Significance
Peripheral nerve injury often occurs at axonal sites remote from the neuronal cell bodies. Information about these remote injuries must be accurately relayed back to the cell body as part of the reprogramming that occurs to transition the neuron to a regenerating state. We provide new insights into how the axonal endoplasmic reticulum (ER) proximal to the injury site generates a critical regeneration signal in sensory neurons. This involves injury-triggered activation and synthesis of an intraaxonal, ER transmembrane transcription factor, Luman/cAMP response element binding protein 3, that is retrogradely transported back into the nucleus. Manipulation of axon-derived Luman expression in injured sensory neurons reveals an important role for Luman in regulation of the intrinsic elongating form of axonal growth associated with the regeneration state.
Keywords: dorsal root ganglion, transcription factor, axotomy, sensory neuron
Abstract
Luman/cAMP response element binding protein 3 is an endoplasmic reticulum (ER) transmembrane basic leucine zipper transcription factor whose mRNA and protein localize to adult sensory axons, the latter with axonal ER components along the axon length. Here we show that axon-derived Luman plays an important role in relaying information about axonal injury to the neuronal cell body. Axotomy induces axonal Luman synthesis and also release from the axonal ER of Luman’s transcriptionally active amino terminus, which is transported to the cell body in an importin-mediated manner. Visualization of the activation and retrograde translocation of Luman into the nucleus in real time both in vivo and in vitro was accomplished using a specially created N- and C-terminal–tagged Luman adenoviral vector. Small interfering RNA used to reduce Luman expression either neuronally or just axonally significantly impaired the ability of 24-h injury-conditioned sensory neurons to extend the regeneration-associated elongating form of axon growth but had no impact on axon outgrowth in naïve neurons. Collectively, these findings link injury-associated axonal ER responses proximal to the site of injury to the intrinsic regenerative growth capacity of adult sensory neurons.
A complex series of cellular and molecular events participates in reprogramming sensory neurons as they transition to a regenerating state following peripheral nerve injury (1–7). Injury discharge and rapid ion fluxes initiate this transition, followed by the loss of target-derived signals and receipt of retrograde signals from the site of injury (8–10). Retrograde signals include both injury-induced activation and axonal synthesis of intraaxonal transcription factors (TFs) (6). Nuclear import of activated axonal TFs is mediated via their nuclear localization signal (NLS) binding to importin-α found throughout the axon. This interaction is heightened by the addition of importin-β, which is only axonally translated in response to axotomy, creating a high-affinity NLS–importin binding complex that retrogradely traffics target proteins to the cell body (11, 12). The identification of multiple axonal TF transcripts in the injured nerve supports the view that many may serve as retrograde regeneration signals linking axonal to nuclear activities. However, although roles for transcription factors and associated signaling pathways in regeneration of peripheral neurons have been elucidated (reviewed in refs. 13 and 14), the role of axon-derived TFs in the sensory neuron response to injury has been limited to axonal STAT3, revealing it to be involved in the survival of injured sensory neurons (6), a role also noted for axonal CREB in developing sensory neurons (15).
In preliminary work, we found that the endoplasmic reticulum (ER) transmembrane basic leucine zipper (bZIP) TF family member Luman (also called cAMP response element binding protein 3, CREB3) localizes to both the somal and axonal ER of dorsal root ganglion (DRG) sensory neurons†. Its transcript was also identified, but was not validated in a transcriptome analysis of injured sensory axons (16). Luman, initially recognized for its role in herpes simplex virus latency (17, 18), is released from the ER in its active form by regulated intramembrane proteolysis, allowing for a rapid response to cellular stresses (19). Target genes of Luman include ER stress-related unfolded protein response elements (20, 21), likely activated to deal with the increased protein synthesis associated with axon regeneration. Mammalian Luman is a CREB3 subfamily member of transmembrane bZIP TFs, recognized for roles beyond predicted ones in ER stress physiology (22). Indeed, in our preliminary studies, Luman was expressed by DRG neurons, regulated by axonal injury, and colocalized with importin-β in injured axons.† We hypothesized that axonal Luman, as an ER-localized TF, may serve to transduce ER stress signals from the site of injury back to the cell body. Here Luman is revealed to be an intraaxonal ER-localized TF that is activated and axonally synthesized in response to nerve injury, and when activated serves as a retrograde injury signal regulating the intrinsic regenerative growth capacity of injured sensory axons.
Results
Two principal forms of neurons were used to create cultures for these experiments: either (i) DRG neurons injured in vivo for 24 h to allow sufficient time to generate and receive a retrograde signal from the injury site or (ii) naïve DRG neurons.
Sensory Axons Contain Luman mRNA and Protein.
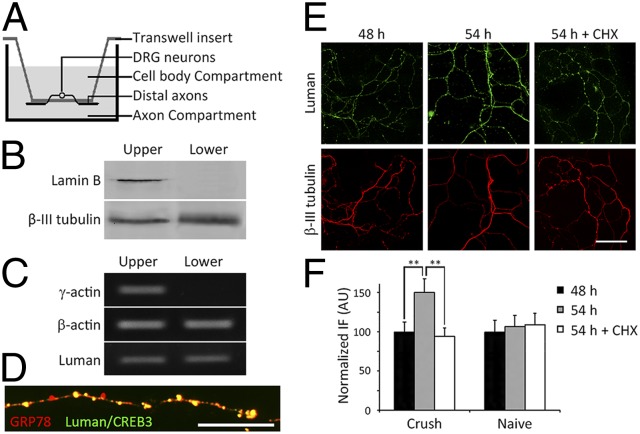
To assess whether Luman mRNA and protein localize to axons, we obtained pure axonal isolates using a Transwell culture insert approach (23). This allows separation of cell bodies from distal axons that grow through 3-μm holes in the insert membrane such that only axons localize to the underside (Fig. 1 and Fig. S1). Both Luman and β-actin mRNAs could be amplified by reverse transcription (RT)–PCR from the pure axonal isolates obtained from the membrane underside, whereas cell body-restricted γ-actin mRNA could not (Fig. 1C), validating the axonal isolate purity. In keeping with Luman as an ER transmembrane TF, its axonal protein expression colocalized with the ER protein GRP78 in a punctate manner characteristic of axonal ER (24) (Fig. 1D).
Fig. 1.
Luman mRNA localizes to axons. (A) Transwell cell-culture insert schematic. DRG neurons seeded on membranes with 3-μm pores extend axons through the pores such that distal axons grow on the lower membrane surface. See also Fig. S1. (B) Western blot of proteins isolated from distal axons (lower) or cell bodies and proximal axons (upper) of naïve DRG neurons cultured for 24 h have no detectable nuclear envelope Lamin B in axonal (lower) samples, whereas β-III-tubulin is detected in both isolates. (C) RT-PCR analysis of RNA isolated from axonal (lower) or cell-body and proximal axon (upper) preparations as in B. β-Actin and Luman mRNAs are detected in axon-only isolates (lower) but not cell body-restricted γ-actin mRNA (upper). (D) Photomicrograph of an axon from a naïve neuron cultured for 24 h on a coverslip showing that Luman IF (green) colocalizes with ER marker GRP78 IF (red). (Scale bar, 10 μm.) (E) Twenty-four–hour injury-conditioned neurons cultured 48 h before desomatization of lower-membrane axons and culturing an extra 6 h ± 10 μg/mL cycloheximide (CHX; translation inhibitor). Luman IF detects protein levels (green) with axons identified by β-III-tubulin IF (red). (Scale bar, 50 μm.) (F) Quantification of changes in IF from experiments as in E. CHX prevented the Luman IF [arbitrary units (AUs) normalized to predesomatization levels at 48 h] increase normally observed in desomatized axons, supporting intraaxonal synthesis of Luman. Mean ± SEM; n = 40 axonal fields per experimental group per time point; **P < 0.01.
Luman Is Synthesized in Axons in Response to Nerve Injury.
Axons have protein synthetic machinery (25). To assess whether injured axons synthesize Luman, rats underwent a unilateral sciatic nerve crush injury 24 h (injury-conditioned) before culturing lumbar (L)4,5,6 DRG neurons on Transwell inserts as above. Distal axons growing on the insert underside were desomatized 48 h later to examine Luman synthesis in axons while excluding cell body-synthesized Luman, a model where axons remain metabolically active for the time frame examined (23). De novo Luman synthesis in the desomatized axons was assessed by immunofluorescence (IF) with IF signal (protein levels) increasing over the 6-h assay and being effectively blocked by cycloheximide protein synthesis inhibition (Fig. 1 D and E). Desomatization is not the signal for de novo axonal Luman synthesis, as levels in axons from naïve neurons did not increase following it (Fig. 1E and Fig. S1B). This implicates molecular events triggered in the 24-h preculture injury-conditioned neurons in the response.
Luman’s Disappearance from Injury-Conditioned Axons Is Not Due to Protein Degradation.
Luman is activated by regulated intramembrane proteolysis, with the cleaved N-terminal cytoplasmic portion translocating into the nucleus (18, 19). In cultures of naïve sensory neurons (that do not undergo 24-h sciatic nerve injury before culturing), Luman IF colocalizes in the somas and axons with calnexin, another ER integral membrane protein, in a punctate pattern (Fig. S2A). However, when 24-h injury-conditioned neurons were cultured, axonal Luman IF had largely disappeared (Fig. S2B). To ascertain whether this disappearance from injury-conditioned axons was due to protein degradation or retrograde transport of Luman in the injury-conditioned axons, experiments were performed using a proteasome inhibitor, MG-132, revealing that the disappearance of Luman was not due to degradation, because MG-132 did not prevent it (Fig. S2B). Further indirect support for retrograde removal of cleaved Luman from the injury site comes from temporal Western blot analysis of in vivo sciatic nerve crush experiments. Here, levels of Luman (with a molecular weight consistent with its cleaved form) in nerve segments proximal to the injury site decreased with increasing time postinjury and increased in a reciprocal manner in corresponding DRGs (Fig. 2A). However, because Luman might be expressed by other cell types, the Transwell insert approach was also used to examine injury-induced disappearance from axons only. Temporal analysis of Luman IF levels in axon isolates from injury-conditioned neurons or naïve controls cultured ± proteasome inhibition was performed (Fig. 2B). In naïve neurons, axonal Luman levels remained relatively constant. Proteasome inhibition did not alter changes in Luman levels detected in the injury-conditioned axons, with axonal Luman levels decreasing to 25% that of naïve axons by 48 h in vitro (72 h after in vivo injury) but rising to preinjury levels by 72 h in vitro.
Fig. 2.
Nerve injury induces Luman removal from DRG axons in an NLS–importin-α–dependent but proteasome-independent manner. (A) Temporal Western blot analysis of proteins from sciatic nerve-injured DRG or associated 10-mm nerve segments proximal to the injury site detects Luman at the indicated hours postinjury. Lamin B, loading control. Cleaved Luman N-terminal (Cl) versus full-length (FL) levels transiently increase and then decline in injured nerves, with a reciprocal gradual increase in DRGs suggestive of retrograde transport. (B) Naïve or 24-h injury-conditioned neurons grown on culture inserts ± proteasome inhibitor MG-132 were fixed at the indicated time points. Axonal Luman levels measured by IF (AUs) and normalized to naïve 12-h levels (n = 40) decrease transiently but do not differ significantly from the proteasome-inhibited group supporting degradation-independent removal from axons. (C) Luman peptide features (26). TAD, transactivation domain; TMD, transmembrane domain. NLS predicts interaction with importin-α. The arrow identifies the N-terminal cleavage site. (D) Western blot (WB) analysis of the interaction between Luman and importin-α by IP performed as indicated. The cleaved cytoplasmic N terminus of Luman (Cl) associates with importin-α in 24-h injury-conditioned but not naïve DRG neurons.This interaction is abolished with 50 μg/mL NLS (Crush+NLS)-treated but not mismatched NLS control (Crush+mNLS)-treated injury-conditioned neurons. First lane, injury-conditioned control sample before IP (input). (E) Representative Western blot of Luman in 24-h injury-conditioned axon isolates cultured for 48 h on tissue-culture inserts ± proteasome (MG-13) + lysosome (chloroquine; CHL) inhibition or colchicine (COL). Note: Injury-conditioned axons have less Luman, most notably for the cleaved form, and are unaffected by protein degradation inhibition, but the disappearance of Luman from an axon can be prevented with the retrograde transport inhibitor colchicine. (F) Quantification of cleaved Luman in E normalized to control naïve levels. Mean ± SEM; n = 3; *P < 0.05; **P < 0.01; ***P < 0.001. See Fig. S2B for additional controls.
Injury-Activated Importin System Assists in Axonal Luman Retrograde Transport.
We next ascertained how axonal Luman is imported into the nuclei of injured neurons. NLS-containing axonal cargo proteins are retrogradely transported to the nucleus by importins in response to injury (11). Our 24-h in vivo sciatic nerve injury model activated the importin system, with increased importin-β and Luman detected in the sciatic nerve proximal to the injury and DRG neurons, most notably in neuronal nuclei, where both colocalized (Fig. S3). This, coupled with Luman’s NLS (Fig. 2C) (26), provides anatomical proof of Luman as an importin cargo candidate.
Immunoprecipitation (IP) studies performed on DRG protein samples from naïve or 24-h injury-conditioned animals revealed an interaction between Luman and importin-α. Using anti–importin-α, a 40-kDa Luman (consistent with the cleaved N terminus) was immunoprecipitated in sciatic nerve-injured but not naïve samples. Similar results were obtained for importin-α when anti-Luman was used for the IP (Fig. 2D). Activated Luman has all known functional domains, including the NLS (17), consistent with an ability of excess NLS but not mismatched NLS peptide to abolish the interaction between importin-α and Luman (Fig. 2D), as seen for other TFs (27).
To examine whether injury-activated Luman translocates from axons to the nucleus by retrograde transport along microtubules, colchicine was used to disrupt microtubule polymerization. This prevented the reductions in axonal Luman normally observed in the injury-conditioned axonal isolates, whereas proteasome (MG-132) coupled with lysosome (chloroquine) inhibition did not (Fig. 2 E and F). We also examined retrograde trafficking of the activated Luman N terminus using an adenoviral vector specially created with green fluorescence protein (GFP) fused to the cytoplasmic N terminus containing the known functional domains and red fluorescence protein (RFP) fused to the C terminus of rat Luman that remains localized to the ER membrane (Ad/GFP-Lu-RFP). The addition of these tags did not alter the ER localization in transduced sensory neurons in vivo or in vitro (Fig. S4 C and D), nor its functional properties, with the fusion protein activating transcription from the unfolded protein response (UPR) element in a manner similar to wild-type Luman (Fig. S4B).
Naïve neuronal cultures were exposed to the vector (100 multiplicity of infection; MOI) for 48 h at the time of plating. RFP and GFP tags colocalized in the somas and axons of the transduced neurons (Fig. 3A, 0 h and Fig. S4A), consistent with an absence of cleavage/activation and retrograde transport signals, as opposed to what was observed for the injury-conditioned neurons (Fig. 2). To visualize whether axonal injury results in Luman cleavage and translocation into the nucleus in vitro, an axonal scratch injury was made with a pin rake (Tyler Research). Axons proximal to the injury site and their cell bodies were imaged with 24-h time-lapse microscopy. Axotomy resulted in the disappearance of GFP from injured axonal tips with a corresponding GFP accumulation in the nucleus in a subnuclear pattern, in agreement with our previous recombinant Luman vectors (28). No change in RFP distribution was noted (Fig. 3 and Movie S1). This is consistent with the cleavage of the Luman N-terminal cytoplasmic portion, whereas the C terminus remains membrane-bound. The axonal disappearance of GFP and corresponding nuclear accumulation were significantly prevented by excess NLS peptide, supporting importin-dependent retrograde transport of the cleaved GFP–Luman in the axon (Fig. 3 B and C and Fig. S5A).
Fig. 3.
Retrograde transport of cleaved dual-tagged Luman in vitro. (A) Naïve DRG neurons cultured on coverslips were transduced with dual-tagged Ad/GFP-Lu-RFP for 48 h before receiving an in vitro scratch injury (arrowhead; a representative neuron is shown). GFP and RFP colocalize in the axon (Left) and corresponding cell body (Right) before injury (0 h). However, over the 24-h postinjury period, GFP signal in the axon proximal to the injury decreased, whereas GFP in the cell body and nucleus increased (bright subnuclear regions; five time points are shown). RFP signal appeared unchanged. The arrow denotes the retrograde transport direction. [Scale bars, 50 μm (axon) and 20 μm (cell body).] See also Movie S1. (B) Quantification of the axonal GFP and RFP signals in A and GFP signal when NLS peptide was added 24 h before the in vitro scratch injury and then monitored for 24 h (see also Fig. S5A). (C) Quantification of the nuclear GFP and RFP signals in A with experimental conditions as in B. Signal is expressed in arbitrary units and normalized to 0 h. Note: RFP remains relatively stable. The decreased GFP in the axon and increased nuclear GFP support that the axonal GFP–N terminus of Luman was cleaved after axonal injury and retrogradely transported to the nucleus. Mean ± SEM; n = 10; **P < 0.01; ***P < 0.001.
Axonal transport of Luman in response to axotomy was also assessed in vivo. The sciatic nerve was crushed and then 24 h later ligated proximal to the injury site for another 24 h before processing for Luman IF. The intense IF signal observed, especially that distal to ligation, is consistent with the retrograde transport of Luman from the initial crush site toward the cell body, as was the decreased IF signal observed proximal to ligation. This was not observed in control nerves that were only ligated (Fig. 4A).
Fig. 4.
In vivo retrograde transport of Luman after injury. (A) Sciatic nerve sections processed for Luman IF from 24-h sciatic nerve ligation (Upper) or sciatic nerve crush, followed by ligation proximal to the injury 24 h later for an extra 24 h as indicated. Endogenous Luman IF is increased and accumulates distal to ligation in the crush+ligation nerve (arrows) versus ligation only. This is consistent with increased Luman synthesis and retrograde transport from the initial injury site, as is the paucity of IF staining proximal to ligation. (Scale bar, 50 μm.) n = 3. (B) Dual-tagged Ad/GFP-Lu-RFP injected intrathecally 7 d before an ipsilateral 48-h nerve crush injury transduced L4,5 DRG neurons with tagged Luman. (Left) Representative sciatic nerve section (1 mm proximal to the injury) and corresponding L5 DRG section (Right). Retrograde direction is indicated by a black arrow. The cleavage and presumed retrograde transport of GFP–Luman N terminus into the nucleus is consistent with the reduced colocalization of GFP and RFP observed in the injured nerve proximal to the injury (white arrows) and the increased GFP in the nuclei of injured sensory neurons (white arrows, DRG sections). [Scale bars, 100 μm (nerve) and 50 μm (DRG).] n = 4. In contrast, GFP and RFP appear to always colocalize in naïve nerves and neurons with no nuclear GFP (Fig. S5B).
To track the movement of cleaved Luman in vivo, Ad/GFP-Lu-RFP or control vectors were intrathecally injected at the lumbar level and left for 7 d before a 2-d sciatic nerve crush injury to efficiently transduce the L4,5 DRG neurons (29). In the DRG and sciatic nerves contralateral to the injury, GFP and RFP colocalized and were absent in neuronal nuclei (Fig. S5B). In contrast, there was a marked loss of GFP, but not RFP, in the nerve proximal to the injury and an accumulation of GFP in corresponding DRG neuronal nuclei (Fig. 4B), supporting activation of the axonal Luman N terminus and its retrograde transport to the nucleus, in agreement with our in vitro data. Collectively, the data reinforce that injury induces importin-mediated removal of N-terminal Luman from axons back into the nucleus.
Axonal Knockdown of Luman Inhibits Regenerative Outgrowth.
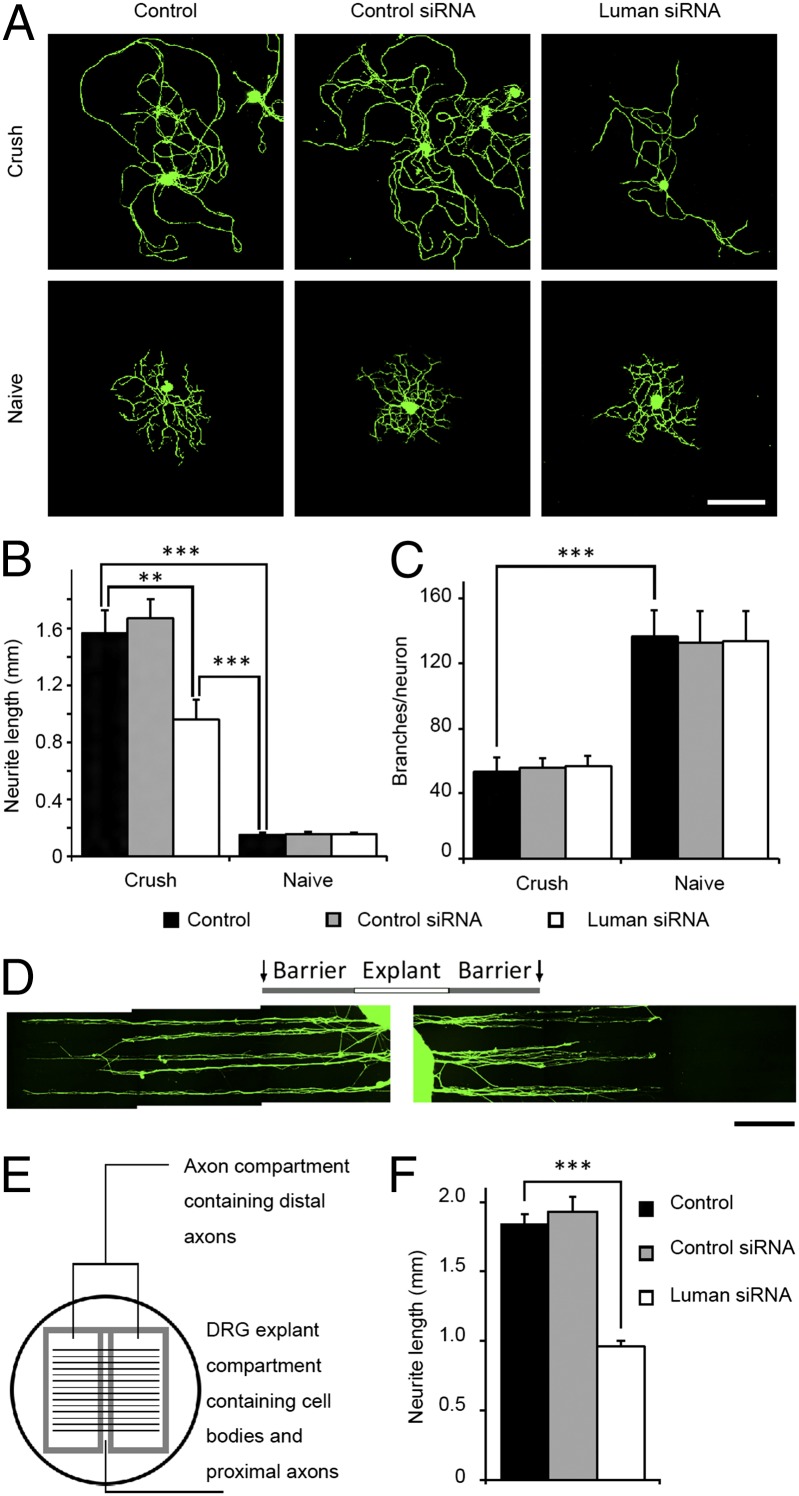
Luman-regulated immune cell migration involves intricate cytoskeletal and membrane rearrangements (30), requisite cellular events for axon regeneration. Thus, we examined whether injury-activated Luman regulates axon growth. Axon outgrowth was assessed in DRG cultures exposed to Luman or control siRNAs, the former significantly reducing overall Luman protein expression (Fig. S6). Injury-conditioned or naïve rat sensory neurons were transfected with Luman or control siRNAs for 48 h at the time of plating, followed by assessment of total axon outgrowth and branching (Fig. 5A). Injury-conditioned DRG neurons had longer, less branched axons relative to naïve (4). Luman siRNA significantly decreased total axon length in injury-conditioned DRG neurons, with no discernible impact on branching. No effect on either parameter was observed for cultures of naïve sensory neurons (Fig. 5 B and C). However, this dissociated culture approach does not allow selective assessment of the effect of axon-derived Luman on the growth capacity of the injury-conditioned neuron.
Fig. 5.
Neuronal or axon-only knockdown of Luman inhibits axonal outgrowth in injury-conditioned DRG neurons. (A) Representative naïve or 24-h injury-conditioned neurons (Crush) assayed for 48 h in medium alone (control), control siRNA, or Luman siRNA with β-III-tubulin IF to detect axons/neurites (green). (Scale bar, 200 μm.) (B) Quantification of total neurite length in A reveals a significant decrease in injury-induced outgrowth with Luman siRNA (n = 250 per group). (C) Quantification of axon branches per neuron in A (n = 50 per group) reveals no impact of siRNA on branching. (D) Representative fluorescence photomicrograph montages of axonal outgrowth from 24-h injury-conditioned DRG mini explants detected by β-III-tubulin IF (green). Explant compartment and silicon barrier regions (gray) are indicated. Compartments were transfected with control siRNA (Left) and Luman siRNA (Right) for 6 d. Luman siRNA axon outgrowth is significantly shorter compared with control siRNA-treated axons. (Scale bar, 1 mm.) (E) Schematic of a compartmented culture dish created by scratching the surface with a pin rake and placing a silicon insert on top 24 h after seeding DRG mini explants in the middle. Only axons grow under the barrier. (F) Quantification of the 10 longest neurite lengths per DRG explant in the right and left compartments; n = 60 (medium alone), 130 (control siRNA), or 70 (Luman siRNA). Note: Knockdown of Luman in the axon compartment only (Fig. S7) significantly decreased neurites/axons from injury-conditioned DRG explants. Mean ± SEM; **P < 0.01; ***P < 0.001.
To better simulate the in vivo state and test the effect of only axon-derived Luman on injury-associated outgrowth, a modified compartmented culture approach was used (31) using injury-conditioned DRG mini explants (Fig. 5E and Fig. S7A). Here, Luman siRNA’s impact on Luman protein expression was restricted to the applied compartment (Fig. S7 B and C), similar to Cox et al. (15), allowing outgrowth assessment from the same explant into differentially treated compartments. Explants also preserve relationships among neurons, Schwann cells, and satellite glial cells implicated in axonal regeneration (32, 33). Injury-conditioned explants were placed in a culture dish for 24 h under a low volume of medium to ensure good adherence before the compartment barriers were put in place. It took ∼1 d for the explants to extend axons beyond the barriers into differentially treated compartments. Axons were allowed to grow an additional 5 d into control (control siRNA or medium alone) or Luman siRNA compartments in the same dish, to ensure adequate growth for assessment of the impact of treatment (Fig. 5D). Axons were visualized by β-III-tubulin. The 10 longest axons/explants (left and right compartments) were measured (Fig. 5F). Axons grown under medium alone or control siRNA conditions were not different, but were significantly longer than axons transfected with Luman siRNA (Fig. 5 D and F). This implicates axon-derived Luman in injury-conditioned regenerative outgrowth.
Discussion
Retrograde injury signals help neurons transition to a regenerative state (10). Here we provide the first insights, to our knowledge, into the role that the axonal ER proximal to the site of axon injury plays in generating an intrinsic regeneration signal through the cleavage/activation and synthesis of Luman, a transmembrane ER TF. The localization of Luman mRNA to axons was validated, with nerve injury inducing axonal Luman cleavage and synthesis and its removal from axons to the cell body via importin-dependent retrograde transport. Finally, a previously unidentified role for this axonal TF in the intrinsic elongating form of axonal growth associated with regeneration was elucidated.
Luman as an Axonal ER-Associated Injury Signal.
Our finding that ER markers extend the axon length agrees with recent findings (34) and provides a mechanism whereby injury at any site along the axoplasmic ER may impact ER-associated events. Although this includes signals derived proximal to the injury site, such as the local activation and synthesis of Luman described here, we cannot exclude that additional signals propagated along the length of the axonal ER are also involved (34). The ER localization of Luman along the axon length in the naïve state ideally positions it for local activation in response to injury through release of its N terminus (Fig. 2), containing known functional domains such as the NLS (17, 26). Whether the C terminus that remains ER-bound subserves a functional purpose is not known, but it does contain a recognition site involved in the cleavage event (19). How Luman mRNA transcripts are transported into the axon is unknown, but probably involves a 3′ UTR interaction with an RNA-binding protein such as ZBP1 that targets transcripts to axons and translationally represses cargo mRNAs until needed (35). The observed injury-induced translation of axonal Luman transcripts likely serves to prolong this form of Luman signaling, because axon outgrowth is impaired when synthesis is prevented (Fig. 5).
Whether Luman is an inductive injury signal or whether sustained Luman signaling is required for regenerative growth is beyond the scope of this study, and necessitates a long-term injury time course be performed. However, the low level of activated Luman in the 24-h naïve neuron cultures coupled with a lack of interaction with the importin retrograde transport machinery supports that Luman’s disappearance from injury-conditioned axons requires processes not activated in the naïve state (Figs. 2 and 3). Further, the low level of activated Luman in the uninjured DRGs (Fig. 2A) should allow axon-derived axotomy-activated Luman to impact transcriptional activities, with identification of target genes the focus of future work. It is also possible that axon-derived axotomy-activated Luman exerts yet unknown local, nontranscriptional effects on axonal growth. Finally, even though the naïve neurons have axons completely removed during dissociation, this rapid process likely does not allow the time for activation and transport back of the axon-derived Luman injury signal, as evidenced by a lack of nuclear Luman (Fig. 3A, 0 h).
Axonal Luman Contributes to Axon Growth After Injury.
Retrograde axonal signaling is linked to optimal axon regeneration (5), with the high capacity of peripheral axons to synthesize proteins in a cell-autonomous way likely factoring into this (31, 36). We discerned distinct modes of axon growth associated with the naïve (branching) versus injury-conditioned states (elongating; Fig. 5) as in Smith and Skene (4). Our siRNA results reveal a mode-specific role for axonal Luman in injury-conditioned neurons with it regulating only the elongating form of axon growth in a manner dependent on local axonal synthesis (Fig. 5). Although other axonally translated TFs influence neuronal viability (6, 15), axonal-derived Luman is the first, to our knowledge, to be directly linked to regulation of the regenerative form of axon outgrowth. The downstream targets of Luman’s effect on axon growth are not known. They may be proteins linked to the elongating form of axonal growth (37) or Luman-regulated UPR-associated genes (20, 21), because the UPR has been linked to spinal cord injury outcomes (38). Finally, the axonal outgrowth inhibition by Luman siRNA was only partial (Fig. 5), likely due to several factors: (i) The Luman signal would go unhindered in vivo in the injury-conditioned neurons until the neurons or mini explants were put into culture; (ii) knockdown of Luman was partial; or (iii) Luman is not the sole regulator of injury-conditioned outgrowth.
In conclusion, Luman mediates an important form of communication between injured distal axons and neuronal cell bodies in the regulation of axon growth. Elucidating key transcriptional regulators of intrinsic regenerative axonal growth provides novel targets for enhancing peripheral nerve repair.
Materials and Methods
Unless stated otherwise, experiments were performed at least in triplicate and reagents were purchased from Sigma-Aldrich. IF, IP, Western blot, chloramphenicol acetyltransferase assay, and RT-PCR method details can be found in SI Materials and Methods.
Surgical Procedure.
Animal procedures were conducted on adult male Wistar rats (∼250 g; Charles River Laboratories) in accordance with the Canadian Council on Animal Care and approved by the University of Saskatchewan Animal Research Ethics Board. Aseptic surgical procedures used 2% (vol/vol) isoflurane anesthetic and pre- and postoperative analgesics (buprenorphine, 0.05 mg/kg). A smooth-jaw ultrafine hemostat (0.6 mm) closed for 10 s was used for unilateral midthigh sciatic nerve crush injuries.
Adult DRG Culture.
L4,5,6 DRGs from CO2-euthanized rats were treated with 0.25% collagenase (1 h, 37 °C) and dissociated (2.5% trypsin, 30 min, 37 °C) before plating on laminin- (1 μg/mL) and poly–d-lysine–coated (25 μg/mL) coverslips at 104 cells per well in a six-well plate (BD Biosciences) in DMEM ± 10 ng/mL 2.5S nerve growth factor (NGF) (Cedarlane Labs). Cytosine β-d-arabinofuranoside (Ara-C; 10 μM) was added to inhibit proliferation of nonneuronal cells.
Proteasome and retrograde transport inhibition.
In select experiments, MG-132 (proteasome inhibitor; 5 μM) and chloroquine (lysosome inhibitor; 100 μM) were added to inhibit protein degradation, or colchicine (10 μg/mL) to disrupt microtubule polymerization. NLS peptide (50 μg/mL; ENZO Life Sciences) competed with Luman for binding to the importin transport complex. Mismatch NLS peptides (50 μg/mL) served as control.
Assessment of de novo axonal protein synthesis.
Neurons grown for 48 h on Transwell insert membranes were desomatized by scraping the upper membrane (23). Axons remaining on the lower membrane were then cultured ± the translation inhibitor cycloheximide (10 μg/mL) for 6 h, followed by processing for Luman IF to assess Luman protein levels.
siRNA treatment.
DRG cultures were transfected with Luman siRNA (5′-CGACUGGGAGGUAGAGGAUUUAC-3′; Integrated DNA Technologies) or negative control nontargeting siRNA using Lipofectamine RNAiMAX Reagent (Life Technologies) according to the manufacturer’s instructions. Luman knockdown efficacy was assessed by Western blot.
Axon Isolation.
Transwell insert.
DRG axon isolation was as per Zheng et al. (23), using a polyethylene tetraphthalate insert coated with poly–l-lysine and laminin with 3-μm instead of 8-μm pores (BD Biosciences).
Compartmented explant culture.
DRGs cut into four or five pieces were grown using an adaptation of Vogelaar et al. (31) compartmented cultures whereby individual DRG explants extend axons into right and left compartments, allowing differential treatments. DRG explants (15–20 per well) were plated in a row on top of scratches made with a pin rake (Tyler Research) in six-well plates coated with poly–d-lysine (25 μg/mL) and laminin (1 μg/mL) in DMEM ± 10 ng/mL NGF. The volume of medium was kept low for 1 d to improve explant adherence, followed by placement of a compartmented insert cut from a 1 mm-thick medical-grade silicon sheet (Rubber Sheet Roll) in the dish, creating leak-proof compartments (Fig. 5). Cultures were maintained for 7 d in total, with Ara-C added every other day to inhibit nonneuronal cell proliferation.
Adenovirus Preparation.
Ad/GFP-Lu-RFP construction: GFP and RFP were fused to the N terminus and C terminus of rat Luman (39), respectively, and the ORF of the fused protein was inserted into the Adeno-X Viral DNA vector (Clontech). Adenovirus was grown and purified using the Adeno-X Expression System (Clontech; per the manufacturer’s instruction). For in vitro infection, neurons were infected with 100 MOI 48 h before axotomy. For in vivo infection, 15 μL Ad/GFP-Lu-RFP (2 × 1011 pfu/mL; n = 4) or control Adeno-LacZ (expressing Escherichia coli β-galactosidase; 2 × 1011 pfu/mL; n = 2) was injected intrathecally via a sterile silicon catheter inserted at the lumbar sacral junction such that the tip lay at the L5 DRG level and was left 1 wk before performing a 48-h sciatic nerve crush injury.
Neurite Growth.
Dissociated DRG neuronal cultures.
Total neurite length per neuron (Northern Eclipse software; Empix Imaging) and total neurite branch endpoints [NeurphologyJ (40)] were calculated for all neurons in ∼50 random fields per experimental group per replicate (n = 3).
Compartmented cultures.
Neurite outgrowth was assessed in right and left axon compartments for the same 24-h injury-conditioned explants. The 10 longest neurites were measured with Northern Eclipse from 13 explants per condition (n = 60, medium alone; n = 130, control siRNA; n = 70, Luman siRNA).
Statistical Analyses.
Statistical analyses were performed with Prism (GraphPad Software). Differences between means were assessed by one-way analysis of variance with post hoc Tukey’s analysis. All values in the figures are means ± SEM with significance at P < 0.05.
Supplementary Material
Acknowledgments
We thank P. M. Richardson for his critical review and R. Zhai, J. Johnston, and N. Rapin for excellent technical assistance. We are grateful for support from the Canadian Institutes of Health Research [CIHR RT733698 and MOP74747 Grants (to V.M.K.V.)], a Natural Sciences and Engineering Research Council Discovery Grant (to V.M.), and China Scholarship Council 2010603017 and a University of Saskatchewan Scholarship (to Z.Y.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
†Hasmatali et al., Society for Neuroscience Annual Meeting, November 12–16, 2011, Washington, DC, abstr 438.1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407462111/-/DCSupplemental.
References
- 1.Lieberman A. Some factors affecting retrograde neuronal responses to axonal lesions. In: Bellairs R, Gray EG, editors. Essays on the Nervous System: A Festschrift for Professor J. Z. Young. Clarendon; Oxford: 1974. pp. 71–105. [Google Scholar]
- 2.Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- 3.Ji RR, et al. Prominent expression of bFGF in dorsal root ganglia after axotomy. Eur J Neurosci. 1995;7(12):2458–2468. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17(2):646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2010;223(1):28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Yaakov K, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31(6):1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niemi JP, et al. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci. 2013;33(41):16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy PG, Borthwick LS, Johnston RS, Kuchel G, Richardson PM. Nature of the retrograde signal from injured nerves that induces interleukin-6 mRNA in neurons. J Neurosci. 1999;19(10):3791–3800. doi: 10.1523/JNEUROSCI.19-10-03791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michaelevski I, et al. Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal. 2010;3(130):ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13(5):308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanz S, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40(6):1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 12.Perry RB, et al. Subcellular knockout of importin β1 perturbs axonal retrograde signaling. Neuron. 2012;75(2):294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zigmond RE. Cytokines that promote nerve regeneration. Exp Neurol. 2012;238(2):101–106. doi: 10.1016/j.expneurol.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raivich G. Transcribing the path to neurological recovery—From early signals through transcription factors to downstream effectors of successful regeneration. Ann Anat. 2011;193(4):248–258. doi: 10.1016/j.aanat.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10(2):149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumy LF, et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA. 2011;17(1):85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu R, Yang P, O’Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;17(9):5117–5126. doi: 10.1128/mcb.17.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R, Misra V. Potential role for Luman, the cellular homologue of herpes simplex virus VP16 (alpha gene trans-inducing factor), in herpesvirus latency. J Virol. 2000;74(2):934–943. doi: 10.1128/jvi.74.2.934-943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raggo C, et al. Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol. 2002;22(16):5639–5649. doi: 10.1128/MCB.22.16.5639-5649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DenBoer LM, et al. Luman is capable of binding and activating transcription from the unfolded protein response element. Biochem Biophys Res Commun. 2005;331(1):113–119. doi: 10.1016/j.bbrc.2005.03.141. [DOI] [PubMed] [Google Scholar]
- 21.Liang G, et al. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26(21):7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CP, Kok KH, Jin DY. CREB3 subfamily transcription factors are not created equal: Recent insights from global analyses and animal models. Cell Biosci. 2011;1(1):6. doi: 10.1186/2045-3701-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng JQ, et al. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21(23):9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González C, Couve A. The axonal endoplasmic reticulum and protein trafficking: Cellular bootlegging south of the soma. Semin Cell Dev Biol. 2014;27:23–31. doi: 10.1016/j.semcdb.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Merianda TT, et al. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40(2):128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu R, Yang P, Padmakumar S, Misra V. The herpesvirus transactivator VP16 mimics a human basic domain leucine zipper protein, Luman, in its interaction with HCF. J Virol. 1998;72(8):6291–6297. doi: 10.1128/jvi.72.8.6291-6297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270(24):14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 28.Misra V, Rapin N, Akhova O, Bainbridge M, Korchinski P. Zhangfei is a potent and specific inhibitor of the host cell factor-binding transcription factor Luman. J Biol Chem. 2005;280(15):15257–15266. doi: 10.1074/jbc.M500728200. [DOI] [PubMed] [Google Scholar]
- 29.Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko J, et al. Human LZIP binds to CCR1 and differentially affects the chemotactic activities of CCR1-dependent chemokines. FASEB J. 2004;18(7):890–892. doi: 10.1096/fj.03-0867fje. [DOI] [PubMed] [Google Scholar]
- 31.Vogelaar CF, et al. Axonal mRNAs: Characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol Cell Neurosci. 2009;42(2):102–115. doi: 10.1016/j.mcn.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst. 2012;17(1):1–18. doi: 10.1111/j.1529-8027.2012.00378.x. [DOI] [PubMed] [Google Scholar]
- 34.Stirling DP, Cummins K, Wayne Chen SR, Stys P. Axoplasmic reticulum Ca(2+) release causes secondary degeneration of spinal axons. Ann Neurol. 2014;75(2):220–229. doi: 10.1002/ana.24099. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly CJ, et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011;30(22):4665–4677. doi: 10.1038/emboj.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma P, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25(2):331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnelly CJ, et al. Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J Neurosci. 2013;33(8):3311–3322. doi: 10.1523/JNEUROSCI.1722-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valenzuela V, et al. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ying Z, Zhang R, Verge VM, Misra V (June 5, 2014) Cloning and characterization of rat Luman/CREB3, a transcription factor highly expressed in nervous system tissue. J Mol Neurosci, 10.1007/s12031-014-0330-7. [DOI] [PubMed]
- 40.Ho SY, et al. NeurphologyJ: An automatic neuronal morphology quantification method and its application in pharmacological discovery. BMC Bioinformatics. 2011;12:230. doi: 10.1186/1471-2105-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.