Abstract
To explore possible mechanisms by which complement membrane attack complexes (MAC) that are deposited in the glomerular mesangium might be pathogenic, we stimulated rat glomerular mesangial cells grown in vitro with nascent MACs formed from the purified human complement components C5b6 and normal human serum and measured production of superoxide ion (O2-) and hydrogen peroxide (H2O2). Mesangial cells incubated with C5b6 + serum, which results in cell membrane interaction with the MAC, produce 0.9 +/- 0.15 nmol O2-/10(5) cells per 30 min, which was significantly greater than the amount produced by cells incubated with C5b6 alone, serum alone, or decayed MACs that can no longer interact with the cell membrane (0.3 +/- 0.2, 0.4 +/- 0.1, 0.3 +/- 0.2 nmol O2-/10(5) cells per 30 min, respectively; P less than 0.02). Production of O2- after stimulation with MACs increased during the first 20 min of incubation but then plateaued. Cells exposed to decayed MACs produced small amounts of O2-, which did not increase from 20 to 60 min. Production of H2O2 was also observed after stimulation with MACs, and continued to increase during 60 min of incubation (1.22 +/- 0.16 nmol H2O2/10(5) cells per 60 min), whereas H2O2 production could not be detected after exposure to decayed MACs. Cell viability was not adversely affected by exposure to nascent MACs as determined by trypan blue exclusion or chromium-51 release. These results demonstrate that glomerular mesangial cell membrane interaction with the MAC stimulates the production of the toxic oxygen metabolites O- and H2O2. Activation of the terminal complement pathway by mesangial immune deposits in vivo might lead to tissue injury by stimulation of local production of toxic oxygen-free radicals.
Full text
PDF

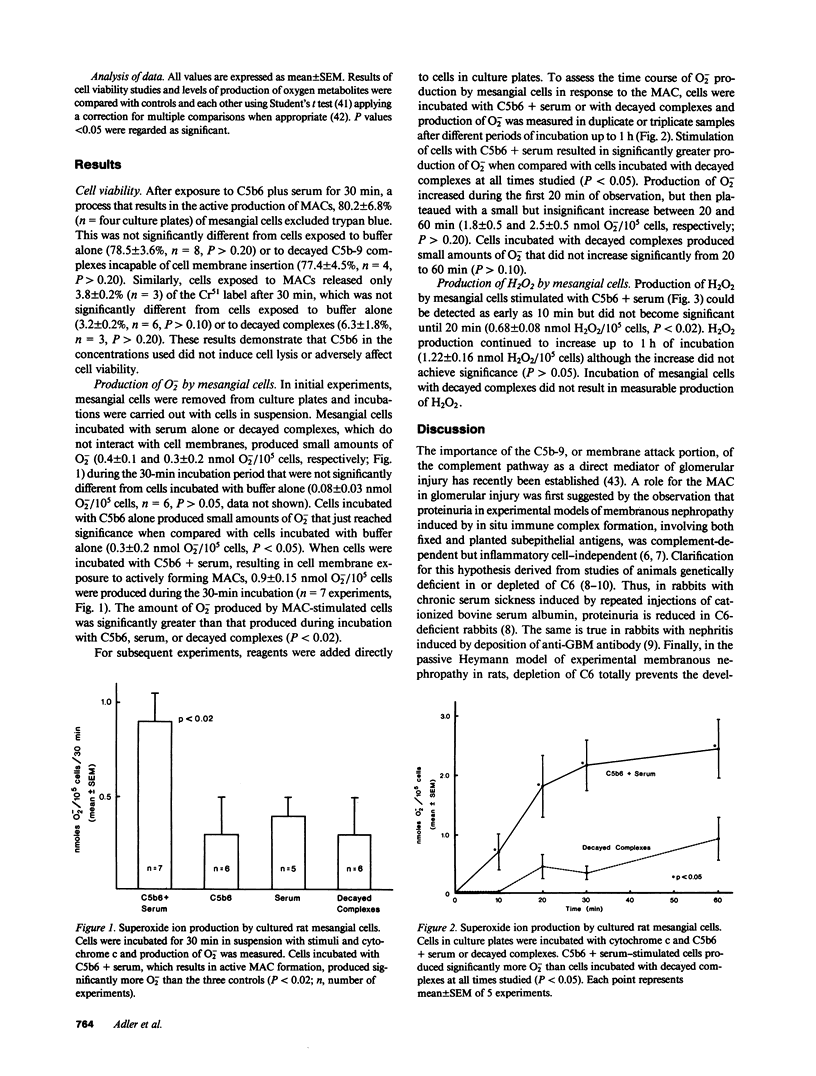


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Baker P. J., Pritzl P., Couser W. G. Detection of terminal complement components in experimental immune glomerular injury. Kidney Int. 1984 Dec;26(6):830–837. doi: 10.1038/ki.1984.225. [DOI] [PubMed] [Google Scholar]
- Adler S., Salant D. J., Dittmer J. E., Rennke H. G., Madaio M. P., Couser W. G. Mediation of proteinuria in membranous nephropathy due to a planted glomerular antigen. Kidney Int. 1983 Jun;23(6):807–815. doi: 10.1038/ki.1983.99. [DOI] [PubMed] [Google Scholar]
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Adler S., Yang Y., Couser W. G. Complement activation by heat-killed human kidney cells: formation, activity, and stabilization of cell-bound C3 convertases. J Immunol. 1984 Aug;133(2):877–881. [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Handley C. J. Oxygen free-radicals mediate an inhibition of proteoglycan synthesis in cultured articular cartilage. Ann Rheum Dis. 1984 Jun;43(3):462–469. doi: 10.1136/ard.43.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Katz S., Koffler D. Renal localization of the membrane attack complex in systemic lupus erythematosus nephritis. J Exp Med. 1981 Dec 1;154(6):1779–1794. doi: 10.1084/jem.154.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G. Membrane attack complex of complement as a pathologic mediator. Lab Invest. 1983 Sep;49(3):237–249. [PubMed] [Google Scholar]
- Biesecker G., Noble B., Andres G. A., Koffler D. Immunopathogenesis of Heymann's nephritis. Clin Immunol Immunopathol. 1984 Dec;33(3):333–338. doi: 10.1016/0090-1229(84)90304-0. [DOI] [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Lee E. C., McCoy J. P., Johnson K. J., Varani J. Protein degradation following treatment with hydrogen peroxide. Am J Pathol. 1984 Jun;115(3):418–425. [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Ward P. A., Johnson K. J., Till G. O. Evidence for a role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984 Jun;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- Foidart J. B., Dechenne C. A., Mahieu P., Creutz C. E., de Mey J. Tissue culture of normal rat glomeruli. Isolation and morphological characterization of two homogeneous cell lines. Invest Cell Pathol. 1979 Jan-Mar;2(1):15–26. [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Groggel G. C., Adler S., Rennke H. G., Couser W. G., Salant D. J. Role of the terminal complement pathway in experimental membranous nephropathy in the rabbit. J Clin Invest. 1983 Dec;72(6):1948–1957. doi: 10.1172/JCI111159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groggel G. C., Salant D. J., Darby C., Rennke H. G., Couser W. G. Role of terminal complement pathway in the heterologous phase of antiglomerular basement membrane nephritis. Kidney Int. 1985 Apr;27(4):643–651. doi: 10.1038/ki.1985.59. [DOI] [PubMed] [Google Scholar]
- Hänsch G. M., Seitz M., Martinotti G., Betz M., Rauterberg E. W., Gemsa D. Macrophages release arachidonic acid, prostaglandin E2, and thromboxane in response to late complement components. J Immunol. 1984 Oct;133(4):2145–2150. [PubMed] [Google Scholar]
- Imagawa D. K., Osifchin N. E., Paznekas W. A., Shin M. L., Mayer M. M. Consequences of cell membrane attack by complement: release of arachidonate and formation of inflammatory derivatives. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6647–6651. doi: 10.1073/pnas.80.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Koffler D., Biesecker G., Noble B., Andres G. A., Martinez-Hernandez A. Localization of the membrane attack complex (MAC) in experimental immune complex glomerulonephritis. J Exp Med. 1983 Jun 1;157(6):1885–1905. doi: 10.1084/jem.157.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I., Karnovsky M. J. Glomerular cells in culture. Kidney Int. 1983 Mar;23(3):439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G., Platt J. L., Falk R. J., Rodriguez-Iturbe B., Michael A. F. Cell populations and membrane attack complex in glomeruli of patients with post-streptococcal glomerulonephritis: identification using monoclonal antibodies by indirect immunofluorescence. Clin Immunol Immunopathol. 1984 Dec;33(3):324–332. doi: 10.1016/0090-1229(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Perkinson D. T., Baker P. J., Couser W. G., Johnson R. J., Adler S. Membrane attack complex deposition in experimental glomerular injury. Am J Pathol. 1985 Jul;120(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Biesecker G., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: reaction with C7, C8, C9. J Immunol. 1978 Aug;121(2):484–490. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Membrane attack by complement. Mol Immunol. 1984 Jul;21(7):589–603. doi: 10.1016/0161-5890(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Nachman R. L., Weksler B. B. Human complement in the arachidonic acid transformation pathway in platelets. J Exp Med. 1981 Feb 1;153(2):257–268. doi: 10.1084/jem.153.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Koski C. L., Shin M. L., Mayer M. M. Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol. 1983 Sep;131(3):1411–1415. [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Wiggins R. C., Kunkel R. G., Ward P. A. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest. 1984 Oct;51(4):396–403. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Belok S., Madaio M. P., Couser W. G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980 Dec;66(6):1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Darby C., Couser W. G. Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest. 1980 Jul;66(1):71–81. doi: 10.1172/JCI109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNANUE E., DIXON F. J. EXPERIMENTAL GLOMERULONEPHRITIS. IV. PARTICIPATION OF COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1964 Jan 1;119:965–982. doi: 10.1084/jem.119.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Peppin G., Ortiz X., Ragsdale C., Test S. T. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985 Feb 15;227(4688):747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- de Heer E., Daha M. R., Bhakdi S., Bazin H., van Es L. A. Possible involvement of terminal complement complex in active Heymann nephritis. Kidney Int. 1985 Feb;27(2):388–393. doi: 10.1038/ki.1985.21. [DOI] [PubMed] [Google Scholar]



