Fig. S4). Collectively, these results indicate a conserved mechanism for intercellular protein movement among the different SHR homologs.
SHR Homologs Interact With SCR, MGP, and JKD Proteins and Rely on SCR Expression for Movement and Function.
In A. thaliana, generation of extra ground tissue layers by ectopic expression of AtSHR is dependent upon SCR. In the absence of SCR, extra cell layers do not form (7). To test whether the phenotype generated by expression of the SHR homologs in A. thaliana is also dependent upon SCR, we crossed the transgenic lines expressing BdSHR-YFP, OsSHR1-YFP, and OsSHR2-YFP into a scr-4 (null) mutant background. In all cases, the scr-4 mutation was largely epistatic to the effects of the expression of the SHR homologs on root patterning (Fig. 3C). Expression of BdSHR and OsSHR1 in scr-4 marginally decreased the time until middle cortex formation (from day 7 to day 4), which normally forms precociously in scr-4 mutants (11); however, there were no other changes in the scr-4 phenotype. Interestingly, nuclear localization and movement of BdSHR-YFP, OsSHR1-YFP, and OsSHR2-YFP appeared decreased in the scr-4 background, which is similar to what we previously reported for AtSHR movement (12, 13), indicating a similar mechanism for the nuclear localization and intercellular movement of the SHR proteins.
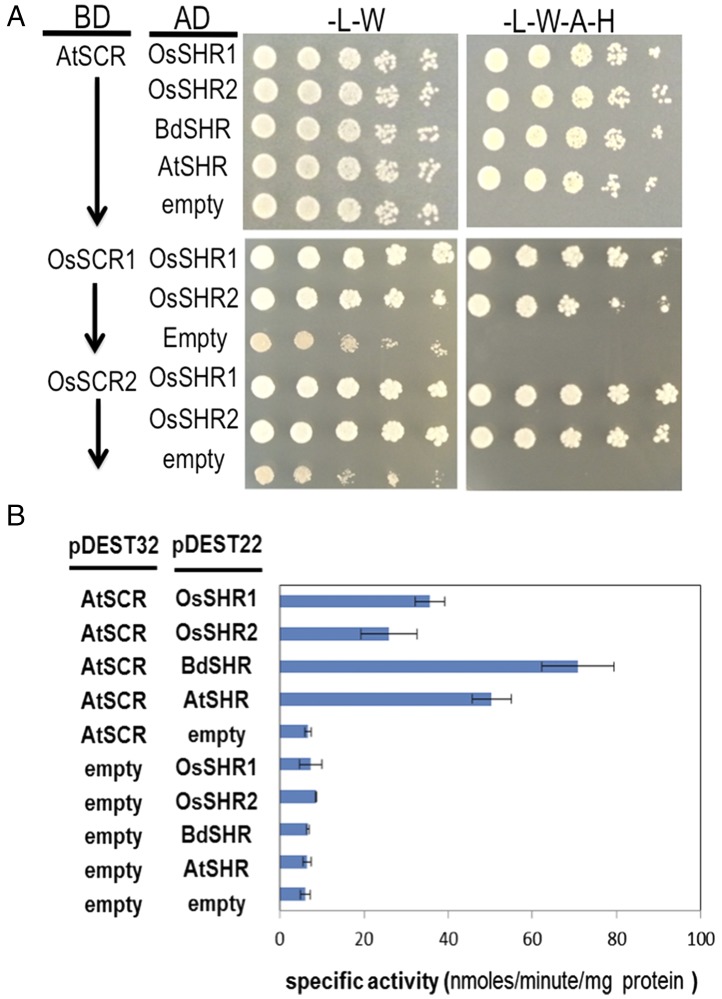
The heart of the SCR-SHR sequestration model is that interaction between SCR and SHR in the presumptive endodermis limits SHR movement and this restricts the root to a single layer of endodermis (Fig. 1) (4). Because BdSHR, OsSHR1 and OsSHR2 all moved beyond the endodermis and their movement and localization is dependent upon SCR, we tested whether these homologs could directly interact with AtSCR using yeast two-hybrid and bimolecular fluorescence complementation (BiFC) assays. Based upon yeast growth assays, all of the SHR homologs interacted with AtSCR, as well as with MGP and JKD, which have been shown to interact with AtSHR (Fig. 4A and Fig. S5) (9). Based upon their growth on selective media, all of the homologs showed similar levels of interaction with AtSCR as did AtSHR. Quantitative BiFC (Fig. S5), as measured in A. thaliana protoplast, showed no significant difference in interaction between any of the SHR proteins and AtSCR. In yeast, using normalized levels of beta-galactosidase activity, of all of the SHR proteins (including AtSHR), BdSHR showed the strongest interaction with AtSCR (Fig. 4B). These results show that the increased movement of BdSHR, OsSHR1, or OsSHR2 compared with AtSHR cannot be explained simply by an inability to interact with AtSCR, nor do the strengths of interactions with AtSCR correlate with the extent of movement. Because rice has two SCR proteins, OsSCR1 and OsSCR2, we tested for interaction between the OsSHR proteins with OsSCR. As shown in Fig. 4, both OsSHR1 and OsSHR2 interacted with OsSCR1 and OsSCR2.
Fig. 4.
The SHR homologs interact with SCR. (A) Fivefold serial dilutions of diploid yeast expressing AtSCR, OsSCR1, or OsSCR2 as bait with the SHR prey proteins (as labeled) grown on selective medium. Medium lacking adenine and histidine was used to select for interaction between the bait and prey proteins. AD, activation domain vector (prey); BD, binding domain vector (bait). BiFC results are shown in Fig. S5A. (B) Quantitative beta-galactosidase assays showing the specific activity of the beta-galactosidase enzyme induced by the interaction of the bait and prey proteins. The interacting proteins are as labeled. Assays were done in diploid yeast and normalized to total protein.
To test further the role of AtSCR in the localization and movement of the monocot SHR proteins, we examined the localization and movement of OsSHR2 in roots that ectopically express AtSCR from the SHR promoter (Fig. 3C). As previously reported, expression of SCR-TFP in the stele (from the AtSHR promoter) results in the nuclear localization of AtSHR-GFP in the stele; the AtSHR-GFP does not move out of the stele, and the endodermis is not formed. When SHR:SCR-TFP was crossed into plants expressing OsSHR2, there was a dramatic increase in the nuclear localization of OsSHR2 in stele cells. However, there were no effects on the movement of OsSHR2 out of the stele or on the production of ground tissue layers. These results indicate the AtSCR promotes the nuclear localization of OsSHR protein but cannot block its movement.
The SHR Homolog Can Partially Substitute for AtSHR.
The roots of shr-2 mutants are significantly shorter than wild type; they have a smaller meristem (based both upon the length of the meristem and cell number) and lack an endodermis. To determine whether the monocot SHR proteins could substitute for AtSHR, OsSHR1, OsSHR2, or BdSHR was crossed into the shr-2 mutant background and both root growth and patterning were assessed (Fig. S6). Expression of either OsSHR1or BdSHR in shr-2 was able to fully rescue root growth and meristem size; OsSHR2 provided a partial rescue of the shr-2 phenotype with restoration of root growth to nearly wild-type levels (Fig. S6D) and a 48.6% rescue in the size of the meristem (Fig. S6E). Although the monocot SHR proteins were able to restore growth of shr-2 roots, all of the SHR homologs produced an “over-rescue” phenotype with the formation of multiple ground tissue layers (Fig. 2A). These results indicate that in most cases, the SHR homologs can functionally substitute for AtSHR.
In both wild-type and shr-2 mutants, expression of the homologous SHR transgenes in A. thaliana resulted in the formation of additional ground tissue layers with OsSHR2 showing the greatest potential for inducing extra cell layers (Fig. 2 A and D and Fig. S1 C−F). In wild-type A. thaliana roots, movement of SHR into the cortical endodermal initial (CEI) and the CED cell up-regulates SCR, which, together with SHR, turns on CYCLIND6;1 (CYCD6;1) (8). In the CED cells, expression of CYCD6;1 triggers the asymmetric periclinal cell division that creates the separate endodermal and cortical cell layers. Consistent with an induction of periclinal cell divisions in the generation of extra cell layers in the roots expressing the rice and B. distachyon SHRs, SCR is expressed beyond the endodermis (Fig. 3D) and CYCD6;1 is expressed not only in the CEI and the CED cells but also in the cortical cell lineages. In 6-d-old roots, expression of CYCD6;1 was particularly enriched in the outer cortex cell layers. Occasionally, expression was also seen in the epidermis (2 out of 10 roots) and the endodermis (1 out of 10 roots) (Fig. S7) (8, 12, 14, 15). These results are consistent with the ectopic movement of the OsSHR1, OsSHR2, and BdSHR proteins inducing SCR and CYCD6;1, which leads to ectopic periclinal cell divisions in the cortex.
The Monocot SHRs Specify a Single Layer of Endodermis and Multiple Layers of Cortex.
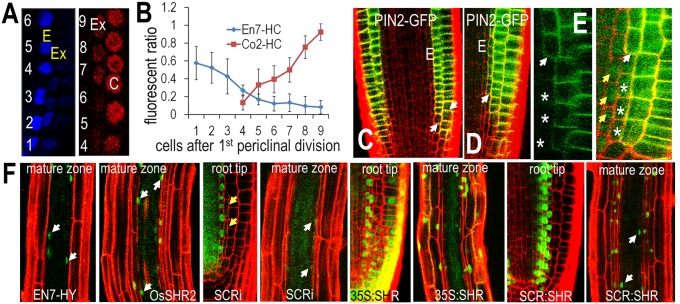
To determine the identity of the additional cell layers created by the expression of OsSHR1, OsSHR2, or BdSHR, we examined cell-type specific markers of the endodermis (ENDODERMIS7:Histone2B:mCherry, referred to here as EN7-HC) and cortex (CORTEX2:Histone2B:mCherry, referred to here as pCO2-HC) (16) in wild-type roots expressing the monocot SHR proteins. We found an inverse correlation between expression of the EN7-HC and the CO2-HC markers, with cells near the root tip transiently expressing EN7-HC and cells in the distal region of the meristem expressing CO2-HC (Fig. 5A and Fig. S8 A−F). To better understand the determination of cell fate within the extra ground tissues, we quantified the fluorescence intensity of the EN7-HC and CO2-HC makers in the first extra cell layer and the results were normalized to the endodermis or the cortex. We found that in the first additional cell layer, EN7-HC expression was highest adjacent to the quiescent center (QC) and then decreased dramatically in the shootward direction (Fig. 5B). The level of EN7-HC was reduced by 80% in cells separated from the CEI by four rounds of anticlinal (transit amplifying) cell divisions. In this region of the root where EN7-HC levels dropped, CO2-HC expression was initiated (Fig. 5B). The intensity of CO2-HC within the first extra cell layer increased sevenfold after an additional five rounds of anticlinal cell division, indicating that the additional ground tissue layers that are not in direct contact with the stele transiently express EN7-HC, but then quickly adopt a cortex cell identity as they are displaced into the distal meristem (shootward) via transit amplifying cell divisions.
Fig. 5.
The extra ground tissue cell layers induced by the SHR homologs have a cortical cell identity. (A) Representative images of expression of EN7-HC (pseudocolored in blue) and CO2-HC (in red) that is quantified in B. C, cortex; E, endodermis; Ex, the extra cell layer that was measured in the calculation of the fluorescence ratio shown in B. The numbers show the location of cells relative to the first divided initial cell at root tip. (B) Quantification of the fluorescence ratio between Ex and E or Ex and C as labeled (n = 8 roots, 41–68 cells for each marker). Co2-HC, pCo2-H2B-mCherry; En7-HC, pEN7-H2B-mCherry. (C) PIN2:PIN2-GFP expression in wild type. PI staining (red) was used to visualize cell boundaries. The arrows point to the cortex cells that start to express PIN2-GFP. E, endodermis. (D) PIN2:PIN2-GFP in roots expressing OsSHR2. Cell layers were shown by PI staining in red. The arrow points to the additional cell layer that starts to express PIN2-GFP. (E) Magnified images of the meristem expressing PIN2:PIN2-GFP in OsSHR2-expressing roots. Left shows just PIN-GFP; whereas Right also shows OsSHR2-YFP. The white arrows point to cells that first start to express PIN2-GFP. Yellow arrows point to the nuclear localization of OsSHR2 in the presumptive endodermis. The asterisks mark the same cells in Left and Right. (F) PI is excluded from the stele and a single ground tissue layer (the endodermis) in all genotypes (as labeled). The white arrows point to nuclei in the endodermis.
To further verify the identity of the extra ground tissues layers, we crossed a functional marker of cell identity, the PIN2:PIN2-GFP (PIN-FORMED 2) auxin efflux carrier into a line expressing OsSHR2. In wild-type A. thaliana roots, PIN2 is absent from the endodermis and is expressed in the cortex and the epidermis (Fig. 5C). In the epidermis, PIN2 localizes to the basal region (the shootward side) of cells, while it is restricted to the apical end (rootward side) of cells in the cortex (17). In roots expressing OsSHR2, which had the most dramatic effect on root patterning, the additional cell layers all expressed rootward-localized PIN2 (Fig. 5 D and E); this is similar to the region of the root meristem in which expression of pCO2-HC was initiated. These results indicate that the ectopic cell layers generated by the expression and movement of the monocot SHRs adopt a cortex cell fate rather than an endodermal or an epidermal cell fate.
One of the distinguishing features of the endodermis is the formation of lignin-rich Casparian strips that encircle each cell in the layer. In the A. thaliana root, the endodermis is the only cell layer to form Casparian strips (i.e., there is no exodermis), so in wild-type roots, there is a single layer of lignified cells that surrounds the stele. In the roots expressing the monocot SHR proteins, we also detected a single lignified cell layer surrounding the stele (Fig. S8 G−J), indicating the presence of a single layer of endodermis (18). Similar to the epithelium in animals, the endodermis establishes a paracellular (apoplastic) diffusion barrier in plants (19). Consistent with an ability to block diffusion, Naseer et al. recently showed that functional Casparian strips (and therefore a functional endodermis) blocks the flux of propidium iodide (PI) solution into the stele so that only the epidermis and cortex are stained with PI in the mature zone of an A. thaliana root; the endodermis and stele cells are unstained (18). In the wild-type roots expressing OsSHR1, OsSHR2, or BdSHR, PI was excluded from the stele and a single cell layer of ground tissue immediately surrounding the stele. These results indicate that there is a single functional layer of endodermis regardless of the number of extra ground tissue layers in the root (Fig. 5F).
These results are consistent with the ability of the monocots SHRs to induce the formation of multiple layers of cortex in rice and brachypodium. In A. thaliana, AtSHR is required for the formation of a functional endodermis. Therefore, we tested whether OsSHR1, OsSHR2, or BdSHR could restore endodermal cell fate in the context of the shr-2 roots. The expression of any of the SHR homologs in shr-2 resulted in the exclusion of PI from the stele and a single cell layer of ground tissue immediately surrounding the stele, but not from any of the additional cell layers of ground tissue. (Fig. S8 N−P). Because shr-2 mutants have no endodermis and cannot exclude PI (Fig. S8M), these results indicate that the SHR homologs have the ability to specify endodermis. However, in all roots examined, the ability to induce endodermis was spatially limited to the single cell layer adjacent to the stele. Thus, the expression of OsSHR1, OsSH2, or BdSHR does not alter the number of endodermal cell layers.
Ectopic Movement or Expression of AtSHR Does Not Result in the Formation of Additional Functional Endodermal Cell Layers.
The results of our analyses with AtSHR and the homologs from rice and B. distachyon suggest that although the movement and regulation of these proteins are similar, the monocot SHR proteins (even in the presence of an increased domain of movement) have a spatially limited ability to specify endodermis. In the wild-type and the shr-2 roots expressing BdSHR, OsSHR1, or OsSHR2, PI penetrated into the root and the extra cell layers surrounding the stele. However, PI did not penetrate the stele cells and was also restricted from a single ground tissue layer surrounding the stele, indicating that these proteins alone are not sufficient to induce the formation of endodermis (18). This is in contrast to the AtSHR, whose increased movement is thought to result in the formation of ectopic endodermis (7, 9, 16, 20). Expression of SHR is thought to be both necessary and sufficient for the induction of endodermal cell fate.
In Cui et al., the presence of extra endodermal layers was shown by the expression of SCR in the supernumerary layers (4). In Nakajima et al. (7), the formation of extra endodermal cell layers in roots expressing SHR from the SCR promoter (SCR:SHR) was shown by expression of SCR in the cells, as well as by the accumulation of suberin, which is associated with Casparian strips. However, no functional assays were performed to test for the presence of additional layers of endodermis in either the SCR RNAi (4) or the SCR:SHR lines. To determine whether ectopic movement or expression of AtSHR can produce a functional endodermis, we examine PI exclusion in SCR RNAi-, 35S:SHR-GFP–, and SCR:SHR-nlsGFP–expressing lines, all of which produce extra ground tissue. In SCR RNAi lines (4), we found that the supernumerary layers did not restrict PI movement, indicating that these roots do not form additional functional endodermal cell layers (Fig. 5F). Likewise, in roots constitutively expressing SHR (35S:SHR-GFP) or roots expressing SHR directly in the ground tissue (SCR:SHR-nlsGFP) (Fig. 5F) (6), the extra layers were not lignified (Fig. S8K) nor did they behave as a functional endodermis (Fig. S8L). These results suggest that AtSHR alone is not sufficient to induce a functional endodermis. In A. thaliana and likely in the roots of other plant species, we propose that an additional factor, perhaps derived from the stele, is required for SHR to specify a functional endodermis.
Discussion
In examining the movement capacity and function of the rice and B. distachyon homologs of AtSHR, we find compelling evidence for a conserved mechanism for SHR movement and ground tissue patterning. The movement of all of the SHR proteins was similar in terms of the kinetics of protein movement, the requirement for a conserved threonine in the VHIID domain of the protein, and a role for SCR in promoting nuclear localization of SHR and movement from the stele into the endodermis. With respect to protein function, expression of any one of the B. distachyon or O. sativa homologs of AtSHR was able to rescue endodermal cell fate in the shr-2 mutant background. In addition, both BdSHR and OsSHR1 were able to complement defects in the meristem of the shr-2 plants, as well as root growth. Unlike AtSHR, movement of the monocot SHRs was not limited to a single layer of ground tissue. The increased movement of the SHR proteins produced multiple layers of cortex cell. In no instances did we observe more than one layer of functional endodermis, as is predicted by Cui et al. (4). It is possible that some of the ground tissue layers in the roots expressing the SHR homologs or AtSHR had mixed cell identity, with partial endodermal and cortex cell character. However, the PI exclusion assays and lignin autofluorescence indicate that while SHR is necessary for specification of a functional endodermis, it is not sufficient.
A major feature of the ground tissue patterning model proposed by Cui et al. (4) was the ability of SCR to inhibit SHR movement via direct protein−protein interaction and sequestration in the nuclei of endodermal cells. While we cannot entirely rule out differences in the levels of SCR expression between the roots expressing the A. thaliana and grass SHR proteins, our results suggest that the direct binding of AtSCR to the monocot SHR proteins is insufficient to inhibit movement from the endodermis. SCR is, however, responsible for the nuclear localization of OsSHR1, OsSHR2, and BdSHR. To minimize the apparent differences between the behaviors of the SHR proteins, we propose a modified model for the regulation of SHR movement, in which SCR directly promotes the nuclear localization of AtSHR (along with the SHR homologs) and indirectly blocks movement through the up-regulation of an unidentified factor, indicated by “?” in Fig. 6, that inhibits movement of AtSHR from the endodermis. This revised model is based upon several lines of evidence. First, all of the SHR homologs can bind to AtSCR and all of the SHR homologs are nuclear localized in the ground tissue, yet they move beyond the endodermis in both shr-2 and in wild-type roots. These results indicate that neither nuclear localization of SHR in the ground tissue nor binding of SHR to SCR is sufficient to block intercellular movement. In addition, previous results showed that the inclusion of a nuclear export signal in AtSHR-GFP caused cytoplasmic localization of AtSHR-GFP in the endodermis, yet the protein was restricted to the endodermis, presumably due to the presence of an SCR-dependent factor that prevents movement of AtSHR, independent of nuclear localization (21). Likewise, Sena et al. showed that when expressed in the epidermis of wild-type roots (where SCR is not normally expressed), AtSHR showed little movement into the cortex; however in the scr mutant background, AtSHR moved freely into the cortex (20). All of these data point to the presence of a SCR-dependent factor that restricts movement of AtSHR (but not OsSHR1, OsSHR2, or BdSHR) in the endodermis. This is in contrast to the original model in which the direct binding of AtSHR by SCR resulted in the nuclear localization of AtSHR, resulting in restricted movement (4).
Fig. 6.
Revised model for ground tissue patterning (4). The green circles are nuclei. Dotted lines indicate protein movement. The size of the font is meant to indicate the relative amount of the protein. (A) The pathways for the formation of a wild-type A. thaliana root and (B) one in which SCR is reduced via RNA interference. The arrows and text shown in red are modifications to the previous model. Unlike the previous model proposed by Cui et al. (4), SCR-dependent nuclear localization and restriction of SHR movement are separable. The ability of the monocot SHR proteins to interact with SCR and localize to nuclei, yet move past the endodermis, indicates that a factor downstream of SCR (indicated by “?”) is important for inhibiting movement of AtSHR.
A conserved mechanism limiting the movement of SHR is used to explain the formation of one endodermal cell layer in plant roots (Fig. 1) (4). This hypothesis was based, in part, upon previous data showing that ectopic expression of AtSHR can change the properties of the cell wall and induce production of suberin, which was used as a marker of endodermis. For example, in Nakajima et al. (7), expression of SHR from the SCR promoter caused the formation of supernumerary ground tissue layers that produced suberin. Recently, work by Naseer et al. has questioned the usefulness of suberin as a marker of endodermis; instead, they have shown that a functional endodermis is lignified and able to exclude PI in the absence of suberin (18). Here we show, using the EN7-HC, CO2-HC, and PIN2-GFP markers (14, 16), as well as lignin autofluorescence and PI exclusion assays, that the expression of the SHR homologs in A. thaliana roots results in the formation of a single endodermal cell layer and multiple layers of cortex. In addition, reexamination of the lines that show either ectopic movement or ectopic expression of AtSHR indicates that AtSHR also has a limited ability to specify functional endodermis, even with the production of suberin.
All of our data are consistent with the SHR proteins having a spatially restricted ability to specify endodermis that is independent of the extent of protein movement. Because it is the SHR-containing ground tissue layer in contact with the stele that develops as endodermis, it may be a stele-derived signal that participates with SHR to induce endodermal specification. It is this signal that is the critical factor in the formation of a single endodermal cell layer next to the stele. In the context of the monocot roots, the spatial restriction of the stele-derived signal in the presence of increased movement of SHR represents a plausible and testable mechanism for the formation of multiple layers of cortex without an expansion of the endodermis. In this context, the regulation of the extent of SHR movement would control the number cortex cell layers, which in rice and B. distachyon differs between root segments on the same plant.
Materials and Methods
All plants were grown on 1% agar plates with 0.5x Murashige and Skoog (MS) medium under a 16-h light/8-h dark cycle at 23 °C. Five- to six-day-old plants were used for all experiments unless otherwise stated. Roots were counterstained in 0.01 μg/mL PI in water. Confocal images were obtained using a 20× water-immersion lens on a Leica TCS SL microscope. The yeast two-hybrid assays were tested in diploid yeast cells by mating the two yeast strains, Y187 and AH109. BiFC assays were performed in protoplasts extracted from A. thaliana. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
J. Ugochukwu and C. Heyworth provided technical support. D. Wagner provided comments on the manuscript. A. Stout manages the confocal facility. S.W. and C.-M.L. are partially supported by National Science Foundation Grant 1243945 awarded to K.L.G.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.V.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407371111/-/DCSupplemental.
References
- 1.Dolan L, et al. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119(1):71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Clark LH, Harris WH. Observations on the root anatomy of rice (Oryza-Sativa-L) Am J Bot. 1981;68(2):154−161. [Google Scholar]
- 3.Watt M, Schneebeli K, Dong P, Wilson IW. The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops. Funct Plant Biol. 2009;36(11):960−969. doi: 10.1071/FP09214. [DOI] [PubMed] [Google Scholar]
- 4.Cui H, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316(5823):421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher KL, Paquette AJ, Nakajima K, Benfey PN. Mechanisms regulating SHORT-ROOT intercellular movement. Curr Biol. 2004;14(20):1847–1851. doi: 10.1016/j.cub.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 6.Helariutta Y, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101(5):555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima K, Sena G, Nawy T, Benfey PN. Intercellular movement of the putative transcription factor SHR in root patterning. Nature. 2001;413(6853):307–311. doi: 10.1038/35095061. [DOI] [PubMed] [Google Scholar]
- 8.Sozzani R, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466(7302):128–132. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch D, et al. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007;21(17):2196–2204. doi: 10.1101/gad.440307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vatén A, et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell. 2011;21(6):1144–1155. doi: 10.1016/j.devcel.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Paquette AJ, Benfey PN. Maturation of the ground tissue of the root is regulated by gibberellin and SCARECROW and requires SHORT-ROOT. Plant Physiol. 2005;138(2):636–640. doi: 10.1104/pp.104.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koizumi K, Hayashi T, Gallagher KL. SCARECROW reinforces SHORT-ROOT signaling and inhibits periclinal cell divisions in the ground tissue by maintaining SHR at high levels in the endodermis. Plant Signal Behav. 2012;7(12):1573–1577. doi: 10.4161/psb.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koizumi K, Wu S, MacRae-Crerar A, Gallagher KL. An essential protein that interacts with endosomes and promotes movement of the SHORT-ROOT transcription factor. Curr Biol. 2011;21(18):1559–1564. doi: 10.1016/j.cub.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi K, Gallagher KL. 2012. Identification of SHRUBBY, a SHORT-ROOT and SCARECROW interacting protein that controls root growth and patterning. Development (140):1292−1300.
- 15.Koizumi K, Hayashi T, Wu S, Gallagher KL. The SHORT-ROOT protein acts as a mobile, dose-dependent signal in patterning the ground tissue. Proc Natl Acad Sci USA. 2012;109(32):13010–13015. doi: 10.1073/pnas.1205579109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidstra R, Welch D, Scheres B. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Genes Dev. 2004;18(16):1964–1969. doi: 10.1101/gad.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433(7021):39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 18.Naseer S, et al. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA. 2012;109(25):10101–10106. doi: 10.1073/pnas.1205726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64:531–558. doi: 10.1146/annurev-arplant-050312-120050. [DOI] [PubMed] [Google Scholar]
- 20.Sena G, Jung JW, Benfey PN. A broad competence to respond to SHORT ROOT revealed by tissue-specific ectopic expression. Development. 2004;131(12):2817–2826. doi: 10.1242/dev.01144. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher KL, Benfey PN. Both the conserved GRAS domain and nuclear localization are required for SHORT-ROOT movement. Plant J. 2009;57(5):785–797. doi: 10.1111/j.1365-313X.2008.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.