Significance
Boron neutron capture therapy (BNCT) for cancer is based on the selective uptake of 10B target compounds by tumor cells followed by neutron irradiation. The capture reaction between 10B atoms and neutrons gives rise to short-range particles, which are highly effective in producing cell damage. Thus, BNCT is designed to damage tumor cells and preserve healthy cells. The boron carrier used is pivotal to the success of BNCT. The present study describes the therapeutic success of BNCT mediated by MAC-TAC liposomes, K[nido-7-CH3(CH2)15-7,8-C2B9H11] (MAC) in the bilayer membrane and encapsulating the hydrophilic species Na3[ae-B20H17NH3] (TAC) in the aqueous core, using the hamster cheek pouch oral cancer model. A sustained tumor response of 70–88% was associated with only mild mucositis in dose-limiting precancerous tissue.
Keywords: oncology, cancer, boronated liposomes, BNCT, neutron radiation
Abstract
The application of boron neutron capture therapy (BNCT) mediated by liposomes containing 10B-enriched polyhedral borane and carborane derivatives for the treatment of head and neck cancer in the hamster cheek pouch oral cancer model is presented. These liposomes are composed of an equimolar ratio of cholesterol and 1,2-distearoyl-sn-glycero-3-phosphocholine, incorporating K[nido-7-CH3(CH2)15-7,8-C2B9H11] (MAC) in the bilayer membrane while encapsulating the hydrophilic species Na3[ae-B20H17NH3] (TAC) in the aqueous core. Unilamellar liposomes with a mean diameter of 83 nm were administered i.v. in hamsters. After 48 h, the boron concentration in tumors was 67 ± 16 ppm whereas the precancerous tissue contained 11 ± 6 ppm, and the tumor/normal pouch tissue boron concentration ratio was 10:1. Neutron irradiation giving a 5-Gy dose to precancerous tissue (corresponding to 21 Gy in tumor) resulted in an overall tumor response (OR) of 70% after a 4-wk posttreatment period. In contrast, the beam-only protocol gave an OR rate of only 28%. Once-repeated BNCT treatment with readministration of liposomes at an interval of 4, 6, or 8 wk resulted in OR rates of 70–88%, of which the complete response ranged from 37% to 52%. Because of the good therapeutic outcome, it was possible to extend the follow-up of BNCT treatment groups to 16 wk after the first treatment. No radiotoxicity to normal tissue was observed. A salient advantage of these liposomes was that only mild mucositis was observed in dose-limiting precancerous tissue with a sustained tumor response of 70–88%.
Boron neutron capture therapy (BNCT) is a binary cancer treatment modality that combines irradiation using a thermal or epithermal neutron beam with 10B target species that are taken up preferentially by neoplastic cells. The high linear energy transfer (LET) α-particles and recoiling 7Li nuclei emitted during the neutron capture reaction have a range of 5–9 µm in tissue (approximately the diameter of one cell) and are known to have a high relative biological effectiveness (1). In this way, BNCT will selectively target tumor tissue while sparing normal tissue. In addition, because BNCT involves biochemical rather than geometric targeting, it is ideally suited to treat infiltrating tumor cells, undetectable micrometastases (2), and the foci of malignant transformation in field-cancerized tissue (3). Clinical trials of BNCT for the treatment of glioblastoma multiforme and/or melanoma and, more recently, head and neck tumors and liver metastases, using boronophenylalanine (BPA) or sodium mercaptoundecahydrododecaborane as the 10B carriers, have been performed or are under way in Argentina, Europe, Japan, Taiwan, and the United States (4–8). These treatments have been marginally more effective than external-beam radiation therapy (9–11).
The requirements for successful BNCT are preferential accumulation and retention of a nontoxic 10B carrier in a tumor, a sufficiently high absolute concentration of 10B in tumor tissue (at least 20 ppm) for a sufficient number of 10B(n,α)7Li reactions to occur, and targeting of all tumor cell populations (12–14). Furthermore, the microlocalization of 10B also determines the therapeutic outcome of BNCT (15). To this end, the development of new, more selective, nontoxic, and effective boron delivery agents that can preferentially deliver a high concentration of boron to the tumor tissue with a high tumor-to-blood boron ratio is probably the single greatest need for the future progress of BNCT (16).
Considerable attention has been focused on the liposomal delivery system as a new targeting modality. Liposomes are efficient drug delivery vehicles that are able to selectively deliver large quantities of a wide variety of 10B agents to the tumor tissue. Small liposomes (<100 nm) pass through aberrant tumor vessels (17) and passively accumulate by the enhanced permeability and retention effect (18, 19). Small unilamellar liposomes in particular are viewed as potentially useful boron delivery vehicles for BNCT and have been extensively studied by Hawthorne and coworkers (20–26) and other groups (27–29). They can encapsulate aqueous solutions of sodium salts of polyhedral borane anions and/or incorporate lipophilic boron-containing moieties embedded within the bilayer membrane. Recently, Hawthorne and coworkers demonstrated the therapeutic efficacy of a boron-rich liposomal system, MAC-TAC liposomes, incorporating K[nido-7-CH3(CH2)15-7,8-C2B9H11] (MAC) in the lipid bilayer and a hydrophilic species Na3[ae-B20H17NH3] (TAC) in the aqueous core, for treating BALB/c mice bearing EMT6 mammary adenocarcinomas (26).
Leveraging on the successful liposome mediated BNCT study in mice, the aim of the present study was to assess the therapeutic efficacy and potential radiotoxicity of BNCT mediated by MAC-TAC liposomes in the hamster cheek pouch oral cancer model. Our previous biodistribution studies with this model using MAC-TAC liposomes showed that tumor boron values peaked at 67 ppm after 48 h with a very favorable 10:1 tumor/normal tissue ratio (30). Thus, this protocol was very attractive for radiobiological study.
We previously proposed and validated the use of the hamster cheek pouch model of oral cancer for BNCT studies (31, 32). Although progress has been made in the understanding and treatment of head and neck malignancies, their management continues to pose a challenge. Squamous cell carcinoma of the head and neck region is the sixth most common cause of cancer deaths worldwide. The relatively poor overall 5-y survival rate for malignancies of the oral cavity of 60% (33) and the fact that radical surgery causes large tissue defects poses the need for more effective and less toxic therapies. These should damage malignant cells selectively, sparing normal cells. Oral mucositis limits the radiation dose that can be administered with BNCT to head and neck and brain tumors (6, 7). It is a frequent, dose-limiting side effect during conventional radiotherapy for advanced head and neck tumors. Approximately 80% of such patients are thus affected (34). Despite its incidence and clinical relevance, no effective way to prevent or treat mucositis is currently available (35). In addition, the inflammatory process associated with moderate mucositis in field-cancerized tissue could favor tumor development from this tissue (3, 36). Within this context, BNCT protocols that minimize mucositis are more likely to deliver therapeutically useful doses to a tumor without exceeding normal and precancerous tissue tolerances.
In the hamster cheek pouch tumor model, carcinogenesis protocols lead to the development of what has been called, globally, “precancerous tissue” (31), from which tumors arise. Thus, this mode of tumor induction provides a tumor model surrounded by precancerous tissue. The possibility of studying precancerous tissue in addition to tumor and normal tissue is clinically relevant in terms of its role as a potentially dose-limiting tissue.
Results and Discussion
In our previous tumor control studies using other boron targeting agents, the follow-up period was established as 4 wk after a BNCT treatment. The treatment protocols assayed in the present study made it possible, to our awareness for the first time, to prolong the follow-up period beyond 4 wk posttreatment (after one treatment of BNCT or after the first treatment in the case of two treatments). No normal tissue radiotoxicity was observed with any of the protocols. In general, body weight (bw) values oscillated slightly over the posttreatment period evaluated. It was occasionally necessary to euthanize the animals as a result of decline as a result of one or more of the following factors, all unrelated to potential BNCT-induced toxicity: overgrowth of nonresponsive treated tumors, overgrowth of tumors that develop from precancerous tissue, and general decline caused by hepatic and digestive disorders caused by the carcinogen dimethyl-1,2-benzanthracene (DMBA) (37). The animals that were followed for longer periods frequently developed s.c. mammary adenocarcinomas in the abdominal region, conceivably related to treatment with DMBA (38). In those cases, the adenocarcinomas were surgically excised, enabling follow-up to continue uneventfully.
Four Week Follow-Up.
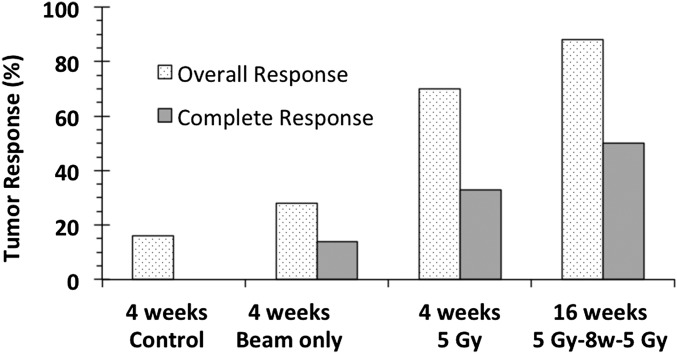
Outcome at 4 wk posttreatment is summarized in Table S1. The data corresponding to later time points (when it was possible to extend the follow-up period) are presented separately. In the case of the control group (cancerized, untreated), only 16% of the tumors underwent a spontaneous reduction in tumor volume and there were no complete tumor responses (CRs). The results from initial screening with various neutron irradiation doses (SI Results, S6) led us to perform subsequent BNCT treatments at 5 Gy to avoid severe mucositis to the precancerous tissue. The overall tumor response (OR) was approximately 70%, of which the CR rate was 33%. Because treatment at a 5-Gy irradiation dose caused only mild mucositis in precancerous tissue (a maximum of grade 2), the subsequent two BNCT treatment studies were performed at 5 Gy per treatment, which corresponds to a per-treatment irradiation dose of 21 Gy to the tumor tissue. Repeated BNCT treatments were performed with 4-, 6-, or 8-wk intervals between each treatment. This resulted in OR rates of 70%, 79%, and 88%, respectively, of which the CR rates were 52%, 37%, and 50%, respectively, higher than with the one-treatment protocol. Mucositis in precancerous tissue in the two-treatment protocol was mild and did not exceed grade 2. In the case of the beam-only group, the OR rate 4 wk posttreatment was only 28%, of which the CR rate was 14%. The difference in OR rates between the beam-only group and the single-treatment group was statistically significant (P = 0.0001).
Tumor response at 4 wk posttreatment is summarized in Table S2, considering stratification for tumor size at the time of irradiation (i.e., pretreatment), an issue of clinical relevance (39). For all protocols, the incidence of CR was higher for the small tumors than for the medium-sized and large tumors (SI Results, S7), stressing the importance of initiating treatment promptly after diagnosis.
Extended Follow-Up.
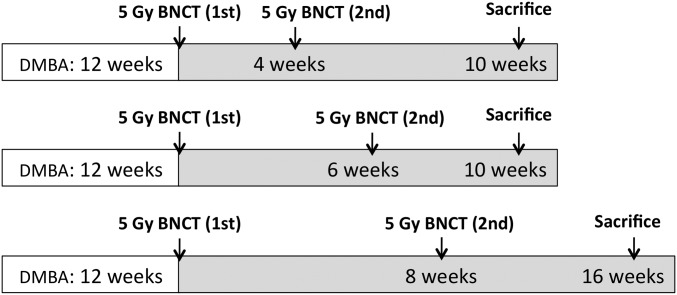
As described earlier, the posttreatment follow-up period for tumor control studies in the hamster cheek pouch oral cancer model was previously established as 4 wk after one treatment of BNCT or after the second treatment in the case of two-treatment protocols (12, 40). For the first time to our awareness, it was possible to extend the follow-up period to 16 wk in the case of two-treatment protocols, mainly as a result of good therapeutic outcome, i.e., good tumor control, and reduced mucositis. Table 1 shows tumor response at the last time point evaluated for two-treatment protocols as shown in the timeline in Fig. 1. No statistically significant difference in tumor response was found between follow-up data corresponding to a one-BNCT treatment and the two-BNCT treatments. This would imply that the two treatment protocols sustain an OR rate of 70–88% over a period of 10–16 wk after the first treatment. This period constitutes ∼13% of the lifespan of a healthy hamster. If a direct extrapolation were possible, this period would correspond to a clinically meaningful period of ∼10 y in a human subject. Fig. 2 graphically presents tumor response for the most representative protocols. Fig. 3 illustrates, using a representative example, the macroscopic appearance of a tumor-bearing pouch before and after treatment.
Table 1.
Percentage of tumor response at last time point evaluated for each 5-Gy neutron irradiation to precancerous tissue
| Group/outcome | 5 Gy single treatment | 5 Gy/4 wk/5 Gy | 5 Gy/6 wk/5 Gy | 5 Gy/8 wk/5 Gy |
| Hamsters | 25 | 5 | 4 | 5 |
| Tumors | 102 | 27 | 19 | 8 |
| Follow-up after first (or single) application, wk | 4 | 10 | 10 | 16 |
| Response, % | ||||
| Partial | 37 | 15 | 42 | 50 |
| Complete | 33 | 56 | 37 | 38 |
| None | 29 | 30 | 21 | 13 |
| Overall | 71 | 70 | 79 | 88 |
Neutron irradiation dose to precancerous tissue quoted in Grays; 5 Gy irradiation dose in precancerous tissue corresponds to 21 Gy in tumor. Interval between BNCT treatments in weeks in the case of repeat treatment. Most of the control animals were euthanized at 4 wk follow-up due to tumor overgrowth. Thus, it was not possible to time-match the control group with the repeat BNCT treatment groups with extended follow-up.
Fig. 1.
Schematic representation of the timeline of the experimental period corresponding to the treatment protocols of 5 Gy absorbed dose per application to the precancerous tissue.
Fig. 2.
Histogram illustrating tumor response (percentage of tumors that exhibit OR or CR) for each group and follow-up time as indicated.
Fig. 3.
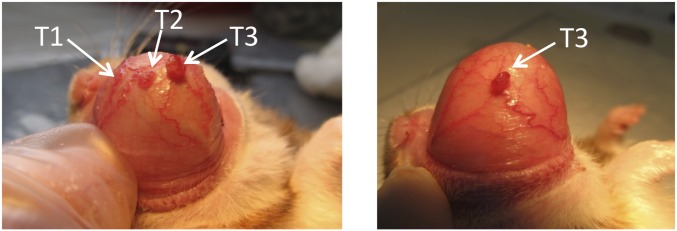
Representative example of a tumor-bearing pouch pretreatment (Left) and 14 d posttreatment (Right) with 5 Gy absorbed dose to precancerous tissue. Tumor 1 (1.3 mm3) and tumor 2 (7 mm3) exhibited CR, whereas tumor 3 (17.9 mm3) exhibited PR, with a reduction in volume to 6.6 mm3. Precancerous pouch tissue surrounding tumor exhibited grade 1 mucositis before and after treatment.
Regarding the development of novel tumors (i.e., tumors not present at the end of the carcinogenesis protocol) from precancerous tissue, 100% of the animals in the control group exhibited novel tumors at 4 wk follow-up (41). In contrast, 64% of the hamsters in the one-treatment protocol at 4 wk follow-up developed novel tumors, whereas approximately 75–80% of the hamsters in the two-treatment protocol (at the longest follow-up time in each case) developed novel tumors. This would imply that the two-treatment protocols sustain a partial inhibitory effect of BNCT on the development of novel tumors from precancerous tissue for 10–16 wk.
Although we previously demonstrated the therapeutic success of BNCT mediated by BPA in the hamster cheek pouch oral cancer model (12, 32), we undoubtedly face the ongoing challenge of optimizing this technique by using approaches that would conceivably also be useful in other experimental models and, eventually, in a clinical scenario. In the case of head and neck cancer, a therapeutic strategy will be successful if it achieves tumor response, minimizes the development of mucositis in the dose-limiting precancerous tissue surrounding the tumor, and reduces the development of novel tumors from precancerous tissue. Within this context, the BNCT treatment protocols mediated by MAC-TAC liposomes assayed herein for the first time in an oral cancer model to our knowledge, induced tumor response in ≥70% of the tumors with extremely mild mucositis (grade 1/2) in precancerous tissue and no normal tissue toxicity. In addition, these protocols partially inhibited the development of novel tumors from precancerous tissue. Although effective tumor control has previously been achieved in this model with the use of other boron carriers (12, 32), this is the first time of which we are aware that the combination of good tumor response, mild mucositis, and partial inhibition of the development of novel tumors from precancerous tissue has allowed for a longer follow-up period beyond the previously established time period. Repeated BNCT treatment yielded a sustained tumor response of 70–88% (37–56% CR) and a sustained partial inhibitory effect on the development of novel tumors from precancerous tissue up to 16 wk after the first treatment of BNCT.
Our working hypothesis to test the repeat BNCT treatment was that this strategy would minimize mucositis in dose-limiting precancerous tissue. We also sought to choose an interval that would minimize tumor cell repopulation that is known to jeopardize therapeutic response. Based on the known fact that mucositis is a multistage process initiated by mucosal injury and associated with an increased production of inflammatory cytokines, which cause direct mucosal damage and initiate positive feedback loops (42), the interval between treatments would allow the inflammatory process to partially subside before the second dose is delivered, preventing enhancement of mucositis. In terms of therapeutic efficacy, lengthening overall treatment time in conventional (i.e., low-LET) radiotherapy is known to reduce toxicity at the expense of reducing tumor control probability (43). However, in the case of BNCT that involves a combination of low and high LET radiation components, a repeat treatment would allow for boron retargeting of cell populations originated in tumor cells that were refractory to the first application and subsequently proliferated (3, 40). Within the time frame evaluated (intervals of 4, 6, and 8 wk between applications), the present data do not reveal statistically significant differences in tumor response attributable to differences in the interval. This gives some flexibility in selecting the best moment for the second application of BNCT in patient-tailored therapy (39). Admittedly, some patients may be in too fragile condition to benefit from a double application protocol. All three intervals would conceivably preclude enhancement of mucositis after the second treatment and similarly minimize repopulation. It is known that inflammation associated with mucositis can induce tumor promotion, activating premalignant lesions (36, 44). It is one of the hallmarks of cancer, acting on any stage of tumorigenesis (45). Within this context, the fact that BNCT can be delivered without enhancing mucositis is not only an asset in terms of preventing toxicity but also in terms of inhibiting the development of novel tumors. The development of novel tumors in precancerous tissue would model the development of second primary tumors in field-cancerized oral mucosa (3) and can thus be considered a parameter that affects medium- and long-term therapeutic outcome.
The fact that MAC-TAC liposomes are not toxic and deliver a high absolute boron concentration to a tumor (67 ± 16 ppm) with extremely favorable ratios of tumor relative to dose-limiting precancerous tissue (∼6:1) and normal tissue (∼10:1) (30) made it an extremely attractive boron carrier to assess in radiobiological studies. It was possible to deliver a 5-Gy absorbed neutron radiation dose to the dose limiting-precancerous tissue (4 Gy to normal tissue) with an associated absorbed tumor radiation dose of 21 Gy. In addition, because of the high absolute boron content in tumor tissue, the boron radiation component of the total tumor dose was 90% (Table 2). This is clearly an asset because it maximizes the tumor-selective component of the total dose.
Table 2.
Prescribed absorbed doses from radiation components for 5-Gy absorbed dose to precancerous tissue and corresponding beam-only irradiation
| Tissue | Boron concentration, ppm | γ-Photons, Gy | Induced protons (14N), Gy | Boron, Gy | Absorbed dose, Gy | |
| Total background | Total | |||||
| MAC-TAC BNCT | ||||||
| Tumor | 66.6 ± 16.1 | 0.9 ± 0.1 | 0.8 ± 0.2 | 19 ± 6 | 1.7 ± 0.2 | 21 ± 6 |
| Precancerous tissue | 11.3 ± 6.2 | 0.9 ± 0.1 | 0.8 ± 0.2 | 3 ± 2 | 1.7 ± 0.2 | 5 ± 2 |
| Normal tissue | 7 ± 5.5 | 0.9 ± 0.1 | 0.8 ± 0.2 | 2 ± 2 | 1.7 ± 0.2 | 4 ± 2 |
| Beam only | ||||||
| Tumor, precancerous tissue, and normal tissue | — | 0.9 ± 0.1 | 0.8 ± 0.2 | — | 1.7 ± 0.2 | 1.7 ± 0.2 |
The mean thermal neutron fluence at the irradiation position was 3.9 × 1012 ± 8 × 1011 n cm−2 and the irradiation time ranged from 8 to 9 min. Values are presented as means ± SD.
We can conclude that, in the hamster cheek pouch oral cancer model, BNCT mediated by MAC-TAC liposomes can achieve sustained, robust tumor control over a long follow-up period of 16 wk. At the end of the 16-wk period, tumor growth was observed in only 13% of the tumors, whereas, over a period of only 4 wk in untreated animals and in animals treated with beam only, 84% and 72% of the tumors, respectively, continued to grow. This tumor control is associated with the mildest mucositis in dose-limiting precancerous tissue, a partial inhibitory effect on the development of novel tumors from precancerous tissue and no normal tissue toxicity.
Materials and Methods
An abbreviated summary of the methods used is given here. SI Materials and Methods provides detailed experimental procedures. Also, please refer to our previously published boron biodistribution study (30) for a detailed description of MAC-TAC liposome administration protocols.
Tumor Induction.
Tumors were induced in the right cheek pouch of young Syrian hamsters by topical application of the carcinogen DMBA 0.5% in mineral oil twice per week for 12 wk as previously described (40, 46) (SI Materials and Methods, S1). All animal procedures were conducted in accordance with protocols approved by the National Atomic Energy Commission Animal Care and Use Committee.
In Vivo BNCT.
A suspension of MAC-TAC liposomes with a mean diameter of 83 nm was administered i.v. at a dose of 18 mg 10B per kilogram bw (SI Materials and Methods, S2). The dose and postadministration times used for neutron irradiation were selected on the basis of a previous study (30). This biodistribution study involved ∼25 hamsters each with multiple tumors (total of 68 tumors of varying size). Boron concentration in various tissues such as blood, tumor, precancerous tissue, normal pouch tissue, spleen, liver, and kidney were determined at 16 h, 30 h, 48 h, 54 h, and 72 h. Boron concentration in blood and tissue was measured by inductively coupled plasma MS. On average, after 48 h postinjection of MAC-TAC liposomes, the boron concentration peaked to 67 ppm in tumor, whereas precancerous tissue incorporated ∼11 ppm of boron. The tumor-to-normal pouch tissue boron ratio was 10:1. Table 2 presents a representative example of dose prescription data. Previously reported boron concentration values (30) were used for dose calculation.
The animals were irradiated 48 h postadministration of MAC-TAC liposomes under i.p. ketamine (140 mg/kg bw)/xylazine (21 mg/kg bw) anesthesia at the Reactor Argentino 3 nuclear reactor (47). Physical dosimetry data has been previously reported (48). The thermal neutron flux was approximately 7.7 × 109 n⋅cm−2⋅s−1 in the center of the irradiation position. The dose rate of γ-rays in air at the irradiation location was 6.5 ± 0.5 Gy⋅h−1 (SI Materials and Methods, S3).
A series of preliminary experiments were performed to establish the optimum radiation dose range. Forty-eight hours after the administration of MAC-TAC liposomes, an absorbed radiation dose was given as prescribed for precancerous tissue. The dose-limiting radiation to precancerous tissue in oral cancer was expected to range from 3.5 Gy to 7 Gy. Having determined that 3.5 Gy exhibited no radiation toxicity whereas 6 Gy and 7 Gy exhibited severe toxicity (SI Results, S6), subsequent experiments were performed at 5 Gy (25 hamsters bearing a total of 102 tumors), which resulted in a total radiation dose of 21 Gy to the tumor tissue. In addition to these experiments, a few experiments were done in which a repeat BNCT treatment was administered (complete with redosing of liposomes) at an interval of 4, 6, or 8 wk between each treatment. Five hamsters bearing a total of 27 tumors with a 4-wk interval between treatments, 4 hamsters bearing a total of 19 tumors with a 6-wk interval between treatments, and 6 hamsters bearing a total of 16 tumors with an 8 wk interval between treatments were evaluated. Group n (6 hamsters bearing a total of 36 tumors) was treated once with beam only (i.e., neutron irradiation, fluence matched with 5 Gy BNCT irradiations, with no prior administration of MAC-TAC liposomes) to evaluate the effect of the background dose. It was not possible to perform two treatments with beam only as a result of animal decline because of tumor overgrowth. An additional group of 12 cancerized, untreated hamsters bearing a total of 77 tumors were followed to assess tumor growth without treatment. This control group was time-matched with the treated groups.
Follow-Up.
To evaluate toxicity, the clinical signs and bw of all animals were monitored twice per week. The tumor and precancerous tissue responses were assessed by visual inspection and tumor volume assay before treatment and at 1, 7, 14, 21, and 28 d posttreatment in keeping with the previously established follow-up period for tumor control studies in this model (12, 32, 40, 49). When possible, the animals were followed for longer periods of time (up to 16 wk from the first treatment). Tumor volume was determined by external caliper measurement of the three largest orthogonal diameters and expressed in cubic millimeters (32). Any degree of reduction from initial tumor volume was considered as partial tumor response (PR) as defined in previous studies (49). A reduction to ≤50% of the initial tumor volume was named PR0.5. CR was defined as disappearance of the tumor on visual inspection. OR was defined as PR plus CR (49). Tumor response was evaluated considering three arbitrary tumor sizes (small, <10 mm3; medium, 10–100 mm3; and large, >100 mm3) defined to categorize tumor size at the time of irradiation and evaluate potential differential response according to size (49). The severity of mucositis was evaluated semiquantitatively in dose-limiting precancerous tissue as previously described (3, 50, 51) (SI Materials and Methods, S4). To evaluate the potential inhibitory effect of the different BNCT protocols on the development of novel tumors (i.e., tumors not present at the end of the carcinogenesis protocol) from precancerous tissue in this model, we used the percentage of hamsters with novel tumors in the treated pouch as a representative end point (SI Materials and Methods, S5) (3, 41).
Statistical analysis of differences in tumor response was performed by Fisher exact test. Statistical significance was set at P = 0.05.
Supplementary Material
Acknowledgments
The authors acknowledge the expert staff of Reactor Argentino 3 nuclear reactor. This study was supported in part by the University of Missouri through the International Institute for Nano and Molecular Medicine, by the US Department of Energy through Idaho National Laboratory, and by grants from Agencia Nacional de Promoción Científica y Tecnológica and Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410865111/-/DCSupplemental.
References
- 1.Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat Res. 1999;151(1):1–18. [PubMed] [Google Scholar]
- 2.Pozzi EC, et al. Boron neutron capture therapy (BNCT) for liver metastasis: Therapeutic efficacy in an experimental model. Radiat Environ Biophys. 2012;51(3):331–339. doi: 10.1007/s00411-012-0419-8. [DOI] [PubMed] [Google Scholar]
- 3.Monti Hughes AM, et al. Boron neutron capture therapy for oral precancer: Proof of principle in an experimental animal model. Oral Dis. 2013;19(8):789–795. doi: 10.1111/odi.12077. [DOI] [PubMed] [Google Scholar]
- 4.González SJ, et al. First BNCT treatment of a skin melanoma in Argentina: Dosimetric analysis and clinical outcome. Appl Radiat Isot. 2004;61(5):1101–1105. doi: 10.1016/j.apradiso.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 5.Zonta A, et al. Clinical lessons from the first applications of BNCT on unresectable liver metastases. J Phys Conf Ser. 2006;41(1):484–495. [Google Scholar]
- 6.Kankaanranta L, et al. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: A phase I study. Int J Radiat Oncol Biol Phys. 2011;80(2):369–376. doi: 10.1016/j.ijrobp.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Kankaanranta L, et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: final analysis of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2012;82(1):e67–e75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 8.Barth RF, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146–166. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksson R, et al. Boron neutron capture therapy (BNCT) for glioblastoma 4 multiforme: A phase II study evaluating a prolonged high-dose of 5 boronophenylalanine (BPA) at the Studsvik facility in Sweden. Radiotherapy Oncol. 2008;88(2):288. doi: 10.1016/j.radonc.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Hopewell JW, et al. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: an assessment of clinical potential. Appl Radiat Isot. 2011;69(12):1737–1740. doi: 10.1016/j.apradiso.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Hawthorne MF, Lee MW. A critical assessment of boron target compounds for boron neutron capture therapy. J Neurooncol. 2003;62(1-2):33–45. doi: 10.1007/BF02699932. [DOI] [PubMed] [Google Scholar]
- 12.Trivillin VA, et al. Therapeutic success of boron neutron capture therapy (BNCT) mediated by a chemically non-selective boron agent in an experimental model of oral cancer: A new paradigm in BNCT radiobiology. Radiat Res. 2006;166(2):387–396. doi: 10.1667/RR3592.1. [DOI] [PubMed] [Google Scholar]
- 13.Heber EM, et al. Homogeneous boron targeting of heterogeneous tumors for boron neutron capture therapy (BNCT): Chemical analyses in the hamster cheek pouch oral cancer model. Arch Oral Biol. 2006;51(10):922–929. doi: 10.1016/j.archoralbio.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Garabalino MA, et al. Boron neutron capture therapy (BNCT) for the treatment of liver metastases: Biodistribution studies of boron compounds in an experimental model. Radiat Environ Biophys. 2011;50(1):199–207. doi: 10.1007/s00411-010-0345-6. [DOI] [PubMed] [Google Scholar]
- 15.Santa Cruz GA, Zamenhof RG. The microdosimetry of the (10)B reaction in boron neutron capture therapy: A new generalized theory. Radiat Res. 2004;162(6):702–710. doi: 10.1667/rr3257. [DOI] [PubMed] [Google Scholar]
- 16.Barth RF. Boron neutron capture therapy at the crossroads: Challenges and opportunities. Appl Radiat Isot. 2009;67(7-8 Suppl):S3–S6. doi: 10.1016/j.apradiso.2009.03.102. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 19.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Shelly K, et al. Model studies directed toward the boron neutron-capture therapy of cancer: Boron delivery to murine tumors with liposomes. Proc Natl Acad Sci USA. 1992;89(19):9039–9043. doi: 10.1073/pnas.89.19.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feakes DA, Shelly K, Knobler CB, Hawthorne MF. Na3[B20H17NH3]: Synthesis and liposomal delivery to murine tumors. Proc Natl Acad Sci USA. 1994;91(8):3029–3033. doi: 10.1073/pnas.91.8.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feakes DA, Shelly K, Hawthorne MF. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc Natl Acad Sci USA. 1995;92(5):1367–1370. doi: 10.1073/pnas.92.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson-Clark RA, Banquerigo ML, Shelly K, Hawthorne MF, Brahn E. Model studies directed toward the application of boron neutron capture therapy to rheumatoid arthritis: Boron delivery by liposomes in rat collagen-induced arthritis. Proc Natl Acad Sci USA. 1998;95(5):2531–2534. doi: 10.1073/pnas.95.5.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawthorne MF, Shelly K. Liposomes as drug delivery vehicles for boron agents. J Neurooncol. 1997;33(1-2):53–58. doi: 10.1023/a:1005713113990. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Hamdi J, Hawthorne MF. Unilamellar liposomes with enhanced boron content. Bioconjug Chem. 2006;17(1):15–20. doi: 10.1021/bc0501350. [DOI] [PubMed] [Google Scholar]
- 26.Kueffer PJ, et al. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc Natl Acad Sci USA. 2013;110(16):6512–6517. doi: 10.1073/pnas.1303437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altieri S, et al. Carborane derivatives loaded into liposomes as efficient delivery systems for boron neutron capture therapy. J Med Chem. 2009;52(23):7829–7835. doi: 10.1021/jm900763b. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H. Liposomal boron delivery for neutron capture therapy. Methods Enzymol. 2009;465:179–208. doi: 10.1016/S0076-6879(09)65010-2. [DOI] [PubMed] [Google Scholar]
- 29.Koganei H, et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjug Chem. 2013;24(1):124–132. doi: 10.1021/bc300527n. [DOI] [PubMed] [Google Scholar]
- 30.Heber EM, et al. Boron delivery with liposomes for boron neutron capture therapy (BNCT): Biodistribution studies in an experimental model of oral cancer demonstrating therapeutic potential. Radiat Environ Biophys. 2012;51(2):195–204. doi: 10.1007/s00411-011-0399-0. [DOI] [PubMed] [Google Scholar]
- 31.Kreimann EL, et al. The hamster cheek pouch as a model of oral cancer for boron neutron capture therapy studies: Selective delivery of boron by boronophenylalanine. Cancer Res. 2001;61(24):8775–8781. [PubMed] [Google Scholar]
- 32.Kreimann EL, et al. Boron neutron capture therapy for the treatment of oral cancer in the hamster cheek pouch model. Cancer Res. 2001;61(24):8638–8642. [PubMed] [Google Scholar]
- 33.Mehrotra R, Ibrahim R, Eckardt A, Driemel O, Singh M. Novel strategies in therapy of head and neck cancer. Curr Cancer Drug Targets. 2011;11(4):465–478. doi: 10.2174/156800911795538039. [DOI] [PubMed] [Google Scholar]
- 34.Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2(1):21–32. [PubMed] [Google Scholar]
- 35.Sonis ST. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009;45(12):1015–1020. doi: 10.1016/j.oraloncology.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eveson JW. Animal models of intra-oral chemical carcinogenesis: A review. J Oral Pathol. 1981;10(3):129–146. doi: 10.1111/j.1600-0714.1981.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 38.Moon RC, Grubbs CJ, Sporn MB. Inhibition of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis by retinyl acetate. Cancer Res. 1976;36(7 Pt 2):2626–2630. [PubMed] [Google Scholar]
- 39.Kato I, et al. Effectiveness of BNCT for recurrent head and neck malignancies. Appl Radiat Isot. 2004;61(5):1069–1073. doi: 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 40.Molinari AJ, et al. “Sequential” boron neutron capture therapy (BNCT): A novel approach to BNCT for the treatment of oral cancer in the hamster cheek pouch model. Radiat Res. 2011;175(4):463–472. doi: 10.1667/RR2148.1. [DOI] [PubMed] [Google Scholar]
- 41.Heber EM, et al. Development of a model of tissue with potentially malignant disorders (PMD) in the hamster cheek pouch to explore the long-term potential therapeutic and/or toxic effects of different therapeutic modalities. Arch Oral Biol. 2010;55(1):46–51. doi: 10.1016/j.archoralbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Mais K. Mucositis from radiotherapy to the head and neck: An overview. Nursing. 2006;1:18–20. [Google Scholar]
- 43.Dörr W, Schlichting S, Bray MA, Flockhart IR, Hopewell JW. Effects of dexpanthenol with or without Aloe vera extract on radiation-induced oral mucositis: Preclinical studies. Int J Radiat Biol. 2005;81(3):243–250. doi: 10.1080/09553000500103033. [DOI] [PubMed] [Google Scholar]
- 44.Pérez MA, Raimondi AR, Itoiz ME. An experimental model to demonstrate the carcinogenic action of oral chronic traumatic ulcer. J Oral Pathol Med. 2005;34(1):17–22. doi: 10.1111/j.1600-0714.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- 45.Multhoff G, Radons J. Radiation, inflammation, and immune responses in cancer. Front Oncol. 2012;2:58. doi: 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shklar G, Eisenberg E, Flynn E. Immunoenhancing agents and experimental leukoplakia and carcinoma of the hamster buccal pouch. Prog Exp Tumor Res. 1979;24:269–282. doi: 10.1159/000402104. [DOI] [PubMed] [Google Scholar]
- 47.Miller M, et al. New irradiation facility for biomedical applications at the RA-3 reactor thermal column. Appl Radiat Isot. 2009;67(7-8 Suppl):S226–229. doi: 10.1016/j.apradiso.2009.03.107. [DOI] [PubMed] [Google Scholar]
- 48.Pozzi E, et al. Dosimetry and radiobiology at the new RA-3 reactor boron neutron capture therapy (BNCT) facility: Application to the treatment of experimental oral cancer. Appl Radiat Isot. 2009;67(7-8 Suppl):S309–312. doi: 10.1016/j.apradiso.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 49.Molinari AJ, et al. Tumor blood vessel “normalization” improves the therapeutic efficacy of boron neutron capture therapy (BNCT) in experimental oral cancer. Radiat Res. 2012;177(1):59–68. doi: 10.1667/rr2729.1. [DOI] [PubMed] [Google Scholar]
- 50.López-Castaño F, Oñate-Sánchez RE, Roldán-Chicano R, Cabrerizo-Merino MC. Measurement of secondary mucositis to oncohematologic treatment by means of different scale. Review. Med Oral Patol Oral Cir Bucal. 2005;10(5):412–421. [PubMed] [Google Scholar]
- 51.Sonis ST, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36(4):373–381. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.