Significance
Depression and anxiety have been linked to increased inflammation. However, we do not know if inflammatory status predates onset of disease or whether it contributes to depression symptomatology. We report preexisting individual differences in the peripheral immune system that predict and promote stress susceptibility. Replacing a stress-naive animal’s peripheral immune system with that of a stressed animal increases susceptibility to social stress including repeated social defeat stress (RSDS) and witness defeat (a purely emotional form of social stress). Depleting the cytokine IL-6 from the whole body or just from leukocytes promotes resilience, as does sequestering IL-6 outside of the brain. These studies demonstrate that the emotional response to stress can be generated or blocked in the periphery, and offer a potential new form of treatment for stress disorders.
Keywords: depression, anxiety, stress, interleukin-6, leukocytes
Abstract
Depression and anxiety disorders are associated with increased release of peripheral cytokines; however, their functional relevance remains unknown. Using a social stress model in mice, we find preexisting individual differences in the sensitivity of the peripheral immune system that predict and promote vulnerability to social stress. Cytokine profiles were obtained 20 min after the first social stress exposure. Of the cytokines regulated by stress, IL-6 was most highly up-regulated only in mice that ultimately developed a susceptible behavioral phenotype following a subsequent chronic stress, and levels remained elevated for at least 1 mo. We confirmed a similar elevation of serum IL-6 in two separate cohorts of patients with treatment-resistant major depressive disorder. Before any physical contact in mice, we observed individual differences in IL-6 levels from ex vivo stimulated leukocytes that predict susceptibility versus resilience to a subsequent stressor. To shift the sensitivity of the peripheral immune system to a pro- or antidepressant state, bone marrow (BM) chimeras were generated by transplanting hematopoietic progenitor cells from stress-susceptible mice releasing high IL-6 or from IL-6 knockout (IL-6−/−) mice. Stress-susceptible BM chimeras exhibited increased social avoidance behavior after exposure to either subthreshold repeated social defeat stress (RSDS) or a purely emotional stressor termed witness defeat. IL-6−/− BM chimeric and IL-6−/− mice, as well as those treated with a systemic IL-6 monoclonal antibody, were resilient to social stress. These data establish that preexisting differences in stress-responsive IL-6 release from BM-derived leukocytes functionally contribute to social stress-induced behavioral abnormalities.
Human studies indicate that psychosocial stressors increase peripheral cytokine production, a potentially important factor in the development of depression or anxiety (1–6). Subsets of patients with major depressive disorder (MDD) and posttraumatic stress disorder have higher levels of multiple inflammatory markers, including the cytokine interleukin 6 (IL-6) (3, 4, 6–8), which meta-analyses indicate is consistently elevated across studies (2, 9–11). Although systemic injection of proinflammatory cytokines induces “sickness behavior” reminiscent of depressive symptoms (12), a causal relationship between peripherally derived cytokines and stress-related disorders awaits confirmation. To directly address whether systemic inflammation functionally contributes to stress vulnerability, we used two social stress paradigms: repeated social defeat stress (RSDS) (13, 14) and a purely emotional stressor (witness defeat) (15). As in humans (16, 17), chronic social subordinations in mice lead to depression-like behavior, including social avoidance, in a subset of mice termed susceptible, whereas resilient mice resist the development of such behavior (18–20). We hypothesize that preexisting differences in the sensitivity of an individual’s peripheral immune system dictate their subsequent vulnerability or resilience to social stress.
Results
Leukocyte-Derived IL-6 Is Predictive of Susceptibility Versus Resilience to Stress.
We examined cytokine and chemokine profiles 20 min after the first exposure to an aggressor in the RSDS paradigm (Table 1 and Fig. S1A). Although acute social stress regulated a number of cytokines and chemokines, IL-6 was the only cytokine significantly elevated in animals that later developed a susceptible phenotype compared with both control and resilient mice. Furthermore, IL-6 levels strongly negatively correlated with social interaction behavior following subsequent RSDS (Fig. S1B). IL-1β was also elevated in susceptible mice compared with resilient mice, however, neither differed from controls. The antiinflammatory cytokine IL-10 and the chemokines chemokine (C-X-C motif) ligand 1 (CXCL1) and chemokine (C-C motif) ligand 2 (CCL2) were similarly elevated in susceptible and resilient animals compared with controls, indicating a general effect of defeat (Table 1). IL-9 was not significantly regulated by stress but did correlate with subsequent social avoidance behavior (Fig. S1C).
Table 1.
Cytokines and chemokines regulated by stress 20 min after the first defeat
| Cytokine | IL-1β pg/mL | IL-6 pg/mL | IL-9 pg/mL | IL-10 pg/mL | Cxcl1/KC pg/mL | CCL2/MCP1 pg/mL |
| Control | 8.73 ± 1.09 | 3.36b ± 0.96 | 131.2 ± 17.7 | 4.24b,c ± 0.37 | 95.25b,c ± 5.32 | 20.58b,c ± 1.88 |
| Susceptible | 11.75c ± 2.47 | 92.27a,c ± 8.91 | 160.8 ± 9.04 | 10.37a ± 2.05 | 575. 6a ± 170.8 | 40.67a ± 7.44 |
| Resilient | 6.49b ± 0.5 | 10.83b ± 2.7 | 130.5 ± 10.1 | 8.45a ± 1.34 | 547. 9a ± 161.5 | 44.94a ± 6.93 |
| F ratio | F2,24 = 3.88, P < 0.05 | F2,21 = 80.11, P < 0.0001 | F2,29 = 1.53, P > 0.05 | F2,26 = 5.05, P < 0.05 | F2,28 = 3.42, P < 0.05 | F2,28 = 4.38, P < 0.05 |
| R | −0.48, P = 0.057 | −0.77, P < 0.001 | −0.47, P < 0.05 | −0.21, P > 0.05 | −0.08, P > 0.05 | −0.04, P > 0.05 |
| N | C (9) | C (5) | C (10) | C (9) | C (9) | C (9) |
| S (6) | S (7) | S (8) | S (7) | S (8) | S (8) | |
| R (10) | R (10) | R (12) | R (11) | R (12) | R (12) |
Animals were behaviorally phenotyped 10 d after RSDS by measuring social interaction (Fig. S1A). Univariate ANOVA analysis was used to determine significant differences in circulating cytokine levels between control, susceptible, and resilient mice. Correlations to the SI ratio were examined in animals that underwent RSDS. Subjects with nondetectable levels of cytokines/chemokines or levels that varied more than 2 SD from the mean were excluded from analysis. Data are displayed as mean ± SEM. Correlations are listed in row R, and sample numbers are listed in row N.
Significant difference from control.
Significant difference from susceptible.
Significant difference from resilient.
Given the 27-fold change in IL-6 detected in susceptible animals, we next examined an in vivo time course of plasma IL-6 levels before and throughout exposure to RSDS in a second group of animals. There were no basal differences in IL-6 before RSDS, however we again observed that susceptible animals had significantly higher serum IL-6 within 20 min of their first defeat and levels remained elevated 48 h after the last defeat (Fig. 1A and Figs. S2A and S3A). Because baseline levels of IL-6 were low, we verified that there were indeed no basal group differences via a high-sensitivity ELISA in a separate cohort of mice (Fig. S4 A and B).
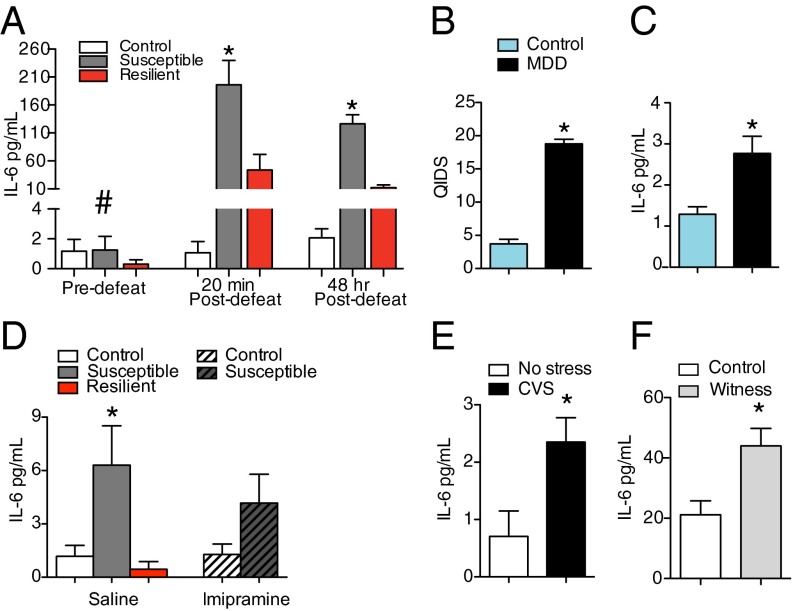
Fig. 1.
Individual differences in the peripheral immune system predict behavioral responses to RSDS. (A) A within-subjects time course of plasma levels of IL-6 indicated a significant interaction between time point of blood draw and behavioral phenotype (F4,19 = 10.83, P < 0.001, control = 9, susceptible = 7, resilient = 6). Post hoc analysis indicated no significant differences in IL-6 levels at baseline (P > 0.05). Twenty minutes after the first defeat, animals that developed a susceptible phenotype had higher circulating levels of IL-6 than control or resilient mice (P < 0.001). Forty-eight hours after the last defeat, susceptible mice had higher levels of IL-6 than resilient or control mice (P < 0.001). (B) Patients with a diagnosis of treatment-resistant MDD (n = 19) scored higher on the QIDS than healthy controls (n = 18) (t35 = 15.68, P < 0.0001, two tailed). (C) Patients with treatment-resistant MDD had higher circulating levels of IL-6 than healthy controls (t35 = 3.17, P < 0.01, two tailed). (D) Thirty-five days after the last defeat, susceptible mice injected with saline (n = 7) had basal elevations in serum levels of IL-6 compared with controls (saline, n = 13, Imipramine, n = 13) and resilient (n = 6) mice (F4,45 = 3.66, P < 0.05) but not susceptible mice injected with Imipramine (n = 11, P > 0.05). (E) Twenty-four hours after the last day of CVS, mice showed significant elevations of IL-6 (t12 = 2.48, P < 0.05, two tailed). (F) Mice exposed to the nonphysical social stress of witness defeat (witness = 10, control = 11) demonstrated elevations in IL-6 30 d after the last stressor, when serum levels were measured immediately after the social interaction test (t19 = 3.1, P < 0.01, two tailed). Bar graphs display mean ± SEM. # denotes a significant interaction. * denotes a significant difference in phenotype. For t tests, * denotes a significant difference between means.
To validate that the increased levels of IL-6 found in susceptible mice were clinically relevant, we examined IL-6 in serum from patients with treatment-resistant MDD using a high-sensitivity human IL-6 ELISA. Severity of depression was quantified using the Quick Inventory of Depressive Symptomatology (QIDS) scale (Fig. 1B). Patients with MDD had higher serum IL-6 than healthy controls (Fig. 1C and Table S1). In a second cohort of patients with treatment-resistant MDD, we examined the effects of antidepressant treatment on circulating levels of IL-6. In this cohort, severity of depression was quantified using Hamilton Depression Scale scores (Table S1). Again levels of IL-6 were elevated in patients with treatment-resistant MDD, and standard antidepressant treatment did not lower circulating levels of IL-6. IL-6 levels were also examined in mice that experienced 10 d of RSDS followed by 35 d of Imipramine treatment (Fig. 1D and Fig. S3 B and C). IL-6 levels were still significantly up-regulated 35 d after the last defeat, and similar to humans, Imipramine did not lower circulating levels of IL-6. These data suggest that standard antidepressants are not acting on immune function to exert their behavioral effects.
To examine whether the effects of stress on IL-6 levels generalize to other stress paradigms, we sampled blood from mice exposed to 21 d of chronic variable stress (CVS). CVS significantly increased basal levels of IL-6 sampled 24 h after the last stress exposure (Fig. 1E). We also examined whether exposure to a purely emotional stressor increased IL-6. Mice witnessed a conspecific undergoing RSDS, but had no physical contact with the aggressor. Circulating levels of IL-6 were elevated in witness mice compared with controls 30 d after the last stressor, when the phenotype of social avoidance emerges (15) (Fig. 1F and Fig. S3D).
Because of the physical nature of RSDS, we performed physical examinations of animals. There were no differences in the number of wounds and no correlation between number of wounds and social-interaction (SI) ratio (Fig. S4 C and D), suggesting that individual differences in susceptibility to RSDS were not due to physical injury. Because IL-6 is involved with the fever response (21), we examined core body temperature after 10 d of RSDS as general sickness behavior could alter social interaction (22). There were no significant effects of RSDS on core body temperature (Fig. S4E). Furthermore, RSDS increased corticosterone levels to the same extent in both susceptible and resilient animals (Fig. S4 F and G). These data suggest that both groups received similar levels of trauma-related stress and further dissociate general hypothalamic pituitary adrenal stress responses from stress-induced levels of IL-6.
To determine whether immune differences in stress-naive mice can predict development of susceptible versus resilient responses to a subsequent RSDS, we conducted an ex vivo leukocyte immune challenge (Fig. S2B). Whole blood was collected by submandibular bleed 4 d before the first physical interaction with an aggressor. Leukocytes were isolated and counted. Mice that later exhibited a susceptible phenotype following RSDS had more circulating leukocytes than those that exhibited a resilient phenotype (Fig. 2A and Fig. S3E) before the stress exposure. The number of leukocytes negatively correlated with the social interaction ratio (Fig. 2B). To determine the specific type of leukocyte that predicts susceptibility, we collected whole blood before RSDS and separated leukocytes via flow cytometry in a separate cohort of mice. We found that before stress exposure, monocytes were elevated in animals that developed a susceptible phenotype after stress exposure (Fig. S5A) and negatively correlated with the social interaction ratio (Fig. S5 B and C). Although there were no baseline differences in B cells (Fig. S5D), there was a modest trend for increased T cells in mice that later developed a susceptible phenotype (Fig. S5E). To measure IL-6 release to an ex vivo immune challenge, leukocytes were collected before RSDS, plated at equal confluence, and stimulated with the toll-like receptor 4 agonist, lipopolysaccharide (LPS), for 24 h. Leukocytes from animals that later exhibited a susceptible behavioral phenotype released more IL-6 in response to LPS before stress than those that exhibited a resilient phenotype (Fig. 2C). Stimulated IL-6 release also negatively correlated with the social interaction ratio (Fig. 2D). These studies confirm preexisting individual differences in leukocyte numbers as well as leukocyte response to an immune challenge before RSDS, suggesting that dysregulated immune physiology and concomitant increased IL-6 release are a risk factor for stress susceptibility.
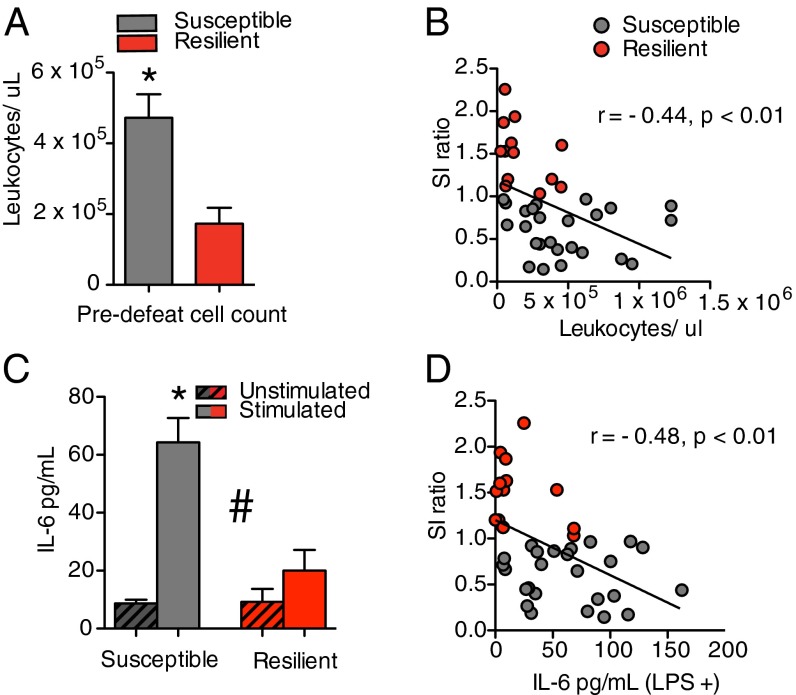
Fig. 2.
Leukocyte profiles before stress. (A) Before RSDS, mice that displayed a susceptible phenotype at the end of RSDS had more circulating leukocytes than mice that displayed a resilient phenotype (t36 = 3.03, P < 0.01, two tailed; susceptible, n = 25, resilient, n = 13). (B) Prestress circulating leukocytes before social defeat stress negatively correlated with the social interaction ratio following RSDS (r = –0.44, P < 0.01). (C) Before RSDS, leukocytes from susceptible animals released more IL-6 than from resilient animals when stimulated with LPS (F1,36 = 15, P < 0.001; susceptible, n = 25, resilient, n = 13). (D) Prestress levels of IL-6 released by leukocytes in response to LPS negatively correlated with the social interaction ratio following RSDS (r = –0.48, P < 0.01). Bar graphs display mean ± SEM. # denotes a significant interaction. * denotes a significant difference in phenotype. Circles denote individual animals. For t tests, * denotes a significant difference between means.
Peripheral Immune System Controls Behavioral Susceptibility.
To examine the functional contribution of increased immune activation to stress, animals were reconstituted with bone marrow (BM) hematopoietic progenitors isolated from susceptible or control mice (Fig. S2C). To exclusively target peripheral immune cells and spare central nervous system (CNS) microglia during whole body irradiation of CD45.2+ C57BL/6 mice, we used lead shields to cover the animals’ heads (Fig. S6A). For the susceptible donors, CD45.1+ C57BL/6 mice were selected based on high release properties of IL-6 in response to an ex vivo leukocyte challenge with LPS and the development of social avoidance following RSDS (Fig. S7A). Control donors did not undergo RSDS and showed low IL-6 release properties to LPS ex vivo. BM hematopoietic progenitor cells from susceptible or control mice were transplanted into the irradiated CD45.2+ C57BL/6 recipients, and animals were given 5–6 wk to allow for full donor hematopoietic cell reconstitution before exposure to social stress. Blood was collected at the end of each experiment to determine donor chimerism. Approximately 70% of the viable leukocytes derived from donor progenitor cells, although proportions of donor-derived leukocytes did not differ between susceptible and control donors (Fig. 3A and Fig. S7B). After transplant, stress-susceptible BM chimeras overall had more circulating leukocytes compared with wild-type control chimeras (Fig. 3B). Examination of brain tissue indicated that proliferation of cells in neurogenic brain regions known to regulate antidepressant responses was not affected by irradiation (Fig. S6 B and C). Further, >98% of the microglia remained of host origin, suggesting that their resident microglia population was left largely intact (Fig. 3C and Fig. S7C). This is in line with previous studies using lead shielding to protect the CNS from radiation (23).
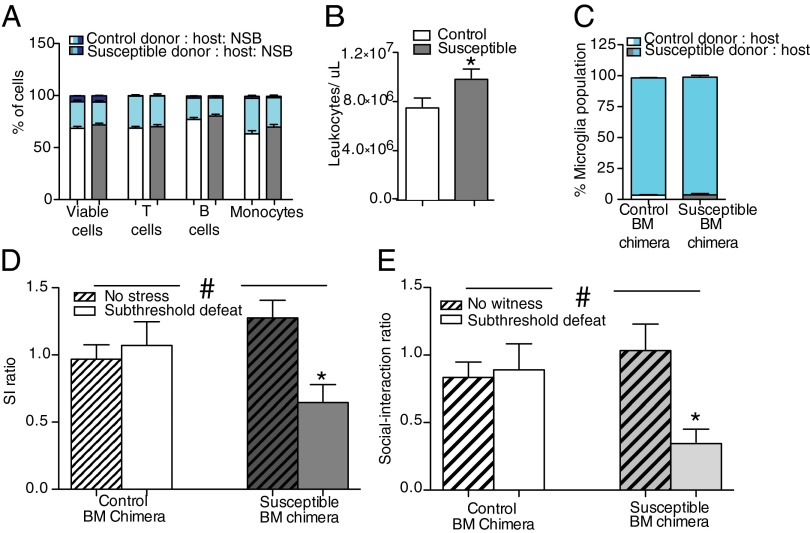
Fig. 3.
BM hematopoietic progenitor cell transplant from a susceptible donor induces depression-like behavior in naïve host animals. (A) A greater percentage of cells came from the donor versus host in BM chimeras across cell types [χ2 (14, n = 58) = 24.11, P < 0.05, two tailed]. The phenotype of the donor did not alter the distribution of leukocytes in either condition [viable cells, χ2 (2, n = 58) = 0.5, P > 0.05, two tailed; T cells, χ2 (2, n = 58) = 1.08, P > 0.05, two tailed; B cells, χ2 (2, n = 29) = 0.04, P > 0.05, two tailed; monocytes, χ2 (2, n = 58) = 1.28, P > 0.05, two tailed]. (B) In a subset of animals tested, naïve host animals that received BM transplants from susceptible donors (n = 14) had higher levels of circulating leukocytes 28 d after transplant and before stress than host mice that received BM grafts from control donors (n = 15) (t25 = 2.34, P < 0.05, two tailed). (C) Donor phenotype did not alter the percentage of microglia from the host or donor measured in a subset of the animals [χ2 (1, n = 15) = 0.0, P > 0.05, two tailed]. More than 98% of the microglia were of host origin. (D) Stress-susceptible BM chimeras that underwent a subthreshold defeat (n = 7) displayed social avoidance behavior, as indicated by a significant interaction and post hoc analysis, compared with control (no defeat, n = 8; subthreshold defeat, n = 7) and stress-susceptible BM chimeras that did not undergo subthreshold defeat (n = 7), as indicated by a significant interaction (F1,25 = 6.94, P < 0.05). (E) Stress-susceptible BM chimeras that witnessed RSDS (subthreshold witness, n = 7) but not animals with transplants from the same donors that did not witness RSDS (n = 8) or control BM chimeras (no witness, n = 6; subthreshold witness, n = 8) displayed greater social avoidance behavior, as indicated by a significant interaction and post hoc analysis (F1,25 = 4.99, P < 0. 05). Bar graphs display mean ± SEM. * denotes a significant main effect of phenotype. # denotes a significant interaction between donor phenotype and exposure to subthreshold defeat/witness. For t tests, * denotes a significant difference between means.
To determine the behavioral consequences of peripheral immune transplantation, stress-susceptible and control BM chimeric mice were subjected to a physical subthreshold defeat that does not induce social avoidance in controls but can reveal a susceptible phenotype if an animal’s stress threshold is shifted by the experimental manipulation (24). Stress-susceptible BM chimeras displayed increased social avoidance following subthreshold defeat compared with controls (Fig. 3D). There were no differences in measures of anxiety-associated exploratory behavior between the two groups as indicated by the elevated plus maze (Table S2). To determine whether the peripheral immune system impacts the behavioral response to a purely emotional stressor (with no physical component), we generated a separate group of susceptible BM chimeric mice and allowed them to witness another mouse undergoing RSDS using a modified subthreshold protocol. Susceptible BM chimeras showed a greater social avoidance behavior compared with control BM chimeras following this subthreshold witness stress (Fig. 3E).
Inhibition of Peripheral IL-6 Promotes Behavioral Resilience.
To determine whether alterations in peripheral IL-6 contribute to individual differences in susceptibility to RSDS, we generated IL-6−/− BM chimeras as described (Fig. S2D). Once again we confirmed ∼70% of viable donor leukocyte chimerism and found no differences in proportions of donor-derived leukocytes between IL-6−/− and WT BM chimeras (Fig. 4A and Fig. S7D). This ruled out nonspecific effects of IL-6 deletion on stem cell engraftment and differentiation into mature leukocytes. The WT control (CD45.2+ C57BL/6) was chosen based on high IL-6 release ex vivo following leukocyte stimulation with LPS, whereas IL-6 levels were undetectable in IL-6−/− mice (Fig. S7E). IL-6−/− BM chimeras and IL-6−/− mice along with WT controls for each were exposed to 10 d of RSDS. IL-6−/− BM chimeras and IL-6−/− mice showed similar levels of resilience measured by the increased social interaction ratio (Fig. 4B), suggesting that leukocyte-derived IL-6 is critical in the development of social avoidance. Neither constitutive IL-6 deletion nor hematopoietic progenitor cell-specific IL-6 deletion affected anxiety-associated exploratory behaviors in the elevated plus maze (Table S2). To determine whether IL-6 from leukocytes impacted the behavioral response to a purely emotional stressor (with no physical component), we exposed IL-6−/− and WT BM chimeric mice to the witness model described previously (15). BM chimeras from high–IL-6 releasing controls displayed robust social avoidance behavior, whereas IL-6−/− BM chimeras demonstrated resilient behavior (Fig. 4C). Lastly, we injected neutralizing IL-6 monoclonal antibodies (mAbs), IgG isotype control antibody (IgG mAb), or saline systemically, 5 min before RSDS (Fig. S2E). IL-6 mAb is too large to enter the brain, but rather acts by sequestering and neutralizing IL-6 in the periphery (Fig. 4D). Consistent with the genetic deletion models, pharmacological blockade with IL-6 mAb, but not IgG mAb or saline alone, prevented the development of social avoidance (Fig. 4E). Treatment with IL-6 mAb did not reduce anxiety-associated exploratory behavior in the elevated plus maze compared with IgG mAb (Table S2).
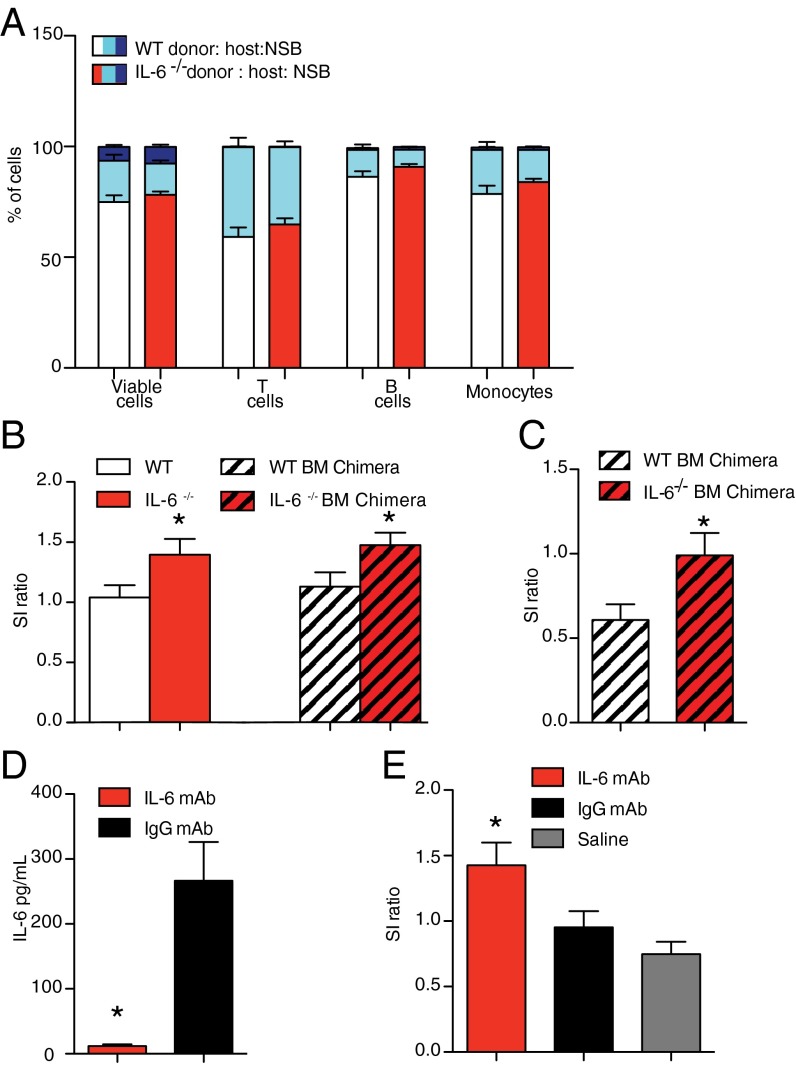
Fig. 4.
Peripheral IL-6 controls susceptibility versus resilience to social defeat. (A) Percent of donor versus host leukocyte concentration in blood of BM chimeras [χ2(14, n = 32) = 70.76, P < 0.001, two tailed]. IL-6−/− BM chimeras had similar distribution of leukocytes in blood compared with WT BM chimeras [viable cells, χ2(2, n = 32) = 0.63, P > 0.05, two tailed; T cells, χ2(2, n = 32) = 1.02, P > 0.05, two tailed; B cells, χ2(2, n = 32) = 1.47, P > 0.05; monocytes χ2 (2, n = 32) = 1.18, P > 0.05]. (B) Constitutive IL-6−/− (IL-6−/−, n = 14; WT, n = 15) or IL-6−/− BM chimeras (IL-6−/−, n = 9; WT, n = 8) are resilient following 10 d of RSDS, as indicated by a significant main effect of IL-6 deletion (F1,43 = 9.18, P < 0.01). (C) IL-6−/− BM chimeras (n = 8) are more resilient to witness defeat (t12 = 2.21, P < 0.05, two tailed) compared with control BM chimera mice (n = 6). (D) An i.p. injection of IL-6 (n = 5), but not IgG (n = 4) mAb, blocked an increase in circulating IL-6 within 20 min of the first defeat (t7 = 4.85, P < 0.01, two tailed). (E) Daily systemic injection of IL-6 (n = 22) but not IgG (n = 23) mAb or saline (n = 28) blocked the development of social avoidance behavior (F3,72 = 7.14, P < 0.01). Bar graphs display mean ± SEM. * denotes a significant main effect of IL-6 deletion. For t test, * denotes a significant difference between means.
Discussion
Here, we demonstrate the validity of a translational social stress animal model that recapitulates aspects of immune dysregulation observed in clinically depressed patients. Individual differences in the sensitivity of the peripheral immune system to social stress are preexisting and confer a greater risk of developing a stress-related disorder. Mice prone to developing a stress-susceptible phenotype had higher prestress levels of circulating leukocytes, largely due to monocyte populations, and these cells produced more IL-6 in response to acute stress and when stimulated ex vivo with LPS. Both in vivo and ex vivo IL-6 levels in response to stimulation were the strongest predictor of the behavioral response to a subsequent social stress. Although previous studies have identified elevated serum IL-6 and circulating leukocytes in stress disorders, this generally followed either depression diagnosis in humans (4, 25) or controlled stress exposure in rodents (26, 27). To our knowledge, this is the first study to show that the IL-6 response before social stress exposure can predict individual differences in vulnerability to a subsequent social stressor.
It is of particular interest that these individual differences in the sensitivity of the peripheral immune system occur within an inbred, genetically similar strain. As genetic differences are likely not driving these alterations, the possibility exists that they are due to epigenetic/environmental factors. Recent work has indicated that there is paternal transmission of stress sensitivity (28). Offspring of fathers that underwent RSDS display increased depression- and anxiety-related behavioral responses to stress (28). Additionally, stability of social hierarchy within the home cage has been demonstrated to induce anxiety-associated behaviors and alter monocyte trafficking to the brain (29). Differences in the social hierarchy within each cage may well affect susceptibility versus resilience to social stress. It is also important to note that our findings dissociate sickness behavior or general malaise from depression-like behavior. Despite large increases in IL-6 in susceptible mice, they do not demonstrate changes in core body temperature that can transiently affect motivated behavior (30). Our work also dissociates general trauma in the RSDS model from development of depression-like behavioral responses. Together, these results point to a peripheral source of IL-6 from leukocytes that is hyperresponsive to stress and serves as a risk factor for development of depression-like phenotypes in vulnerable individuals.
Although studies examining the functional contribution of microglia-dependent inflammatory signaling in the CNS controlling mood-related symptoms are well documented (31–33), an outstanding question in the field has been whether individual differences in peripheral immune responses described above are functionally related to stress-induced depression- or anxiety-like behavior. Transplantation of BM-derived hematopoietic progenitor cells from susceptible donors promoted susceptibility, whereas transplantation of IL-6–deficient BM-derived hematopoietic progenitors promoted resilience to both physical and emotional social stressors in host mice. The degree of resilience in IL-6−/− BM chimeras was similar to that observed in full body knockouts, providing strong evidence that peripheral IL-6 is critical in regulating stress-related behaviors. This evidence builds upon a previously study that demonstrated an antidepressant-like response to acute stress in constitutive IL-6 knockouts and suggests that leukocyte-derived sources of IL-6 may be critical in mediating these behavioral effects (34). Our results are interesting in light of recent studies showing that social defeat stress induces downstream inflammatory signaling pathways, such as NFkB (26, 35–37), and increases monocyte trafficking (29) throughout a number of CNS structures known to control depression and anxiety behavior. It will be important for future studies to define the brain regions and molecular pathways induced by stress-responsive IL-6 from leukocytes that mediate adverse behavioral responses to chronic stress.
Lastly, we show that sequestration and elimination of peripheral IL-6 using mAbs mitigates the maladaptive behavioral coping strategies adopted by susceptible mice. It is possible that more direct therapeutic interventions targeting IL-6 in treatment-resistant patients will be effective in ameliorating symptoms of depression. Indeed, the literature describing the effects of standard antidepressants on systemic inflammation is highly mixed. Although some studies have demonstrated that antidepressants decrease inflammatory cytokines (38, 39), others report either no effect (31, 40, 41) or even increased levels (42, 43). This discrepancy is likely due to a number of factors, including the class of antidepressant used for treatment or the heterogeneity of the patient population (44). In our studies, we only included treatment-resistant patients that typically exhibit greater proinflammatory profiles (45), which may contribute to decreased treatment efficacy and increased relapse. It will be interesting to test more direct anti–IL-6 therapeutics in these patient populations. Similar strategies to block TNF-α with infliximab have shown promise in MDD patients with heightened inflammatory load (45). Although the direct clinical application of our findings remains unknown, prophylactic therapeutic strategies to inhibit IL-6 may reduce stress-related relapse events for MDD patients in remission.
Materials and Methods
Animals.
All subjects were male. CD45.1+/CD 45.2+ C57BL/6 mice were used for RSDS and witness studies. BM transplant hosts (CD45.1+/CD45.2+ C57BL/6) were obtained at 3 wk. IL-6−/− mice (B6.129S2-Il6tm1kopf/J) on a CD45.2+ C57BL/6 background were bred at the Icahn School of Medicine at Mount Sinai from stock obtained from Jackson Laboratory. CD-1 mice (Charles River Laboratories) used as aggressors were sexually experienced retired breeders at least 4 mo of age. Aggressors were singly housed at all times other than during the social defeats. All other animals were group housed before social defeat and single housed following social defeat.
RSDS/Subthreshold Defeat.
RSDS and subthreshold defeat were performed as previously described, and animals were tested for social interaction (13, 19).
Witness Defeat.
Witness defeat was performed as previously described (15). Subthreshold witness defeat used a 14-d incubation period.
IL-6 mAb.
Mice were given daily i.p. injections of mouse anti–IL-6 mAb (R&D Systems, Clone MP5-20F3), rat IgG1 isotype control (R&D Systems, Clone 43414), or saline vehicle. Antibodies were given at a dose of 4 µg per mouse per day in 0.2 mL of saline vehicle. Antibodies/saline were injected 5 min prior to RSDS. The last injection was 24 h before SI.
Human Participants (Cohort 1).
Following informed consent, male and female healthy volunteers or clinical patients underwent a medical and psychiatric evaluation and were required to be medically healthy to participate in the study. Clinical patients were diagnosed with MDD based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and a diagnostic interview with a study clinician. Depression severity at the time of sample collection was determined using QIDS–Self-Report. For complete demographics, see Table S1.
Cytokine Measurement.
Circulating levels of cytokines following the first defeat were measured using Multiplex ELISA (Luminex). Human IL-6 levels (cohort 1) and mouse basal IL-6 levels were measured using a high-sensitivity ELISA (eBioscience). IL-6 levels from subjects used in the time course, leukocyte stimulation, and witness study were detected with a solid phase sandwich IL-6 ELISA (BD Biosciences). Corticosterone was measured using a solid phase sandwich ELISA (Immunodiagnostic Systems).
Leukocyte Isolations.
Leukocytes were isolated from 200 μL of whole blood using Ficoll-Paque Plus (GE Healthcare). A sample of cells was stained with trypan blue (Sigma) to assess viability, counted with a hemocytometer, and samples were plated at 1 × 106 per well in 1 mL of media or media + 34 μg/mL LPS for 24 h.
Generation of BM Chimeras.
Generation of BM chimeras was performed as previously described (46). Animals were given an injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and their heads were placed in lead shields (Nuclead) to protect the brain. BM hematopoietic progenitor cells from a donor mouse were introduced through a retro-orbital injection. The degree of repopulation by donor was determined by flow cytometry.
Flow Cytometry and Cell Cycle Analysis.
Flow cytometry studies were performed using a Fortessa and LSRII (Becton Dickinson) and subsequently analyzed using FlowJo software (Tree Star).
Statistical Analysis.
Differences between two groups were compared using t tests. Comparisons of three or more groups were analyzed by univariate ANOVAs when appropriate, with Newman–Keuls used for post hoc analysis. Comparisons of multiple factors or repeated measures were analyzed using bivariate ANOVAs with a Bonferroni post hoc test. Percentages of leukocyte populations were analyzed using χ2 tests. All statistical analyses were performed using Graph Pad Prism 5.0 software (Graphpad Software Inc.). Statistical significance was set at P < 0.05.
Additional detailed information about procedures used in these studies is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Cristina Costantino for her guidance in separating leukocytes. Additionally they thank Eric J. Nestler and Ming-Hu Han for helpful discussions about this project. This research was supported by US National Institute of Mental Health Grants RO1 MH090264 (to S.J.R.) and RO1 MH104559 (to S.J.R. and M.M.), the Johnson and Johnson International Mental Health Research Organization Rising Star Award (to S.J.R.), Irma T. Hirschl/Monique Weill-Caulier Trust Research Award (to S.J.R.), a grant from Janssen Pharmaceuticals (to S.J.R.), the Brain and Behavior Research Foundation (G.E.H.), and US National Institutes of Health Grants T32 MH087004 (to M.L.P. and M.H.) and T32MH096678 (to M.L.P.), as well as a generous gift from Ms. Julia Jackson.
Footnotes
Conflict of interest statement: This work was supported by a research grant from Janssen Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415191111/-/DCSupplemental.
References
- 1.Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol Lett. 2011;32(1):7–24. [PubMed] [Google Scholar]
- 2.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, et al. Leukocytosis, monocytosis and neutrophilia: Hallmarks of severe depression. J Psychiatr Res. 1992;26(2):125–134. doi: 10.1016/0022-3956(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 5.Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive symptoms enhance stress-induced inflammatory responses. Brain Behav Immun. 2013;31:172–176. doi: 10.1016/j.bbi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole SW, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clara I, et al. The Manitoba IBD Index: Evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009;104(7):1754–1763. doi: 10.1038/ajg.2009.197. [DOI] [PubMed] [Google Scholar]
- 8.Baune BT, et al. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: The prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37(9):1521–1530. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Newton TL, Fernandez-Botran R, Miller JJ, Burns VE. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: Associations with lifetime diagnostic status and psychological context. Biol Psychol. 2014;99:150–159. doi: 10.1016/j.biopsycho.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DG, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9(4):209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- 11.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21(6):530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 15.Warren BL, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73(1):7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert P, McEwan K, Bellew R, Mills A, Gale C. The dark side of competition: How competitive behaviour and striving to avoid inferiority are linked to depression, anxiety, stress and self-harm. Psychol Psychother. 2009;82(Pt 2):123–136. doi: 10.1348/147608308X379806. [DOI] [PubMed] [Google Scholar]
- 17.Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73(3):435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 18.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeMay LG, Vander AJ, Kluger MJ. Role of interleukin 6 in fever in rats. Am J Physiol. 1990;258(3 Pt 2):R798–R803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- 22.Yee JR, Prendergast BJ. Endotoxin elicits ambivalent social behaviors. Psychoneuroendocrinology. 2012;37(7):1101–1105. doi: 10.1016/j.psyneuen.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 24.Golden SA, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19(3):337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 26.Monje FJ, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-κB signaling pathway. J Neurosci. 2011;31(25):9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godbout JP, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33(10):2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz DM, et al. Paternal transmission of stress-induced pathologies. Biol Psychiatry. 2011;70(5):408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plata-Salamán CR, Peloso E, Satinoff E. Cytokine-induced fever in obese (fa/fa) and lean (Fa/Fa) Zucker rats. Am J Physiol. 1998;275(4 Pt 2):R1353–R1357. doi: 10.1152/ajpregu.1998.275.4.R1353. [DOI] [PubMed] [Google Scholar]
- 31.Sukoff Rizzo SJ, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatr. 2012;2:e199. doi: 10.1038/tp.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chourbaji S, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23(3):587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Christoffel DJ, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31(1):314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christoffel DJ, et al. Effects of inhibitor of κB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37(12):2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Słuzewska A, et al. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- 39.Mutlu O, et al. Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci. 2012;91(25-26):1252–1262. doi: 10.1016/j.lfs.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Jazayeri S, et al. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178(1):112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Maes M, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34(4):301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 42.Kubera M, et al. Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol. 2004;4(2):185–192. doi: 10.1016/j.intimp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Munzer A, et al. Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins (Basel) 2013;5(11):2227–2240. doi: 10.3390/toxins5112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogelzangs N, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatr. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raison CL, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogunovic M, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203(12):2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.