Significance
One of the more intriguing but controversial ideas in psychology is that unconscious information can influence our decisions without us even knowing it. Here, we explicitly tested these controversial ideas with a novel behavioral task and computational models of decision-making. We report that unconscious information can be accumulated in a similar manner but less effectively than conscious information. However, unlike conscious information, unconscious information does not seem to boost decision confidence. Our findings cannot be accounted for using existing models of priming or adaptation.
Keywords: conscious awareness, decision-making, metacognition, continuous flash suppression, binocular rivalry
Abstract
The controversial idea that information can be processed and evaluated unconsciously to change behavior has had a particularly impactful history. Here, we extend a simple model of conscious decision-making to explain both conscious and unconscious accumulation of decisional evidence. Using a novel dichoptic suppression paradigm to titrate conscious and unconscious evidence, we show that unconscious information can be accumulated over time and integrated with conscious elements presented either before or after to boost or diminish decision accuracy. The unconscious information could only be used when some conscious decision-relevant information was also present. These data are fit well by a simple diffusion model in which the rate and variability of evidence accumulation is reduced but not eliminated by the removal of conscious awareness. Surprisingly, the unconscious boost in accuracy was not accompanied by corresponding increases in confidence, suggesting that we have poor metacognition for unconscious decisional evidence.
One of the fundamental challenges to understanding and predicting human behavior is that we are nonrational and often do not act in our own best interest. Indeed, many thinkers have struggled to conceptualize and model illogical and unexpected behavioral choices. One compelling proposition is that unconscious information can push and pull everyday decisions—without our explicit knowledge or permission. However, despite their popularity, such propositions lack strong experimental support.
Research exploring the role of unconscious information processing in the context of decision-making has typically focused on the role of deliberation in the absence of conscious attention directed at a problem. Within this framework, inattentional thought has been argued to be superior to focused attentional deliberation when making complex decisions (1–4) because of the proposed larger information processing capacity of unconscious thought (5). However, these findings have faced much controversy, because several studies have failed to find evidence for inattentional deliberation (6–10). A particularly important criticism here is that the tasks typically used do not allow for the adequate control of decision variables and hence, have little traction in assessing deliberation without attention. Moreover, these studies do not directly manipulate conscious awareness. Consequently, it is difficult to determine whether any unconscious decisional processes actually occur during the period of inattention.
Here, we developed a task in which we can control the amount of decision-relevant information available during both conscious and nonconscious processing. This task allowed us to investigate the idea that unconscious information can predictably alter conscious decisions. We used a simple perceptual decision paradigm, which has produced many neurobiological and computational insights into decision-making in humans and nonhuman animals (reviewed in ref. 11). Cross-species behavioral and neural data support simple accumulation models, according to which a conscious decision is made after enough noisy evidence has accumulated to a particular criterion level (12–15). These models can explain a variety of different behaviors, such as the relationship between accuracy, difficulty, and response time and even changes of mind (16). However, it is unknown if these models can account for unconscious decisional evidence that might affect behavior.
Results and Discussion
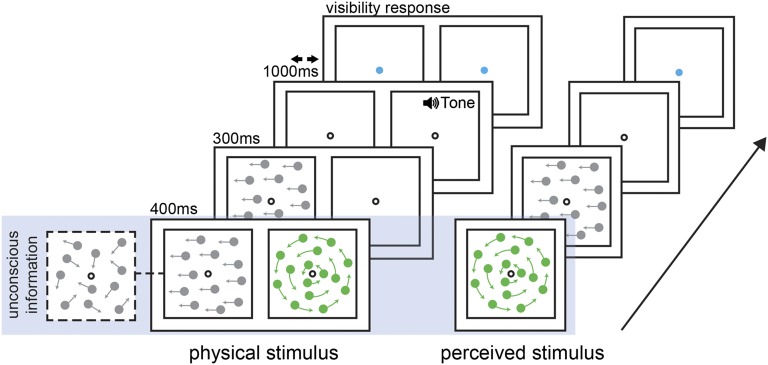
We suppressed noisy visual stimuli from conscious awareness using a dynamic dichoptic mask consisting of bright dots spinning concentrically around the fixation point (Fig. 1 shows the timeline). Participants were asked to decide the direction (left or right) of a coherent dot motion stimulus presented to one eye for 700 ms. In the first experiment, the mask was presented to the other eye for the first 400 ms, suppressing the dot motion stimulus from conscious awareness for this duration. Participants were asked to report on each trial whether they saw any part of the suppressed stimulus; unless indicated otherwise, all subsequent analyses include only trials in which the stimulus was suppressed (Fig. S1 shows suppression break data).
Fig. 1.
General experimental protocol. Left shows the stimulus presentation timeline as it was physically presented on the screen; Right illustrates a subject's perception of the presented stimuli. The blue-shaded region highlights the unconscious portion of the trial. The mask consisted of colored dots spinning concentrically around the central fixation point at a rate of 1.67 revolutions per second. The dot motion stimulus consisted of a number of randomly moving dots, with a fraction of the dots (10–60%) moving coherently in one of two directions. At stimulus offset, a tone was sounded to prompt participants to report the direction of motion as quickly and accurately as possible. Participants had a response window of 1,000 ms in which to make their choice. After their response, the fixation point changed color. Participants were then asked to indicate whether they saw any part of the dot motion stimulus during the first one-half of the stimulus presentation (when it was presented concurrently with the mask stimulus; 1 = saw part/all of the stimulus; 2 = stimulus was completely suppressed for the entire duration).
In a coherent condition, signal strength of the suppressed stimulus was constant throughout the entire presentation. In a noncoherent random condition, the suppressed stimulus only contained purely random motion (0% coherence). This method allowed us to keep the amount of conscious information constant, while adjusting the amount of decision-relevant information presented outside of conscious awareness.
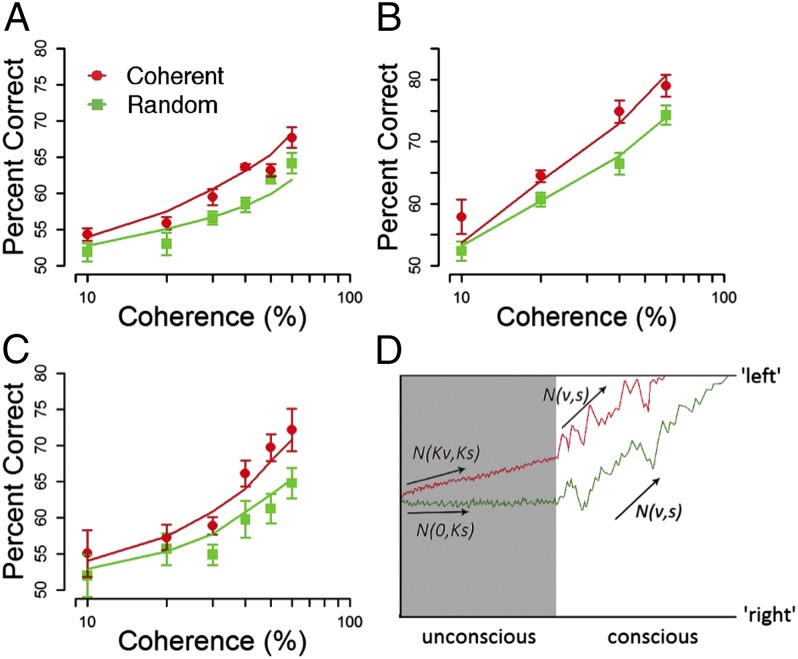
In experiment 1, we found that decision accuracy was significantly higher when the unconscious stimulus contained coherent information than in the random condition across a range of coherence levels [F(1,6) = 23.44, P = 0.003] (Fig. 2A, data points). These data suggest that decisional information during the first one-half of the trial might be processed without conscious awareness, resulting in an overall increase in decision accuracy. To facilitate computational modeling of the data, we replicated this effect in a second experiment, in which the dot motion stimulus remained consciously visible until the participant gave a response. Performance again improved when the unconscious information was coherent rather than random [F(1,4) = 23.44, P = 0.008] (Fig. 2B).
Fig. 2.
Information is accumulated in the absence of conscious awareness, improving decision accuracy. (A) Experiment 1: mean percentage correct (± SEM) is plotted for seven participants for the coherent (red) and random (green) conditions, where the suppressed coherent/random motion (400 ms) was followed by unsuppressed coherent motion (300 ms). Overall, accuracy was lower in the random condition (M = 57.49, SD = 3.54) than in the coherent condition (M = 60.78, SD = 4.12). (B) Experiment 2: mean percentage correct (± SEM) is plotted for five participants, two of whom had also participated in experiment 1. Here, suppressed coherent/random motion (300 ms) was followed by visible coherent motion that remained on the screen until the participants made a response. Again, accuracy was lower in the random (green) condition (M = 63.36, SD = 5.81) than in the coherent (red) condition (M = 69.14, SD = 5.67). (C) Experiment 3: mean percentage correct (± SEM) is plotted for six participants, three of whom had also participated in experiment 1. Here, the visible coherent motion (300 ms) preceded the suppressed random/coherent motion. Overall, accuracy was lower in the random (green) condition (M = 58.11, SD = 5.05) than in the coherent (red) condition (M = 63.56, SD = 7.44). (D) Information flow diagram illustrating the effects of evidence accumulation in the absence of conscious awareness on decision accuracy. In this example, the trial begins with the motion stimulus suppressed from conscious awareness. During the suppression, the rate and variability of evidence accumulation in the coherent (red) condition are reduced by a factor (K) with a mean accumulation rate of Kvi and an SD of Ks. In the random (green) condition, the accumulation rate is zero, with the same reduced variability (Ks). After the suppression period, evidence is accumulated at the full rate (v) and variability (s) in both conditions.
Motion priming has been found for stimuli in the absence of attention (17) and awareness (18). Hence, we sought to determine whether this increase in accuracy could simply be the result of the suppressed information priming or facilitating the subsequent visible stimulus. To test this, we conducted a third experiment, in which the order of presentation was reversed (i.e., the visible stimulus was followed by suppressed coherent/random motion). Again, accuracy was significantly higher in the coherent condition than in the random condition [F(1,5) = 8.43, P = 0.03] (Fig. 2C), ruling out priming as an account for the boost in decision accuracy.
Across all of the experiments, the accuracy boost from unconscious information was well-explained by a simple evidence accumulation model, which assumes that the unconscious information carried less decisional evidence than conscious information (Fig. 2D, steeper slope for the red plot and Methods). Furthermore, we found it necessary to assume that unconscious evidence accumulation was less noisy: the variability in the unconscious information was reduced by the same factor as the rate of accrual of actual decisional evidence. Fits of the model are shown in Fig. 2 (solid lines), and estimated parameters (Table 1) suggest that, when the unconscious stimulus was presented before the conscious stimulus, the quality and variability of evidence accumulation during unconscious processing were about 20% of that when the participant was aware of the stimulus presentation. Interestingly, when the suppressed stimulus was shown after the conscious stimulus, participants carried on collecting evidence at 95% efficiency during the suppression period. This finding fits well with recent work showing that information held in sensory memory can boost decisional accuracy (19) or allow for a change of mind (16). In experiment 3, it is possible that such a memory trace combines with the unconscious evidence, boosting the overall quality of decisional evidence.
Table 1.
Best-fitting parameters estimated for the simple diffusion model for experiments 1–3
| Experiment | Parameter | |||||
| a | Ter | K | β0 | β1 | STer | |
| 1 | 0.078 | 0.197 | 0.216 | 0.0043 | 0.00086 | 0.07 |
| 2 | 0.078 | 0.837 | 0.177 | −0.0246 | 0.00399 | — |
| 3 | 0.170 | 0.130 | 0.957 | −0.0008 | 0.00333 | 0.08 |
Note that we did not need to include variability in nondecision for experiment 2.
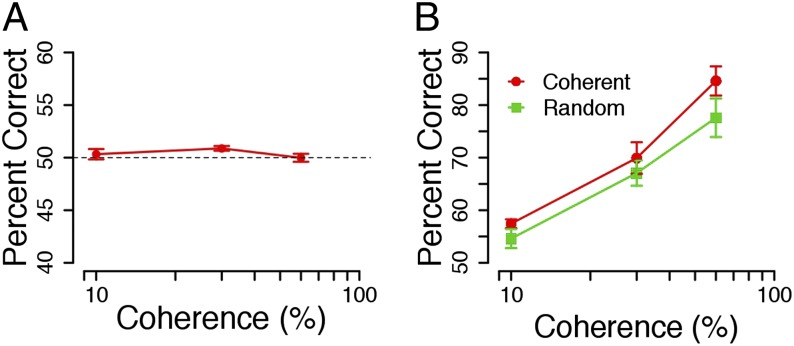
To obtain an objective account of the extent of conscious awareness during the suppression period, we ran a control experiment, in which all of the decisional information was rendered unconscious (experiment 4). Suppressed coherent motion (400 ms) was followed by random visible motion (300 ms). We found that decision accuracy was not significantly different from chance [t(8) = 1.56, P = 1.57] (Fig. 3A). This finding provides additional evidence against a simple priming account of the data: if priming was responsible for the increases in accuracy, then we would expect to find above-chance performance here. The more fascinating implication, however, is that, when there was no conscious coherent signal, participants were unable to use the coherent unconscious information.
Fig. 3.
(A) Experiment 4: mean percentage correct (± SEM) is plotted for nine participants, all of whom had participated in at least one of the previous studies. Here, suppressed coherent motion was followed by unsuppressed random motion. Overall, accuracy for three tested coherence levels was at chance level (M = 50.68, SD = 1.3). (B) Experiment 5: mean percentage correct (± SEM) is plotted for six participants, three of whom had participated in at least one of the previous experiments. Here, the suppressed coherent/random gray dot motion stimulus was followed by a visible coherent yellow dot motion stimulus. Overall, accuracy was higher in the coherent condition (M = 70.65, SD = 4.4) than in the random condition (M = 66.41, SD = 5.09).
We believe that there are two possible explanations for why participants were unable to use the unconsciously processed information when conscious directional information was removed. First, it is possible that the unconscious information is processed but requires conscious information to bind to for us to be able to access and use it. Second, the suppressed information is simply not presented for long enough to reach above-chance performance. Because we show that suppressed information is accumulated at a slower rate than visible information, it is possible that the decision threshold is not being reached fast enough to yield accurate responses. Finally, there is evidence to suggest that participants need some degree of training with feedback to learn how to use unconscious information (20).
We next considered the possibility that participants may have been misattributing the visibility of the gray dots during the suppression period to the subsequent visible motion segment of the stimulus. We tested for this possibility in experiment 5; we presented a suppressed coherent/random gray dot motion stimulus for 400 ms followed by a visible yellow dot motion stimulus for 400 ms. Six participants were instructed to report if they saw any gray dots on each trial after their motion direction response. Overall, decision accuracy was significantly higher when the unconscious stimulus contained coherent information (M = 70.65, SD = 4.4) than when it contained random information (M = 66.41, SD = 5.09) across the range of coherence levels [F(1,5) = 11.82, P = 0.02], replicating our earlier results (Fig. 3B). We also included mock catch trials, where we simulated both breaks in suppression (gray dots were presented to both eyes) and successful suppression (gray dots not presented at all). All participants missed less than 5% of the catch trials (M = 1.98, SD = 1.7). Together, these data indicate that participants are able to accurately identify and report breaks in suppression.
In experiment 6, we tested the possibility that decision accuracy was impeded in the random condition rather than enhanced in the coherent condition. In this control experiment, visible coherent motion (200 ms) was followed by either a random suppressed dot motion stimulus or the mask stimulus alone (250 ms; participants also completed trials on which the order of coherent motion and mask was reversed). There were no significant differences in accuracy between trials where the random dot motion stimulus was present (M = 57.91%, SD = 5.99%) and absent [M = 58.1%, SD = 6.54%; F(1,4) = 0.02, P = 0.89]. This finding suggests that the higher accuracy in the coherent conditions illustrated in Figs. 2 and 3B cannot be attributed to the suppressed random motion impeding performance by adding noise to the decision. Order of presentation also had no effect on accuracy [F(1,4) = 0.085, P = 0.79], indicating that the visibility of the unsuppressed motion was not disrupted when preceded by the mask (as in Fig. 2 A and B).
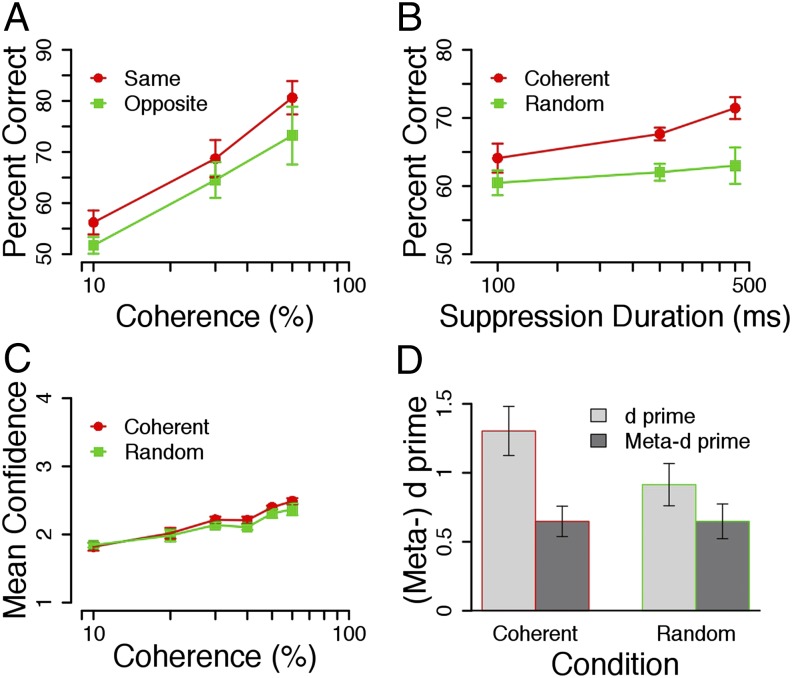
We next wanted to see whether presenting suppressed motion traveling in the opposite direction to the visible motion would result in reduced accuracy compared with motion traveling in the same direction throughout. In experiment 7, suppressed motion was presented for 400 ms followed by visible motion presented for 400 ms that was traveling in either the same or opposite direction. The coherence level remained constant in both conditions. We found that accuracy was significantly higher when the motion was traveling coherently in the same direction than when it was moving in the opposite direction during suppression [F(1,8) = 6.83, P = 0.03] (Fig. 4A). This result suggests that information processed outside of awareness can also have a negative impact on decision-making performance. Here again, we included catch trials and found that all participants missed these catch trials on less than 5% of trials (M = 1.98, SD = 1.7).
Fig. 4.
(A) Experiment 7: mean percentage correct (± SEM) is plotted for nine participants, three of whom had participated in at least one of the previous experiments. Here, the suppressed motion was traveling in either the same or opposite direction of the subsequent visible motion. Overall, accuracy was higher when the suppressed motion was traveling in the same direction (M = 68.51, SD = 8.26) than when it was moving in the opposite direction during the suppression (M = 63.15, SD = 9.87). (B) Experiment 8: accuracy rates (± SEMs) from eight participants were averaged across coherence level for the random (green) and coherent (red) conditions and plotted as a function of suppression duration. Overall, accuracy was higher in the coherent condition (M = 67.73, SD = 1.61) than in the random condition (M = 61.83, SD = 2.25). (C) Experiment 10: mean confidence ratings (± SEMs) are plotted for five participants and averaged across six coherence ratings. Confidence was reported on a scale of 1–4, where 4 = very confident and 1 = not at all confident. There were no significant differences in confidence between the coherent (M = 2.19, SD = 0.24) and random (M = 2.12, SD = 0.2) conditions [F(1,4) = 3.75, P = 0.13]. (D) Experiment 10: mean d′ and meta-d′ scores (± SEMs) are shown for the coherent and random conditions. Although there were no significant differences in the meta-d′ scores between the coherent (M = 0.65, SD = 0.22) and random (M = 0.65, SD = 0.25) conditions, d′ was significantly larger in the coherent (M = 1.3, SD = 0.36) than in the random condition [M = 0.91, SD = 0.31; F(1,3) = 12.65, P = 0.04].
Participants were also asked on each trial to indicate whether they thought that the dot motion stimulus had been presented. To establish participants’ abilities to accurately discriminate whether the suppressed random motion had been presented, we calculated their sensitivity (d′) (21). We found that, for both order conditions, d′ was not significantly different from 0 (individuals ranged from −0.02 to 0.62), indicating that participants were unable to distinguish trials in which the random motion was present from trials in which it was not [unsuppressed first: t(4) = 1.46, P = 0.22; mask first: t(4) = 1.63, P = 0.18]. This finding provides additional objective evidence that the dot motion stimuli were effectively suppressed during the mask presentation.
Sensory decision-making involves the gradual accumulation of information over time, which leads to higher accuracy for longer stimulus presentations (15, 22–24). In experiment 8, we manipulated the duration of the suppression period to see whether the benefits to accuracy observed in the coherent condition were caused by a similar accumulation of evidence over time. Unconscious random or coherent motion was presented for 100–500 ms followed by visible coherent motion (200 ms). We found that there was a significant difference in accuracy between the coherent and random conditions [F(1,7) = 12.77, P = 0.009] (Fig. 4B), and although accuracy in the random condition remained constant across durations [F(2,14) = 0.28, P = 0.76], accuracy in the coherent condition increased with longer unconscious presentation times [F(2,14) = 3.91, P = 0.045]. Furthermore, the difference in accuracy between the coherent and random conditions increased with longer durations [F(2,14) = 2.73, P = 0.02]. This finding suggests that more information was accumulated during longer durations, although that information was outside conscious awareness. When we reversed the presentation order, we did not find the same pattern of improvement with longer durations (experiment 9) (Fig. S2).
Previous studies have uncovered a close relationship between decision confidence and accuracy (reviewed in ref. 25). For example, Kiani and Shadlen (26) found that, for nonhuman primates, confidence, like accuracy, was directly proportional to the quality and amount of evidence available in noisy visual displays. In other words, conscious decisions are typically accompanied by good metacognition. If unconscious decisional information alters confidence, we would expect to find more consistent metacognition in the coherent condition than in the random condition, because the drift rate (and consequently, accuracy) is substantially higher in the former (Fig. 2D). We tested this hypothesis in experiment 10, where we presented visible coherent motion (250 ms) followed by either suppressed coherent or random motion (250 ms). In addition to making their direction and visibility responses, participants were asked to rate how confident they were on a scale from one to four. Results indicated that, although accuracy was again higher in the coherent than in the random condition [F(1,5) = 22.15, P = 0.005] (Fig. S3), participants did not report feeling any more confident (Fig. 4C). These data suggest that participants were unaware of their better performance in the coherent condition.
Metacognition was assessed at the individual level for each condition by calculating the difference between participants’ meta-d′ and d′ performance in accordance with the work by Maniscalco and Lau (27) (SI Experimental Procedures). This meta-d′ score ensures that metacognitive scores are not confounded by variation in objective or subjective decision criteria and provides a measurement of metacognition that is in the same units as the standard signal detection measure of d′, allowing us to directly compare them. If meta-d′ = d′, it would indicate that participants are rating their confidence using all of the available sensory evidence. If meta-d′ < d′, it means that some of the signal that was available for processing during the motion discrimination task was lost for metacognition.
One participant was excluded from this analysis because he or she did not use the full scale of confidence ratings. As shown in Fig. 4D, meta-d′ scores did not significantly differ between the coherent (M = 0.65, SD = 0.22) and random (M = 0.65, SD = 0.25) conditions [F(1,3) = 0, P = 0.99], whereas the difference score (meta-d′ − d′) was significantly larger in the coherent condition (M = −0.65, SD = 0.17) than in the random condition [M = −0.35, SD = 0.26; F(1,3) = 24.02, P = 0.02]. This result indicates that metacognitive sensitivity was greater in the random condition; more of the available motion signal was used when making the confidence rating in the random condition. Because the amount of visible information was the same in both conditions, this finding suggests that participants were not using all of the information accumulated outside of awareness for their confidence judgments. This result poses a challenge to existing models of confidence based on the diffusion model (28, 29). Furthermore, this lack of metacognitive insight suggests that conscious awareness during the accumulation process is necessary for the accurate evaluation of one’s own state of knowledge.
In summary, our results provide evidence that we are able to process and accumulate information in the absence of conscious awareness and use it to improve or diminish decision accuracy. The role of unconscious decisional information can be modeled by existing evidence accumulation models, with the assumption that unconscious information carries less decisional evidence than conscious information. We show that this information can only be accessed and used when it is integrated with consciously processed decision-relevant information. We further present the intriguing finding that we are not aware that we possess this information or that it has affected our decisions. These findings contradict previous research proposing the superiority of unattended information processing and decision-making (1–4), because we show that the rate with which we are able to process information unconsciously is limited compared with conscious deliberation. Nevertheless, we show that information processed outside of awareness can be effectively used to substantially increase (or decrease) decision accuracy without us even realizing it.
Methods
Participants consisted of experienced psychophysical observers with normal or corrected-to-normal vision. Informed written consent was obtained from all participants, and all experiments were approved by the University of New South Wales Human Research Ethics Advisory Panel.
Participants were seated on a height-adjustable chair at a distance of 42 cm from a 20-in SONY Multiscan G520 CRT monitor with a resolution 1,280 × 960 and a refresh rate of 75 Hz. Participants’ heads were stabilized by a chin and headrest housing a mirror stereoscope apparatus adjusted for each observer so that the stimuli presented to each eye overlapped to form visual rivalry. This apparatus uses circular mirrors to display images separately to each eye, which overlap one another to form a single image when viewed binocularly. Stimuli were presented using Psychtoolbox, version 3 (30) for MATLAB on a Macintosh MacPro machine running Mac OSX.
The motion stimuli used in this study were dynamic random dot motion (RDM) displays commonly used in research in perceptual decision-making (reviewed in ref. 12). The RDM consisted of 100 gray dots (10.1 cd/m2), each a 1 × 1-pixel square, moving at a speed of 6.1° per second on a black background. In each trial, the direction of motion was randomly chosen from a pool of an equal number of leftward and rightward directions. Three uncorrelated sequences of dot movement were generated, and frames were interleaved so that, for example, the positions of the dots in frame 4 were correlated only with the dots in frames 1 and/or 7 and none of the other frames. That is, each frame was correlated only with a frame that was either three frames backward or forward and not the subsequent frame (24, 31).
Dots were displayed within an invisible 8.2°-diameter circular aperture with a central 0.7°-diameter fixation point. Participants were instructed to maintain fixation on this point throughout the experiments to facilitate fusion. On average, dot density was 1.9 dots per degree2, and to conserve dot density, any of the signal dots that moved along a trajectory that would place them outside of the circular aperture were wrapped around to appear from the opposite side, thus ensuring that motion energy was uniform across the different levels of motion coherence.
In each trial, the motion strength (the fraction of the dots that moved coherently in one of two possible directions) was randomly selected from a pool of possible coherence values. The range of coherence levels was determined during pilot testing and chosen to span the range of behavioral ability. The direction of coherently moving dots was chosen randomly from a pool of equal numbers of leftward and rightward directions on each trial.
The mask stimulus consisted of 250 green dots (59.5 cd/m2), each a 1 × 1 pixel. Dots were displayed within an invisible 9.8°-diameter circular aperture around a central 0.7°-diameter fixation point, with an average dot density of 3.3 dots per degree2. The dots moved clockwise at a speed of 6.1° per second in a circular motion path around the fixation point. For individual experiment parameters see SI Experimental Procedures.
Modeling
The model that we fit to data was an adaptation of the standard Wiener diffusion model (32). Evidence is assumed to accumulate noisily toward one of two boundaries corresponding to the available responses (left and right in our experiments). A response is triggered when the evidence reaches the respective boundary, and response time is the time taken for the decision plus nondecision time, which incorporates time to make a motor response and encode the stimulus (defined as a normal distribution with mean Ter and SD STer). Evidence begins at the midpoint between the two boundaries (given values of a and 0) and accumulates with a mean rate of v but normally distributed noise (with an SD of s fixed at a value of 0.1).
We assumed that the coherence of the random dot motion would be linearly related to the accumulation rate v in the model, and therefore, the drift rate for the ith coherence condition was vi = β0 + β1Coh, where Coh is the percentage coherence value. Furthermore, we assumed that, when stimuli were presented outside of awareness, the rate and variability of evidence accumulation would be reduced by a factor, K. As such, when the unconscious stimulus was coherent, evidence accumulated with mean rate Kvi and SD Ks. However, when the unconscious evidence was random, the lack of objective evidence meant that the accumulation rate was zero, but the noise in the process was still reduced to Ks.
We fit the model to the aggregate across participants of choice probabilities and distribution of correct and incorrect response times (summarized by the 0.1, 0.3, 0.5, 0.7, and 0.9 quantiles) by minimizing a χ2 statistic (33). Model predictions were generated by simulation using the Euler method (34). There were six free parameters in the model (a, Ter, β0, β1, STer, and K), and they were estimated using differential evolution optimization (35). Table 1 contains the parameter estimates of the model for each of the three experiments.
Supplementary Material
Acknowledgments
This work was supported by Australian National Health and Medical Research Council Project Grants APP1024800 and APP1046198 (to J.P.); Career Development Fellowship APP1049596 (to J.P.); and Australian Research Council Discovery Projects DP140101560 (to J.P.), DP130100124, and DE130100129 (both to C.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403619111/-/DCSupplemental.
References
- 1.Dijksterhuis A. Think different: The merits of unconscious thought in preference development and decision making. J Pers Soc Psychol. 2004;87(5):586–598. doi: 10.1037/0022-3514.87.5.586. [DOI] [PubMed] [Google Scholar]
- 2.Dijksterhuis A, van Olden Z. On the benefits of thinking unconsciously: Unconscious thought can increase post-choice satisfaction. J Exp Soc Psychol. 2006;42(5):627–631. [Google Scholar]
- 3.Nordgren LF, Dijksterhuis A. The devil is in the deliberation: Thinking too much reduces preference consistency. J Consum Res. 2009;36(1):39–46. [Google Scholar]
- 4.Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. On making the right choice: The deliberation-without-attention effect. Science. 2006;311(5763):1005–1007. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- 5.Dijksterhuis A, Nordgren LF. A theory of unconscious thought. Perspect Psychol Sci. 2006;1(2):95–109. doi: 10.1111/j.1745-6916.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 6.Newell BR, Wong KY, Cheung JCH, Rakow T. Think, blink or sleep on it? The impact of modes of thought on complex decision making. Q J Exp Psychol (Hove) 2009;62(4):707–732. doi: 10.1080/17470210802215202. [DOI] [PubMed] [Google Scholar]
- 7.Newell BR, Rakow T. Revising beliefs about the merit of unconscious thought: Evidence in favor of the null hypothesis. Soc Cogn. 2011;29(6):711–726. [Google Scholar]
- 8.Acker F. New findings on unconscious versus conscious thought in decision making: Additional empirical data and meta-analysis. Judgm Decis Mak. 2008;3(4):292–303. [Google Scholar]
- 9.Calvillo DP, Penaloza A. Are complex decisions better left to the unconscious? Further failed replications of the deliberation-without-attention effect. Judgm Decis Mak. 2009;4(6):509–517. [Google Scholar]
- 10.Rey A, Goldstein RM, Perruchet P. Does unconscious thought improve complex decision making? Psychol Res. 2009;73(3):372–379. doi: 10.1007/s00426-008-0156-4. [DOI] [PubMed] [Google Scholar]
- 11.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 12.Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27(3):161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Smith PL, Vickers D. The accumulator model of two-choice discrimination. J Math Psychol. 1988;32(2):135–168. [Google Scholar]
- 14.Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychol Sci. 1998;9(5):347–356. [Google Scholar]
- 15.Ratcliff R, McKoon G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Comput. 2008;20(4):873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461(7261):263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahnev DA, Huang E, Lau H. Subliminal stimuli in the near absence of attention influence top-down cognitive control. Atten Percept Psychophys. 2012;74(3):521–532. doi: 10.3758/s13414-011-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blake R, Ahlström U, Alais D. Perceptual priming by invisible motion. Psychol Sci. 1999;10(2):145–150. [Google Scholar]
- 19.Vlassova A, Pearson J. Look before you leap: Sensory memory improves decision making. Psychol Sci. 2013;24(9):1635–1643. doi: 10.1177/0956797612474321. [DOI] [PubMed] [Google Scholar]
- 20.Roseboom W, Arnold DH. Learning to reach for ‘invisible’ visual input. Curr Biol. 2011;21(13):R493–R494. doi: 10.1016/j.cub.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 22.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: A comparison of neuronal and psychophysical performance. J Neurosci. 1992;12(12):4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newsome WT. The King Solomon Lectures in Neuroethology. Deciding about motion: Linking perception to action. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1997;181(1):5–12. doi: 10.1007/s003590050087. [DOI] [PubMed] [Google Scholar]
- 24.Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22(21):9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung N, Summerfield C. Metacognition in human decision-making: Confidence and error monitoring. Philos Trans R Soc Lond B Biol Sci. 2012;367(1594):1310–1321. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324(5928):759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cogn. 2012;21(1):422–430. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Pleskac TJ, Busemeyer JR. Two-stage dynamic signal detection: A theory of choice, decision time, and confidence. Psychol Rev. 2010;117(3):864–901. doi: 10.1037/a0019737. [DOI] [PubMed] [Google Scholar]
- 29.Ratcliff R, Starns JJ. Modeling confidence and response time in recognition memory. Psychol Rev. 2009;116(1):59–83. doi: 10.1037/a0014086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 31.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86(4):1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 32.Stone M. Models for choice-reaction time. Psychometrika. 1960;25(3):251–260. [Google Scholar]
- 33.Ratcliff R, Tuerlinckx F. Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychon Bull Rev. 2002;9(3):438–481. doi: 10.3758/bf03196302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown SD, Ratcliff R, Smith PL. Evaluating methods for approximating stochastic differential equations. J Math Psychol. 2006;50(4):402–410. doi: 10.1016/j.jmp.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullen K, Ardia D, Gil D, Windover D, Cline J. DEoptim: An R package for global optimization by differential evolution. J Stat Softw. 2011;40(6):1–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.