Significance
Magnetotactic bacteria produce morphologically precise magnetite nanoparticles within organelles termed “magnetosomes.” Biomineralization proteins tightly regulate crystallization of these nanoparticles. A master protein regulator of particle morphology in vivo, magnetosome membrane specific F (MmsF), has recently been discovered. In this study, we purified MmsF and two homologous proteins from Magnetospirillum magneticum strain AMB-1. MmsF imposes strict control over the formation of magnetite nanoparticles when added to chemical precipitation reactions, whereas the highly similar homologues produce alternative iron oxides with less desirable magnetic properties. Remarkably, these intrinsic membrane proteins with three membrane-spanning regions are water-soluble and self-assemble in vitro into nanoscale “proteinosomes.” We speculate that self-assembly exists in vivo and might be required for the activity of the protein.
Keywords: MmsF, proteinosome, magnetite, magnetosome, in vitro precipitation
Abstract
Magnetotactic bacteria synthesize highly uniform intracellular magnetite nanoparticles through the action of several key biomineralization proteins. These proteins are present in a unique lipid-bound organelle (the magnetosome) that functions as a nanosized reactor in which the particle is formed. A master regulator protein of nanoparticle formation, magnetosome membrane specific F (MmsF), was recently discovered. This predicted integral membrane protein is essential for controlling the monodispersity of the nanoparticles in Magnetospirillum magneticum strain AMB-1. Two MmsF homologs sharing over 60% sequence identity, but showing no apparent impact on particle formation, were also identified in the same organism. We have cloned, expressed, and used these three purified proteins as additives in synthetic magnetite precipitation reactions. Remarkably, these predominantly α-helical membrane spanning proteins are unusually highly stable and water-soluble because they self-assemble into spherical aggregates with an average diameter of 36 nm. The MmsF assembly appears to be responsible for a profound level of control over particle size and iron oxide (magnetite) homogeneity in chemical precipitation reactions, consistent with its indicated role in vivo. The assemblies of its two homologous proteins produce imprecise various iron oxide materials, which is a striking difference for proteins that are so similar to MmsF both in sequence and hierarchical structure. These findings show MmsF is a significant, previously undiscovered, protein additive for precision magnetite nanoparticle production. Furthermore, the self-assembly of these proteins into discrete, soluble, and functional “proteinosome” structures could lead to advances in fields ranging from membrane protein production to drug delivery applications.
Magnetic nanoparticles (MNPs) represent an area of intense research due to their diverse and pertinent applications across a range of disciplines and industries. Applications for MNPs include biomedical diagnostics and therapies (1–3), such as MRI contrast reagents, tumor hyperthermia treatments, and magnetically targeted drug delivery, as well as data storage (4) and biotechnology. However, specific magnetic and physical properties of MNPs are critical to the success of each application, with specific size and morphology (with a narrow distribution) being essential considerations. Pure magnetite MNP synthesis under ambient conditions is notoriously difficult to control, with simple precipitations often resulting in a mixture of differently sized and shaped particles with other iron oxide contaminants. This situation can be improved somewhat by using more extreme processes, such as high-temperature incubations or capping surfactants, which favor certain MNP types (5, 6). However, the use of toxic or organic reagents and extreme conditions comes with a high energy and monetary cost, and can limit the biocompatibility of the MNPs for subsequent applications.
Magnetotactic bacteria (MTB) (7) are a diverse, phylogenetically unrelated class of bacteria that have evolved to produce chains of single-domain crystals of magnetite (or greigite in some cases) enveloped within lipid bilayers (8). These specialized organelles are termed “magnetosomes,” and the magnetosomes from different species display particles with unique size and morphological constraints, indicating a strict genetic control over the growth and development of the crystals (9). There are more than 100 genes associated specifically with the magnetosome in a region of the chromosome termed the “magnetosome island” (MAI) of Magnetospirillum magneticum AMB-1 (10), and a small number of the encoded proteins were found to be tightly bound to the magnetite particles in vivo (11). Subsequent studies of these proteins showed that one in particular, the magnetosome membrane specific protein of 6-kDa molecular mass (Mms6) from M. magneticum AMB-1, plays a role in the morphological control of the particle (11, 12). Addition of this purified protein into synthetic magnetite precipitation reactions produces improvement in the homogeneity of the MNPs (13), illustrating the potential to produce superior MNPs using chemical precipitations under ambient conditions with the presence of key biomineralization protein additives.
Recent elegant genetic studies have uncovered a further protein, magnetosome membrane specific F (MmsF), which has been described as the master regulator for magnetite biomineralization in vivo (14). A ΔmmsF mutant displays a phenotype with much smaller, misshapen particles compared with the WT cells (14). The mmsF sequence resides in the same gene cluster as mms6. When the gene cluster (mms6cl, including the mmsF gene) is deleted, a similar but slightly more severely misshapen particle phenotype is observed (14). By reintroducing just the mmsF gene back into the Δmms6cl strain, the crystal morphology is rescued and a near-normal magnetosome is observed (14). These experiments indicate a critical role for MmsF in controlling both the growth and shape of the formed magnetite crystals.
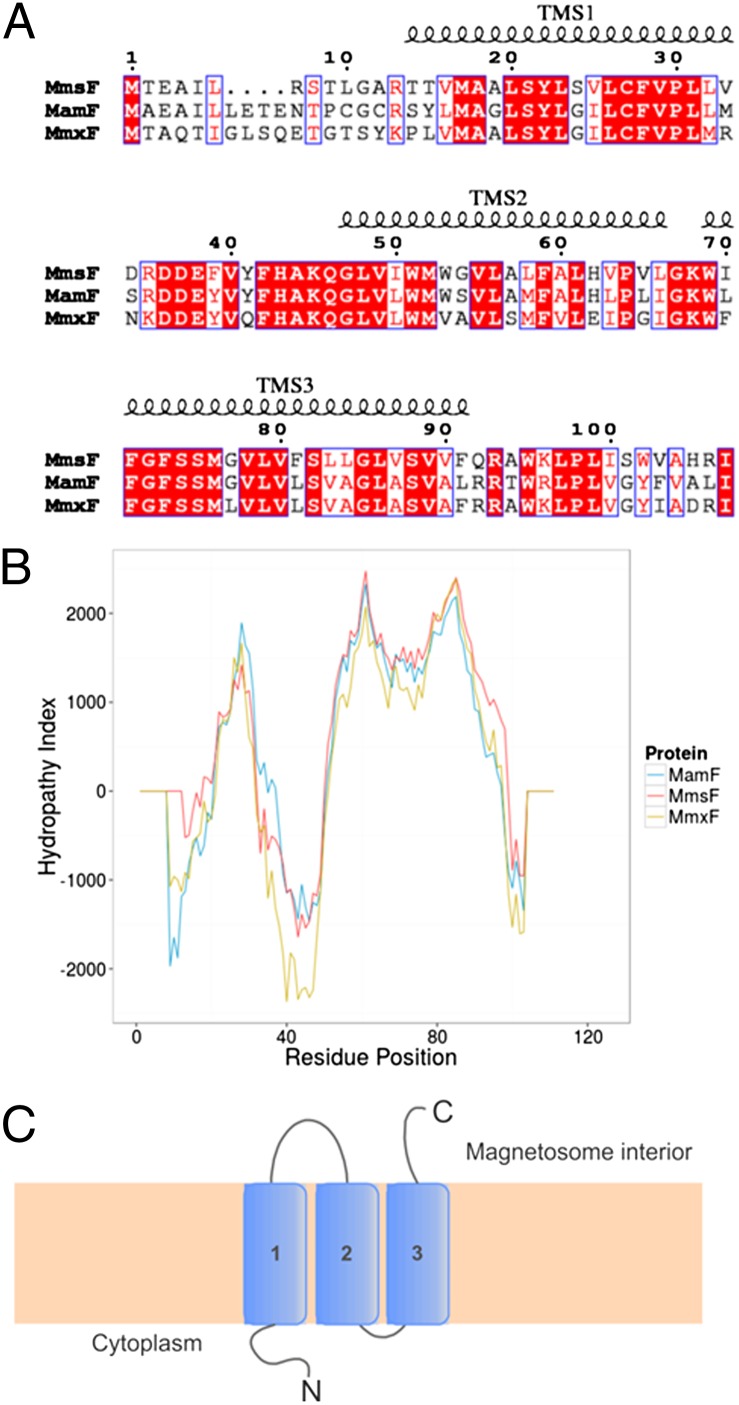
Originally identified in Magnetospirillum magnetotacticum MS-1, MmsF homologs are present in a number of different MTB and show no significant homology to any other known membrane protein family. Analysis of the sequence indicates the presence of three transmembrane spanning (TMS) helices. GFP tagging experiments of MmsF have demonstrated that the C terminus is likely to be located on the inside of the magnetosome membrane (MM), with the N terminus residing in the cytoplasm (14), adding weight to the premise that there are three membrane spanning regions. The N- and C-terminal regions display lower sequence conservation, whereas the TMS regions and, most importantly, the loops residing between them show high levels of conserved residues (Fig. 1). Interestingly, two genes encoding proteins with considerable sequence similarity are found in the MAI of M. magneticum AMB-1. These genes are also found in other species of MTB. In this study, we have isolated MmsF (amb0957) and its two homologs amb0953 and amb1026, henceforth denoted as mamF (magnetosome-associated membrane protein F) and mmxF (MM unknown function F), respectively, to study their effect on MNP synthesis. mamF is in the neighboring gene cluster to mmsF and shares 65% identity, whereas mmxF is located ∼70 genes downstream in the MAI from mmsF and has 66% identity. Significantly, when mmsF is deleted, these genes appear unable to rescue the magnetosome crystallization (assuming these genes are expressed) in vivo (14), suggesting that although they share a high degree of sequence similarity, they might have very different functions.
Fig. 1.
(A) Sequence alignment of MmsF, MamF, and MmxF prepared in ESPript (33). Conserved residues are highlighted in red, similar residue types are depicted with a blue outline, and TMS helices are indicated. (B) Prediction of transmembrane helices using TMPred for the three proteins. MmsF is shown in red, MamF is shown in blue, and MmxF is shown in gold. (C) Topology diagram of the protein spanning the MM.
Here, we report the purification of these three similar proteins, which we show display unusual self-assembly properties, into vesicle-like structures. When included in synthetic magnetite precipitation reactions, the MmsF protein results in highly crystalline, larger, magnetite particles, mimicking the function of the protein in vivo. In contrast, the MamF and MmxF yield a mixture of iron oxides with overall less magnetic material. These results demonstrate a range of previously unidentified biomineralization effects.
Results
MmsF and Its Homologous Proteins Have Three Predicted Transmembrane Helices.
MmsF has been previously identified as an integral membrane protein (14, 15) in M. magneticum AMB-1. We analyzed the primary sequence of MmsF, MamF (65% identity), and MmxF (66% identity) by TMPred and TMHMM (16, 17) transmembrane prediction servers, both of which produce a strongly preferred model with three membrane spanning helices that are thought to be embedded in the MM (Fig. 1). An alignment of the sequences reveals that the majority of the conserved residues occur in the predicted TMS regions, with the greatest variability present at the protein termini and the loop regions between the helices (Fig. 1). A similar arrangement of helical topology and protein conformation between these three proteins is therefore highly likely. The loop connecting the first and second TMS regions has a high proportion of amino acids with acidic side chains, which, like those acidic residues found in the Mms6 protein, are thought to interact with the iron ions and the growing magnetite crystal, and are exposed to the magnetosome interior (Fig. 1).
MmsF and Mms-Like Protein Production.
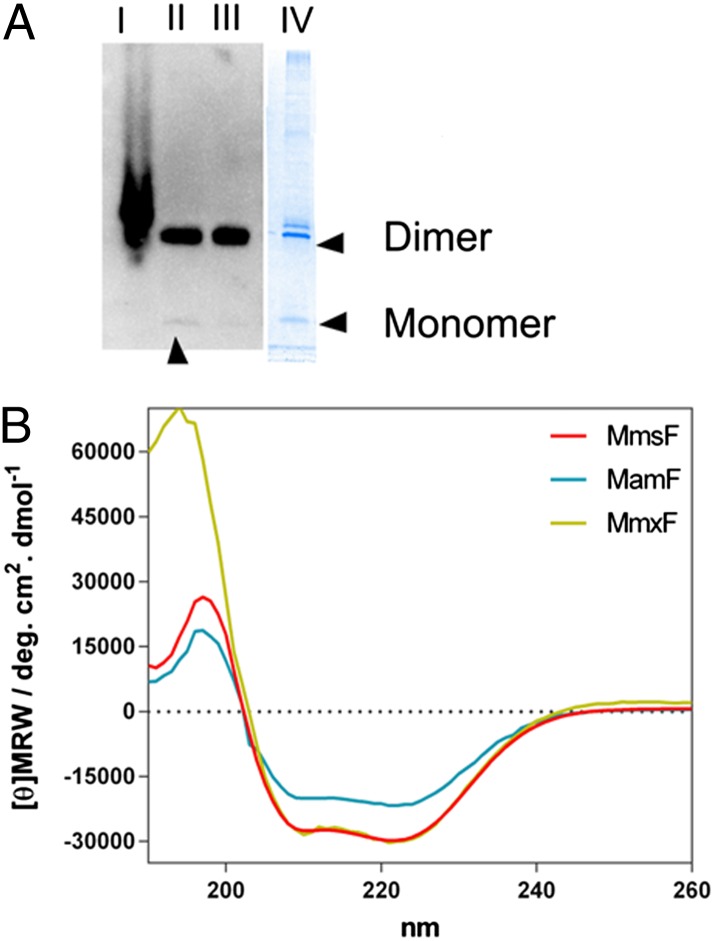
To explore the activity of these proteins in vitro, we successfully cloned the genes into expression vectors to produce StrepII tag fusions (sequence WSHPQFEK) to the N termini of the target proteins in Escherichia coli. We selected StrepII tags to avoid possible effects from polyhistidine tags, which are able to bind metal ions during magnetite formation, and have been shown to slightly alter size of formed nanoparticles in synthetic precipitation reactions (18), potentially masking or subtly altering the effect of the protein on the formed particles. The proteins were expressed in E. coli cells, and the presence of tagged protein in the total and soluble proteomes was determined by Western blot analysis with an antibody against the StrepII sequence, as shown in Fig. 2. The blot revealed that tagged proteins were present in the cell lysate and were all well expressed. Startlingly, the proteins were highly soluble, with almost all of the tagged protein present in the soluble fraction of the cell lysate. This behavior was completely unexpected, considering the extremely hydrophobic nature of the protein sequences. The apparent molecular mass of the intense protein band in all cases was ∼26 kDa, compared with migration of standard markers on SDS/PAGE. This apparent molecular mass is approximately double the theoretical molecular mass of the monomers. Although unusual for globular proteins, it is not unusual for membrane proteins, which often interact in a more variable way with SDS, giving rise to anomalous migration distances (19). It is also possible that the proteins could be forming extremely stable dimers that are resistant to the SDS treatment.
Fig. 2.
(A) Western blot image of MmsF production. Lane I is the total cell lysate, lane II is the soluble lysate after centrifugation, and lane III is the unbound protein after passage through Strep-Tactin Sepharose resin. A Coomassie-stained protein gel image of purified MmsF is shown alongside in lane IV. The assumed dimer and monomer bands are indicated. The faint monomer band on the blot is highlighted by an arrowhead. (B) CD spectra of the three proteins. MmsF is shown in red, MamF is shown in blue, and MmxF is shown in gold. [θ]MRW, mean residue molar ellipticity.
The soluble supernatant was passed through Strep-Tactin Sepharose (IBA) resin to purify the StrepII-tagged protein. The majority of the MmsF and homologous proteins did not appear to interact with the resin (Fig. 2), with only a small fraction of the available material binding to the column and with the monomer band now the dominant species, appearing to show that the monomer is selectively purified. We confirmed that the eluted material was, in fact, the desired protein by analyzing each of the proteins by electrospray ionization MS.
The secondary structure of the proteins was measured by CD (Fig. 2). As expected, based on the prediction of transmembrane helices, the spectrum of each sample gave a characteristic and intense α-helical profile. DichroWeb analysis (20) of the MmsF spectrum using CDSSTR structure fitting gave an estimate of 58% α-helical content (normalized root mean square deviation = 0.002) in agreement with the 52% α-helical content estimated from the TMPred model. MamF has a lower degree of α-helical content, as observed in the CD spectrum, suggesting that a higher proportion of the proteins present might be unstructured. MmxF matches the α-helical content of MmsF precisely. The MmsF protein was also screened for thermal stability by monitoring the A at 222 nm (principle α-helical peak) during temperature ramping to ascertain the unfolding of the protein. The protein lost approximately half of the α-helical content upon heating to 85 °C, with a thermal transition midpoint appearing at 64 °C (Fig. S1). Upon cooling, the protein regained its initial α-helical content, indicating the protein can refold.
MmsF and Homologs Self-Assemble into Water-Soluble Proteinosomes.
We hypothesized from the unusual soluble behavior of the proteins that they are most likely aggregating to shield the hydrophobic regions of the polypeptide chain from the surrounding aqueous environment. The most obvious explanation is that the protein assembles into a structure whereby the hydrophobic regions of a number of the protein subunits pack together, stabilizing one another, with the hydrophilic termini and loop regions exposed to the surrounding solution. It might also explain the lack of interaction with the Strep-Tactin Sepharose resin if the StrepII tag is buried within the structure. To ascertain if the proteins were indeed self-assembling into larger oligomers, we investigated their aggregation by dynamic light scattering (DLS).
From initial DLS analysis, the MmsF protein appeared to be forming discrete species with an average size of 100 (±25) nm in water with a polydispersity of 0.3. The two protein homologs are comparatively larger, with sizes of 130 nm for MmxF and 238 nm for MamF (Fig. S2). This size represented a far larger assembly than we anticipated, bringing it within the size range of transmission electron microscopy (TEM).
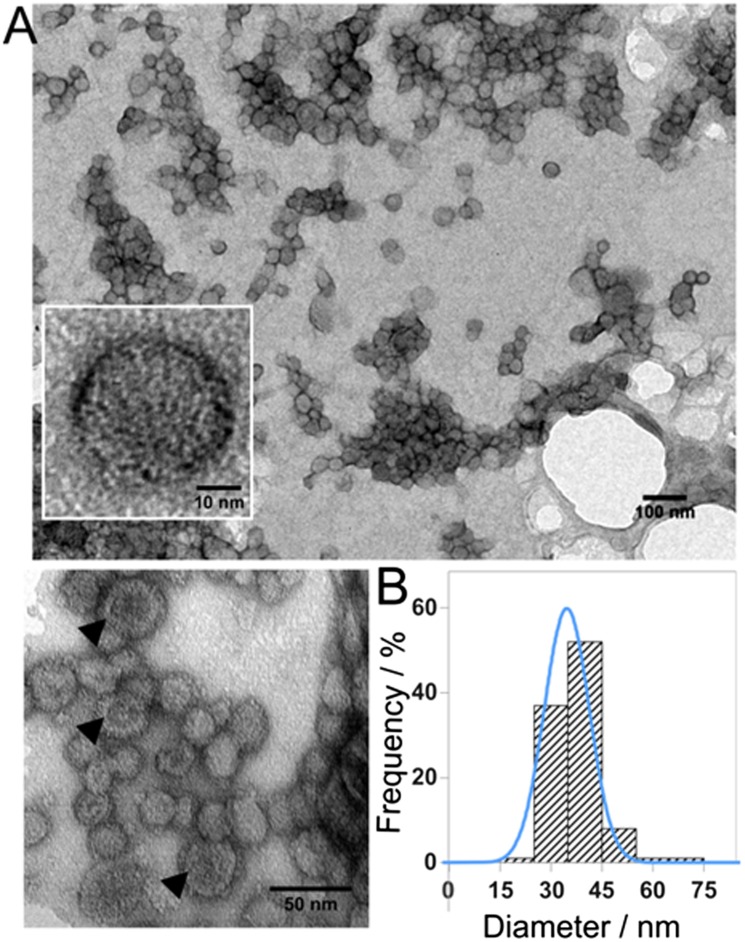
We visualized negatively stained MmsF and its homologs using TEM. The images clearly show discrete, uniform species. The size of each object was measured, with a mean diameter of 36 nm for MmsF and 25 nm for both MamF and MmxF (Fig. 3 and Fig. S3). The overall staining pattern is reminiscent of that typically observed in TEM images of liposomes and polymersomes, where the stain is most intense around the perimeter of each object. Some of the objects showed central pooling of the stain, giving rise to a distinctive donut shape, which we surmise is created by a collapsed hollow structure resulting from sample dry-down for TEM grid preparation (21). To ascertain if this effect was simply an artifact of the TEM preparation, we used cryoelectron microscopy (Fig. S4). Cryoelectron microscopy revealed the presence of the same vesicle-like structures, albeit with less contrast due to the absence of staining. This finding suggests that MmsF is not a solid aggregate of uniform protein density but might actually be a protein shell, termed a “proteinosome.”
Fig. 3.
(A) TEM image of negatively stained MmsF proteinosomes at various magnifications. Black arrowheads highlight donut staining. (B) Size analysis histogram of MmsF proteinosomes with Gaussian fitting overlaid in blue.
We surmised that if these vesicles are assemblies of proteins, then the action of a protease would affect the overall stability of the structure. This hypothesis is shown to be the case, because the vesicle-like structures that we observed in DLS and TEM are sensitive to digestion by proteinase K. After addition of this promiscuous protease, which does not degrade lipids, the vesicle structures underwent a large increase in size with eventual precipitation. These results are likely due to digestion of surface-exposed loops and termini, which alters the water compatibility of the structures. TEM analysis of the proteinase K reaction products (Fig. S5) reveals highly aggregated species with much less structure. We therefore conclude that the integrity of the protein is crucial to the stability of these structures. To confirm the presence or absence of E. coli lipid within the proteinosomes, we analyzed them by means of electrospray ionization MS. No species were detected with the molecular mass that corresponded to lipids typically associated with the E. coli cell membrane (22) (Fig. S6), suggesting that the vesicles are pure protein complexes rather than protein–lipid assemblies.
MmsF Improves Magnetite Nanoparticle Homogeneity in Vitro.
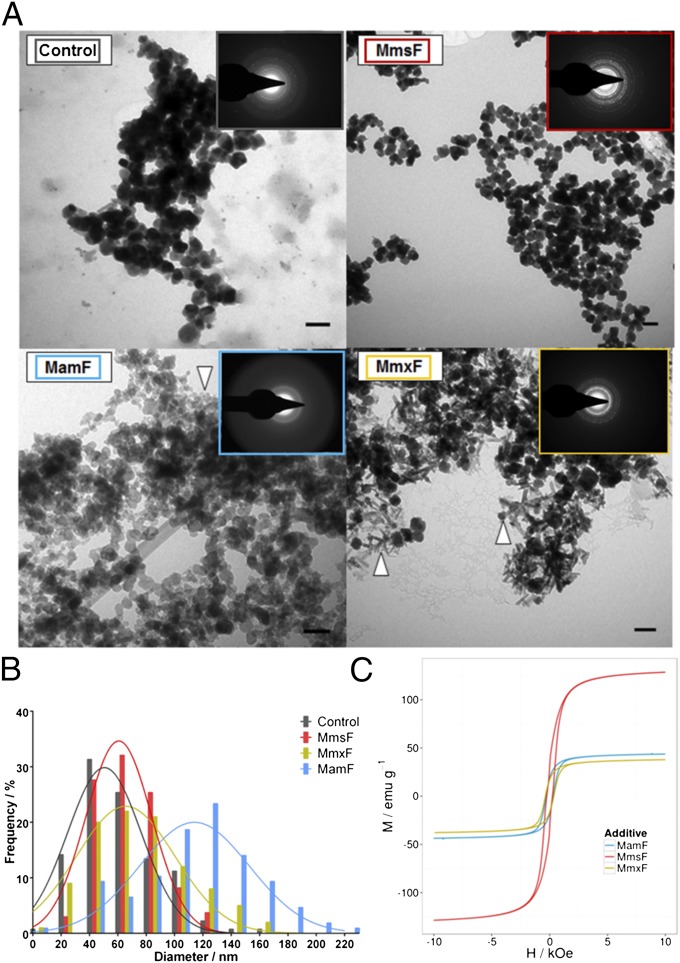
To determine if the isolated proteinosomes were capable of controlling the synthesis of magnetite nanoparticles, we introduced them into room temperature magnetite precipitation reactions. A mixture of ferrous and ferric ions was precipitated by the addition of hydroxide. These reactions typically produce a mixture of iron oxides, but they are predominantly composed of magnetite and have a broad spectrum of different morphologies and sizes, making them particularly sensitive to the effects of additives. In our experiments, the three proteins each had a profound effect upon the resulting nanoparticle products (Fig. 4).
Fig. 4.
(A) TEM images of MNP products in the presence of no protein (control), MmsF, MamF, or MmxF. (Scale bars: 100 nm.) White arrowheads indicate material not observed in the MmsF or control reaction samples. (Insets) Selected area electron diffraction image for each sample is shown. (B) Magnetite nanoparticle size analysis histogram comparing MmsF (red), control products (gray), MmxF products (blue), and MamF products (gold). (C) VSM hysteresis data at 295 K with MmsF shown in red, MamF shown in blue, and MmxF shown in gold.
The addition of MmsF improved the homogeneity of the MNPs. The particles are larger than the control particles and display a more defined morphology, both of which are features consistent with biogenic M. magneticum AMB-1 magnetosome crystals (the particles have a mean length of 56 nm in the longest axis). The addition of the MamF and MmxF results in particles with diverse shapes (needles and plates) that are not present in the control and MmsF samples (Fig. 4), and they also display a much broader size distribution. Particularly in the MmxF particle TEM images, the presence of high numbers of needle-shaped crystals, typical of iron oxyhydroxides, is clear. The overall reaction products with the homologous proteins are brown in color compared with the characteristic black particles of magnetite, again indicating the production of alternative iron oxides (Fig. S7). This finding was confirmed by vibrating sample magnetometry (VSM) measurements. In VSM, the dried MNPs were subjected to an external magnetic field so that the magnetic moment of the material could be measured. The applied field produces a flux in the material, with a maximum flux density (saturation magnetization) being measured at a high applied field, when all of the MNPs are aligned with the externally applied field. By ramping between high positive and negative values of the applied field, the field required to reverse the magnetization and the rate at which this process occurs are established. This effect creates a hysteresis loop (Fig. 4). The MmsF-templated MNPs have a much higher saturation magnetization (129 EMU/g), than those MNPs prepared with either MamF or MmxF (44 EMU/g and 38 EMU/g). Because crystalline magnetite has the highest saturation magnetization of all the iron oxides (23), poor crystallinity of nanoparticles, small particle size, and stoichiometry away from magnetite all reduce the saturation magnetization of iron oxide MNPs. The MNPs produced with the addition of MmsF have the highest saturation magnetization, indicating a high-quality magnetite was produced in the presence of MmsF compared with the iron oxide minerals formed with MamF and MmxF.
Selected area electron diffraction was used to compare the crystallinity and iron oxide composition of the samples. Particles produced with MmsF gave the most intense diffraction pattern, which matched the principal magnetite peaks present within the control particles. MamF gave a diffuse pattern with apparently little magnetite material, consistent with the amorphous particles observed in TEM. MmxF did give clear diffraction rings but yielded some peaks that corresponded to magnetite, maghemite, and iron hydroxides (Fig. 4 and Fig. S8).
During these studies, we have observed that these membrane proteins form remarkably stable and soluble self-assembled structures. MmsF and its homologs have differing effects on the production of magnetite nanoparticles made in simple coprecipitation reactions. We have observed that MmsF mediates the formation of magnetite nanoparticles of increased size and with an absence of alternative iron oxide/oxyhydroxide species, whereas MamF and MmxF are responsible for the production of mixtures of magnetite and iron oxyhydroxides, hindering the production of magnetite even compared with no protein controls.
Discussion
The proteins present within MTB represent a rich source of additives for inclusion in synthetic MNP synthesis to aid in the development of precision nanoparticles for a variety of industries. We set out to analyze the effect of MmsF and its two homologs located within the MAI of M. magneticum AMB-1 on magnetite nanoparticle formation in vitro. We discovered that the MmsF protein and the homologs were overexpressed in a highly soluble form in E. coli, despite being predicted to have three TMS helices. This finding is a very unusual result, with only a limited number of membrane proteins capable of even existing in a stable form in aqueous environments without the addition of detergent or lipid (24). Magnetosome-associated proteins that have been previously characterized, such as Mms6 and, recently, Mms13 (MamC), are also predicted to span the MM. These proteins are expressed in E. coli as insoluble inclusion bodies but, interestingly, can be denatured and refolded into soluble aggregated structures, displaying iron binding capabilities (13, 25, 26). Coupled with our data, this finding points to a pattern of soluble behavior between various magnetosome-associated membrane proteins. Our work on MmsF and its homologous proteins presented here is unique among reported isolations of other MM proteins studied so far in that the protein expresses in the cytoplasmic cell fraction in E. coli rather than in inclusion bodies. The precise self-assembly into folded, well-defined, vesicle-like structures is, in our opinion, extremely unlikely to be a serendipitous form of the protein but might be an alternative and significant native conformation to the expected membrane-bound form. We have searched the available literature for references to similar membrane protein assemblies, and we have not been able to find any previously recorded discoveries of this particular type. We did find a designed synthetic protein/polymer assembly reported by Huang et al. (27) that produced ordered vesicle structures, which they termed “proteinosomes,” to describe protein–polymer assembled compartments. We believe this term can also be applied to the MmsF, MamF, and MmxF structures.
MmsF demonstrated a stark improvement in particle homogeneity, with a larger size, uniform mineral type, and more consistent morphology, with high magnetization completely in keeping with the properties of biogenic particles produced in M. magneticum AMB-1. In complete contrast, the addition of MamF and MmxF resulted in particles of alternative mixed iron oxides. These results show that despite their similar self-assembly properties and sequence conservation, these proteins have markedly different activities in MNP formation. The purity and size distribution of magnetite indicate that the MmsF proteinosomes are an active form of the protein, with a function consistent with that identified in previous in vivo studies (14). It was noted in those genetic studies that the deletion of mmsF alone produced a smaller and even more misshapen particle morphology phenotype than when it was deleted as part of the wider deletion of the mamCDF and mms6 gene clusters (14). This finding led the authors to the conclusion that an unidentified gene present within those two clusters might actually inhibit the ability of the magnetosomes to produce full-sized, mature particles. Our studies indicate that MamF and MmxF do not have the same function as MmsF, and actually appear to favor the production of alternative iron oxides in vitro. We surmise therefore that a likely candidate for the unidentified gene is amb0953 (mamF).
In the protein sequence of MmsF and its homologs, and particularly in those parts of the sequences that are predicted to form transmembrane helices, we see a number of unexpected residues. There is a preponderance of Gly, Ser, Cys, and Thr residues, which might be significant (Fig. 1). Gly is usually considered to be a helical breaker in globular proteins but is often found in membrane proteins, where it is involved in the close packing of different helices (28). Likewise, hydrophilic amino acids are also not commonly associated with membrane insertion, but there are instances in the literature where residues, such as Ser and Thr, are found at the interface between tightly packed transmembrane helices (29). All of these residue types are found in the helices of MmsF and its homologs, and are strongly conserved between them. We hypothesize from our CD data, DLS, and TEM that the helical sections might pack closely with their neighbors and could potentially form the basis for the self-assembly of the defined proteinosomes we observe.
The loop connecting transmembrane helices 1 and 2 (Fig. 1) is the prime region that is likely to be responsible for controlling magnetite formation, because it is displayed on the magnetosome interior (14) and contains charged residues ideal for binding iron ions from solution, magnetite precursors, or crystal facets. Significantly, this region is a conserved region, with very little variation between MmsF and its homologs. One interesting difference between the proteins is the extra aspartate residue in the MmsF loop, which is replaced with an Asx and Ser in MamF and MmxF, respectively. Acidic residues are considered crucial to the function of the biomineralization protein Mms6, and might therefore play a role in the function of MmsF. Mms6 has an acidic-rich cluster with the sequence “acid-acid-acid-X-acid.” MmsF is the only one of the three proteins to have the same motif (albeit running in the opposite direction). The only other differences between these loops are Phe to Tyr and Tyr to Gln substitutions (Fig. 1). Compared with the absence of the highly charged aspartate side chain, we do not consider these substitutions to be significant. Could this one acidic amino acid subtle difference between MmsF and its homologs be responsible for such dramatic effects in nanomagnetite crystallization? It is also important to consider that the C-terminal region of these proteins is thought to be displayed on the magnetosome interior if the membrane topology predictions are correct. These regions display much more variation in sequence, and could therefore affect the function of these proteins. With such a relatively small number of differences between the proteins, the exact sites could be accessed through mutagenesis, offering a clear model for beginning to understand the exact nature of how proteins can control the magnetite biomineralization process.
Proteomic analysis of Magnetospirillum gryphiswaldense (30) shows that MamF is an abundant protein in the MM fraction. It was also noted in that study that MamF produced large, SDS-resistant, oligomeric species in SDS/PAGE experiments (30), which hinted that these proteins might assemble into aggregated stable species in vitro. Coupled with the work described in this paper and the self-assembly properties of other magnetosome proteins (25, 26), we speculate that MmsF and its homologs might assemble within the MM, forming strong packing interactions between themselves. This assembly may tessellate a tightly packed and ordered island of protein within the lipid membrane that could display the active surface loops and termini discussed above in a precise pattern on the interior face of the magnetosome. The presence of such a protein island with displayed acidic residues might aid crystal nucleation, growth, and maturation. It should be noted that the expression levels in MTB will be under careful genetic regulation as opposed to the massive overproduction we generate in E. coli. The artificially high levels of protein in our experiments could give rise to the continuous packing of the individual subunits into the proteinosome shells we observe.
The ability to express predicted integral membrane proteins into ordered, stable proteinosomes might have far-reaching implications for protein assembly, compartmentalization, and membrane protein research. By understanding the basis for the assembly, it might be possible to mutate other disparate proteins to have similar self-assembly properties, potentially allowing membrane proteins that are difficult to produce to be expressed in a soluble form and studied without the need for detergent. With this understanding, proteinosomes could have implications as far-reaching as novel carriers for drug delivery and other innovative encapsulation strategies.
Methods
Protein Expression and Purification.
The mmsF gene (amb0957) and the two MmsF homologs (amb0953 and amb1026) were amplified from M. magneticum AMB-1 genomic DNA using PCR. The reaction products were then introduced into pPR-IBA2 (IBA) expression vectors that enable production of the encoded protein from a T7 promoter. The resulting proteins were expressed as a fusion with an N-terminal StrepII tag from BL21 RP (DE3) E. coli cells (Stratagene) grown for 40 h at 37 °C with vigorous shaking in Terrific broth autoinduction media with trace elements (Formedium). Cells were harvested by centrifugation, resuspended in PBS at a 20% (wt/vol) ratio, and lysed via sonication. Insoluble debris was removed by further centrifugation using a Fiberlite F15 rotor (Thermo Scientific) at 12,000 rpm for 45 min. Supernatant was passed through a gravity flow column packed with Strep-Tactin Sepharose resin (IBA), followed by washing with PBS. StrepII-tagged protein was eluted by application to the column of 5 mM d-desthiobiotin prepared in PBS. The eluent was collected and dialyzed against ultrapure water using 3.5-kDa molecular mass cutoff membranes (Snakeskin dialysis membrane; Thermo Scientific). The protein was quantified by absorption at 280 nm, aliquoted, and frozen at −80 °C.
CD.
Purified protein samples were diluted with ultrapure water to give a concentration of 0.1 mg/mL based on the A at 280 nm. A Jasco J810 CD instrument was used to acquire spectra using a 2-mm path-length cuvette. Wavelength scans of 260 nm to 190 nm were collected using a 1-nm slit width and 1-s intervals. Each sample was analyzed three times, and data were averaged before subtracting a blank water spectrum. For thermal stability, the A at 222 nm (a marker of helical content) was monitored as the temperature of the sample was ramped from 20–85 °C. Data analysis was performed using DichroWeb (20).
Western Blotting.
Protein samples were analyzed by SDS/PAGE using 4–20% TGX gradient gels (Bio-Rad). The protein was transferred to a 0.1-μm nitrocellulose membrane (Whatman) by semidry transfer using tris-glycine-methanol transfer buffer. The membrane was blocked with 3% (mass/vol) BSA in PBS-T (PBS supplemented with 0.1% Tween) before a 1-h incubation with Strep-Mab classic HRP conjugate (IBA). The membrane was then washed with four changes of PBS-T before detection (Immuno-star HRP kit; Bio-Rad) and visualization.
DLS.
Each of the purified protein samples was analyzed by DLS using a Zeta sizer (Malvern Instruments) to estimate its size and polydispersity. One milliliter of protein at 0.1 mg/mL (as estimated by A at 280 nm) in ultrapure water was measured at room temperature. Immediately before analysis, the samples were subjected to centrifugation at 10,000 × g for 10 min to remove any particulates that might affect the readings.
Proteolysis.
Purified protein (1 mg/mL) was incubated at room temperature with 2 units of Proteinase K (New England Biolabs) in 10 mM Tris (pH 8.0) and 1 mM CaCl2 overnight, and the material was analyzed by negatively stained TEM.
MS.
Protein at 1 mg/mL in water was analyzed by electrospray ionization to detect lipids in the size range of 400–1,700 kDa.
Protein TEM.
Negative stain EM was initially used to visualize purified MmsF protein. Five microliters of the purified protein at a concentration of 1 mg/mL was applied to 400 mesh copper-coated holey carbon grids (Agar Scientific). To enhance contrast, the protein grids were stained for 15 s with 0.75% uranyl formate. Protein was then imaged using a FEI Tecnai G2 Spirit transmission electron microscope. Images were subsequently analyzed using ImageJ (National Institutes of Health) (31). Cryogenic TEM was used to confirm the proteinosome structure visualized in negative stain TEM. Lacey carbon film grids were prepared by placing 3 μL of sample onto 300 mesh. The grids were subsequently blotted for 5 s (at 100% humidity) and plunged into liquid ethane using a Vitrobot Mark IV. Samples were visualized on an FEI Tecnai F20 microscope fitted with a Gatan 4K × 4K CCD camera.
Magnetite Nanoparticle Formation.
Magnetite nanoparticles were synthesized via room temperature coprecipitation (RTCP) of mixed-valence iron salts. During these preparations, a NaOH solution was injected slowly into a solution containing Fe(II) and Fe(III) sulfate. To describe RTCP conditions, we use the nomenclature of Ruby et al. (32), where X denotes the molar ratio of Fe(III) to total Fe and R is the ratio of OH to total Fe. We perform experiments at a value of X = 0.3 and until R = 4 at a rate of 0.03 R/min. A total iron concentration of 20 mM was used for all experiments. The molecular masses of Fe(II) and Fe(III) sulfate were predetermined by inductively coupled plasma mass spectrometry (ICP-MS) to ensure that accurate quantities and ratios of iron were used. Ultrapure (MilliQ) water used for nanoparticle synthesis was sparged with nitrogen for an hour, and precipitations were carried out in oxygen-free conditions. All chemicals were purchased from Sigma–Aldrich.
Protein was added at a concentration of 10 μg per 1 mL of reaction solution, and the volume of the reactions was 10 mL. This value was chosen to be consistent with previous studies of biomineralization in vitro (11, 13).
TEM Analysis of Nanoparticles.
MNPs were first dispersed in water, drop-cast, and dried onto carbon-coated copper TEM grids (Agar Scientific) under a gentle stream of nitrogen. All EM was performed on an FEI Technai G2 Biotwin instrument at 120 kV operating at 80 kV and equipped with a wide-angle Gatan MS600CW camera. At least 10 images distributed around the TEM grid were obtained. Particle size analysis was performed with ImageJ (31) data processing. Selected area electron diffraction of particle clusters was carried out on the same samples and on the same instrument. Peaks were assigned by calibration with the diffraction pattern of gold nanoparticles.
Magnetic Measurements.
MNPs were washed and then dried with N2 and then weighed into a gelatin capsule and sealed to minimize exposure to air. Magnetic measurements were taken using an Oxford Instruments Maglab vibrating sample magnetometer at 295 K using a varying external field of between −2 T and 2 T for hysteresis loops.
Supplementary Material
Acknowledgments
We thank Prof. Stephen Baldwin for useful discussions during the early stages of this project. We also thank Victoria Mico Egea for assistance during preliminary protein work, Simon Thorpe for MS, Stephen Muench for cryo-TEM assistance, and Svet Tsokov for help with electron diffraction. This work was funded by the Biotechnology and Biological Sciences Research Council (BB/H005412/2).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409256111/-/DCSupplemental.
References
- 1.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36(13):R167–R181. [Google Scholar]
- 2.Tartaj P, et al. Biomedical applications of magnetic nanoparticles. In: Buschow KHJ, et al., editors. Encyclopedia of Materials: Science and Technology. Elsevier; Oxford: 2007. pp. 1–7. [Google Scholar]
- 3.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112(11):5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 4.Sun SH. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv Mater. 2006;18(4):393–403. [Google Scholar]
- 5.Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124(28):8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 6.Ho CH, et al. Shape-controlled growth and shape-dependent cation site occupancy of monodisperse Fe3O4 nanoparticles. Chem Mater. 2011;23(7):1753–1760. [Google Scholar]
- 7.Blakemore R. Magnetotactic bacteria. Science. 1975;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill DL, Maratea D, Blakemore RP. Ultrastructure of a magnetotactic spirillum. J Bacteriol. 1980;141(3):1399–1408. doi: 10.1128/jb.141.3.1399-1408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murat D, Quinlan A, Vali H, Komeili A. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci USA. 2010;107(12):5593–5598. doi: 10.1073/pnas.0914439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsunaga T, et al. Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res. 2005;12(3):157–166. doi: 10.1093/dnares/dsi002. [DOI] [PubMed] [Google Scholar]
- 11.Arakaki A, Webb J, Matsunaga T. A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1. J Biol Chem. 2003;278(10):8745–8750. doi: 10.1074/jbc.M211729200. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Mazuyama E, Arakaki A, Matsunaga T. MMS6 protein regulates crystal morphology during nano-sized magnetite biomineralization in vivo. J Biol Chem. 2011;286(8):6386–6392. doi: 10.1074/jbc.M110.183434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amemiya Y, Arakaki A, Staniland SS, Tanaka T, Matsunaga T. Controlled formation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomaterials. 2007;28(35):5381–5389. doi: 10.1016/j.biomaterials.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Murat D, et al. The magnetosome membrane protein, MmsF, is a major regulator of magnetite biomineralization in Magnetospirillum magneticum AMB-1. Mol Microbiol. 2012;85(4):684–699. doi: 10.1111/j.1365-2958.2012.08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nudelman H, Zarivach R. Structure prediction of magnetosome-associated proteins. Front Microbiol. 2014;5:9. doi: 10.3389/fmicb.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6:175–182. [PubMed] [Google Scholar]
- 17.Hofmann K, Stoffel W. TMbase—A database of membrane spanning proteins segments. Biol Chem Hoppe Seyler. 1993;374:166. [Google Scholar]
- 18.Galloway JM, et al. Magnetic bacterial protein Mms6 controls morphology, crystallinity and magnetism of cobalt-doped magnetite nanoparticles in vitro. J Mater Chem. 2011;21(39):15244–15254. [Google Scholar]
- 19.Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA. 2009;106(6):1760–1765. doi: 10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(web server issue):W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhakdi S, Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci USA. 1978;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweetman G, et al. Electrospray ionization mass spectrometric analysis of phospholipids of Escherichia coli. Mol Microbiol. 1996;20(1):233–238. doi: 10.1111/j.1365-2958.1996.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 23.Peters C, Dekkers MJ. Selected room temperature magnetic parameters as a function of mineralogy, concentration and grain size. Phys Chem Earth. 2003;28(16-19):659–667. [Google Scholar]
- 24.Korepanova A, et al. Cloning and expression of multiple integral membrane proteins from Mycobacterium tuberculosis in Escherichia coli. Protein Sci. 2005;14(1):148–158. doi: 10.1110/ps.041022305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashyap S, et al. Visualization of iron-binding micelles in acidic recombinant biomineralization protein, MamC. J Nanomater. 2014;2014:320124. [Google Scholar]
- 26.Wang L, et al. Self-assembly and biphasic iron-binding characteristics of Mms6, a bacterial protein that promotes the formation of superparamagnetic magnetite nanoparticles of uniform size and shape. Biomacromolecules. 2012;13(1):98–105. doi: 10.1021/bm201278u. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, et al. Interfacial assembly of protein-polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat Commun. 2013;4:2239. doi: 10.1038/ncomms3239. [DOI] [PubMed] [Google Scholar]
- 28.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: Role of glycine in transmembrane helix association. Biophys J. 1999;77(3):1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson JP, Weinger JS, Engelman DM. Motifs of serine and threonine can drive association of transmembrane helices. J Mol Biol. 2002;316(3):799–805. doi: 10.1006/jmbi.2001.5353. [DOI] [PubMed] [Google Scholar]
- 30.Grünberg K, et al. Biochemical and proteomic analysis of the magnetosome membrane in Magnetospirillum gryphiswaldense. Appl Environ Microbiol. 2004;70(2):1040–1050. doi: 10.1128/AEM.70.2.1040-1050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruby C, Géhin A, Abdelmoula M, Génin J-MR, Jolivet J-P. Coprecipitation of Fe(II) and Fe(III) cations in sulphated aqueous medium and formation of hydroxysulphate green rust. Solid State Sci. 2003;5(7):1055–1062. [Google Scholar]
- 33.Gouet P, Courcelle E, Stuart DI, Métoz F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15(4):305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.