Significance
Hepatosteatosis or nonalcoholic fatty liver disease (NAFLD) is an important health problem affecting approximately 20% of the population of the United States and is associated with cardiovascular risk factors such as insulin resistance and obesity. Using a Delta-like homologue (Dlk1) [also known as preadipocyte factor 1 (Pref1)] transgenic model, we identified a new player in the growth hormone (GH) signaling pathway that, when overexpressed, is protective against obesity and hepatosteatosis. Because the dosage of circulating DLK1 is naturally elevated in early life and during pregnancy, we believe that our transgenic model mimics endogenous mechanisms of DLK1-mediated GH signaling modulation that are used during periods of metabolic stress to protect from steatosis and alter fuel utilization in the whole organism.
Keywords: Delta-like homologue 1, hepatosteatosis, genomic imprinting, growth hormone, adipose tissue
Abstract
Nonalcoholic fatty liver disease (NAFLD) is associated with insulin resistance and obesity, as well as progressive liver dysfunction. Recent animal studies have underscored the importance of hepatic growth hormone (GH) signaling in the development of NAFLD. The imprinted Delta-like homolog 1 (Dlk1)/preadipocyte factor 1 (Pref1) gene encodes a complex protein producing both circulating and membrane-tethered isoforms whose expression dosage is functionally important because even modest elevation during embryogenesis causes lethality. DLK1 is up-regulated during embryogenesis, during suckling, and in the mother during pregnancy. We investigated the normal role for elevated DLK1 dosage by overexpressing Dlk1 from endogenous control elements. This increased DLK1 dosage caused improved glucose tolerance with no primary defect in adipose tissue expansion even under extreme metabolic stress. Rather, Dlk1 overexpression caused reduced fat stores, pituitary insulin-like growth factor 1 (IGF1) resistance, and a defect in feedback regulation of GH. Increased circulatory GH culminated in a switch in whole body fuel metabolism and a reduction in hepatic steatosis. We propose that the function of DLK1 is to shift the metabolic mode of the organism toward peripheral lipid oxidation and away from lipid storage, thus mediating important physiological adaptations associated with early life and with implications for metabolic disease resistance.
Hepatosteatosis or nonalcoholic fatty liver disease (NAFLD) is the accumulation of hepatic triglyceride (TAG) and results from excessive fatty-acid (FA) synthesis relative to oxidative clearance and/or elevated adipose tissue lipid hydrolysis (reviewed in ref. 1). FA synthesis, clearance, and release are regulated by neuroendocrine factors including GH, whose levels vary under conditions of changing energy supply (2). Recent animal studies have underscored the important role of hepatic GH signaling in the development of NAFLD and the metabolic syndrome (3–6).
The Delta-like homolog 1 (Dlk1) gene encodes a single pass membrane protein that is homologous to the Notch pathway ligand Delta but lacks canonical Notch interaction sequences (7–9). DLK1 was described simultaneously by several groups before it was identified as the product of a single gene (9–11), and, consequently, it is also referred to as fetal antigen 1 (FA1) and preadipocyte factor 1 (PREF1). Dlk1 encodes a complex protein that can produce both cleaved (circulating) and membrane-tethered isoforms, a result of alternative splicing (12). Consequences of Dlk1 overexpression have been explored previously in a limited isoform-specific and/or tissue-specific manner. For example, ectopic overproduction of the cleaved ectodomain of DLK1 causes a lipodystrophic phenotype associated with triglyceride deposition and glucose intolerance (13, 14). However, recent work has shown that tightly regulated dosage control of both forms is crucial for its biological function (15–17).
The expression of Dlk1 is highly dynamic. Indeed, as an imprinted gene, Dlk1 is subject to multiple levels of epigenetic dosage control beyond conventional mechanisms of tissue- and temporal-specific regulation (7, 8). The expression dosage of Dlk1 is functionally important because even modest elevation of normal Dlk1 transcription during embryogenesis is incompatible with the completion of intrauterine development (18), and modulation of expression in a stem-cell niche can profoundly alter the self-enewal properties of cells (16). Importantly, circulating and membrane-tethered DLK1 levels are normally physiologically up-regulated during embryogenesis (19), during suckling (20, 21), and in the mother during pregnancy (20). The normal function of this dynamic DLK1 dosage modulation during these periods is not known.
Here, we show that, in contrast to previous Dlk1 tissue and splice form overexpression models (13, 14), Dlk1 overexpression from endogenous control elements causes improved whole-body glucose homeostasis with no primary defect in adipose-tissue expansion, even under extreme metabolic stress. This surprising finding challenges the current dogma that the normal function of DLK1 is to negatively regulate adipogenesis. Moreover, in both a genetic and a dietary model of positive energy balance, DLK1-overexpressing mice were resistant to steatosis, primarily due to a reduction of hepatic lipid synthesis and an increase in skeletal muscle lipid oxidation. Moreover, we found that DLK1-overexpressing mice had elevated production of pituitary growth hormone due to a local defect in IGF1 feedback. Because impaired GH signaling is known to cause steatosis by affecting pathways of hepatic lipid import and synthesis (3–5), as well affecting peripheral FA oxidation (22), we propose that increased dosage of DLK1 acts primarily on the GH axis to shift the metabolic mode toward FA oxidation, with overall beneficial effects on hepatic lipid deposition.
Results
A 70-kb Transgene Overexpresses Dlk1 in an Authentic Isoform-Specific and Spatiotemporal Manner.
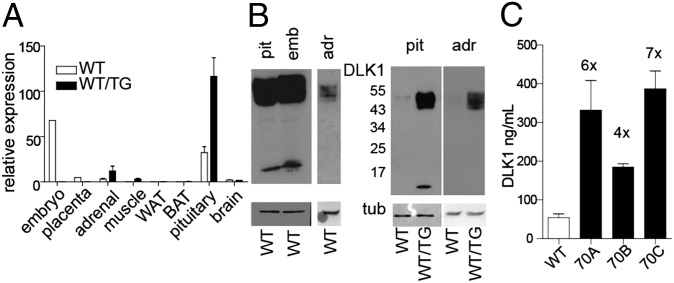
We used a BAC transgenic model containing the Dlk1 gene within 70 kb of the surrounding sequence and generated three independent transgenic lines (70A, 70B, and 70C) (18). Postnatally, Dlk1 is expressed in the adrenal gland (23), the somatotrophs of the pituitary (24), in a small number of cells in the brain (25), and in stromal-vascular cell populations associated with muscle and adipose tissues (11, 26, 27). Dlk1-transgenic hemizygous animals (WT/TG) recapitulated this postnatal restriction of Dlk1 expression, (Fig. 1 A and B and Fig. S1). Moreover, the proportion of transcripts encoding cleavable (A and B) to membrane-tethered (C and D) DLK1 in adult organs was similar in tissues from transgenic mice compared with their WT littermates (Fig. S1). Finally, we measured the amount of cleaved DLK1 ectodomain in the serum of adult mice (Fig. 1C). We found that DLK1 levels were elevated in the three transgenic lines to a similar extent (185–387 ng/mL) and four- to sevenfold compared with the WT adults (∼54 ng/mL). This level is similar to that found in maternal serum in late pregnancy of rodents and humans (20, 28). We concluded that the presence of the transgene in the 70A to -C lines resulted in normally localized but elevated isoform and temporal-specific expression of Dlk1, with no ectopic expression.
Fig. 1.
A 70-kb transgene overexpresses Dlk1 in an authentic isoform-specific and spatiotemporal manner. (A) Real-time quantitative PCR (RT qPCR) of total Dlk1 transcripts in adult tissue. (B) Western blotting of an intracellular epitope of DLK1 in WT tissues (Left), ∼50 kDa both membrane-bound and cleaved ectodomain in pituitary (pit), whole e16 embryo (emb), and adrenal (adr); <20 kDa band is the membrane-bound remnant of cleavage. (Right) Relative protein expression between WT and WT/TG animals (70C line) in pit and adr samples (run on separate gels). (C) Serum levels of cleaved DLK1 ectodomain in adult mice from three transgenic lines and WT measured by ELISA (females at 6 mo of age, mean ± SEM). Numbers above the columns show concentrations relative to WT. WT, n = 10; 70A, n = 4; 70B, n = 3; 70C, n = 9.
Overexpression of Dlk1 Limits Accumulation of White Adipose Tissue, with No Evidence for Failure of Adipogenesis, and Improves Glucose Tolerance and Whole-Body Insulin Sensitivity.
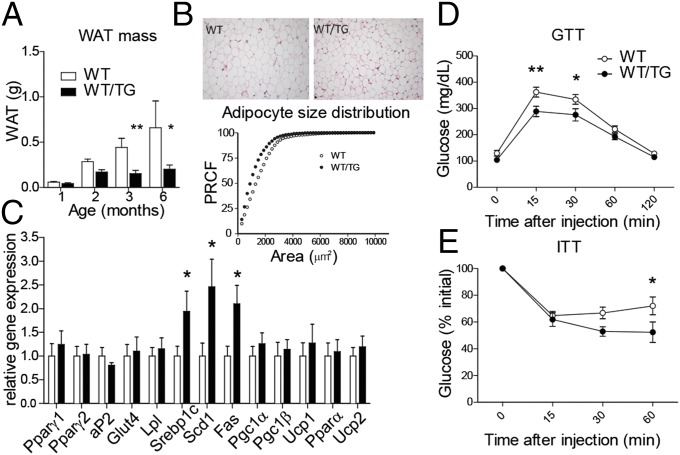
WT/TG mice accumulated white adipose tissue (WAT) at a slower rate than their WT littermates (Fig. 2A), and, at 6 mo of age, had significantly reduced gonadal, renal, and interscapular fat stores in both sexes (Fig. 2A and Table S1). WT/TG mice of both sexes maintained an ∼10% reduction in total body weight up to 1 y of age (Fig. S2A), and the reduction in body weight was attributable to a reduction in both lean and adipose mass, but not skeletal parameters such as length and bone density (Fig. S2B). The relative weight reduction was not due to reduced food intake (Fig. S2C).
Fig. 2.
Overexpression of Dlk1 limits accumulation of WAT, with no evidence for failure of adipogenesis, and improves glucose tolerance and whole-body insulin sensitivity. (A) Abdominal WAT accumulation in female mice from 1–6 mo of age 70B mice and WT littermates (n > 3 per time per genotype), P < 0.001 overall by one-way ANOVA, and at each time point by Bonferroni’s multiple comparison post hoc test, *P < 0.05, **P < 0.01. (B, Upper) Representative H&E staining of epididymal WAT from 26-wk-old mice. (Lower) Percent relative cumulative frequency (PRCF) analysis (PCRF, n = 4 mice per genotype). (C) RT-qPCR of WAT genes from abdominal WAT at 6 mo (data from 70B free-fed male mice), normalized to reference gene expression and then presented relative to WT = 1.0. WT and WT/TG, *P < 0.05 by Mann–Whitney U test, n = 6 per genotype, *P < 0.05. Glucose tolerance test (GTT) (D) and insulin tolerance test (ITT) (E) on 6-mo-old male mice from the 70B line. *P < 0.05 and **P < 0.01 by one-way ANOVA with Bonferroni’s post hoc test; WT, n = 13; WT/TG, n = 8. Values are mean ± SEM.
Histologically the adipose cell size in WT/TG animals was reduced, and the proportion of small fat cells was increased (Fig. 2B). Elevated levels of the cleaved ectodomain of DLK1 in vitro and in vivo has been correlated with reduced expression of the key adipogenic gene Pparγ (12, 13). Surprisingly we did not observe a reduction in either Pparγ1 or Pparγ2 expression in WAT from WT/TG animals compared with their WT littermates (Fig. 2C). Consistent with this finding, we found no evidence of impaired adipogenesis, such as reduced expression of markers of mature fat such as the fatty acid binding protein 4 (aP2), the insulin-sensitive glucose transporter (Glut4), or lipoprotein lipase (Lpl). Gene expression of markers associated with brown or beige fat (Pgc1α and Pgc1β, Ucp1), beta oxidation (Pparα), or uncoupling (Ucp2) were also not affected by Dlk1 overexpression. In contrast, the expression of genes normally positively regulated by insulin, sterol receptor binding protein 1c (Srebp1c), steroyl-coA desaturase (Scd1), and fatty acid synthase (Fas) were all increased in WT/TG animals (Fig. 2C). These gene expression changes were not secondary to elevated circulating insulin levels (Table S2), indicating that WT/TG adipose tissue may instead have increased insulin sensitivity. In addition, the reduction in circulating leptin levels observed in WT/TG animals (Table S2) was consistent with their reduced WAT stores but cannot account for the differences in the expression of lipogenic genes in WAT (29). WT/TG mice had normal circulating levels of adiponectin and resistin (Table S2), suggesting that the alterations we observed in insulin sensitivity were not secondary to altered levels of these adipokines.
We concluded that overexpression of Dlk1 in the context of its normal regulatory environment does not cause primary lipodystrophy because adipose differentiation pathways were not impaired, insulin sensitivity was increased, and there was no evidence of ectopic adipose deposition either in the liver or in skeletal muscle (Table S2). This finding was supported by an overall improvement in glucose tolerance and whole-body insulin sensitivity in WT/TG adults (Fig. 2 D and E). In the fed state, circulating lipid levels were not different between WT and WT/TG animals. However, in fasted animals, serum free-fatty acids (FFAs) and glycerol were elevated (Table S2), suggesting that, when insulin levels were low, the rate of WAT lipolysis was increased in WT/TG animals. An elevated rate of WAT lipolysis could therefore account for the reduced adipocyte cell and depot size that we observed in Dlk1-TG mice.
Dlk1-Overexpressing Mice Are Capable of Adipose Expansion and Are Resistant to Hepatosteatosis, on an Obesogenic Genetic Background.
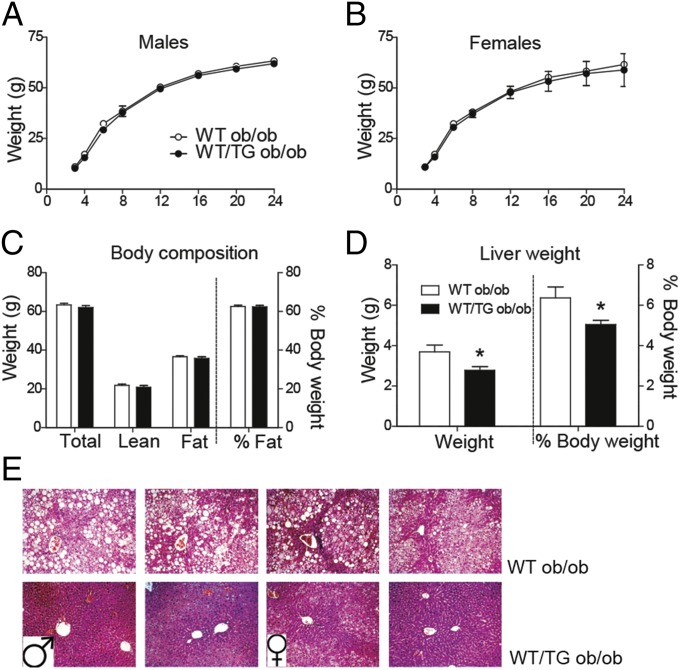
Overexpression of the cleaved form of DLK1 from an adipocyte- or hepatocyte-specific promoter has previously been shown to result in impaired fat expansion and misexpression of adipogenic genes, with reduced glucose tolerance and insulin resistance (13, 14). In contrast, our model of isoform and tissue-authentic overexpression did not show impairments in WAT adipogenic gene expression, and glucose homeostasis was improved. To test whether we had missed a defect in adipose expandability in the Dlk1-TG mice, we measured their body composition and other metabolic parameters in the context of extreme positive energy balance. We hypothesized that, if increased DLK1 from the transgene impairs adipose tissue expandability, WT/TG mice would have reduced WAT mass, and potentially increased ectopic lipid storage with associated impairments in glucose tolerance, compared with WT when crossed onto the hyperphagic ob/ob background. Surprisingly WT/TG ob/ob animals gained weight at the same rate as WT ob/ob littermates and, at 6 mo, had identical body composition (Fig. 3 A–C). Similar to WT ob/ob mice, WT/TG ob/ob mice were hyperglycemic and hyperinsulinemic, but there was no exacerbation of the glucose intolerance, as would be expected if overdose of DLK1 caused lipodystrophy (Table S3). We therefore concluded that overexpression of Dlk1 from endogenous control elements does not compromise adipose expandability in the face of extreme positive energy balance.
Fig. 3.
Dlk1-overexpressing mice are capable of adipose expansion and are resistant to hepatosteatosis, on an obesogenic genetic background. Body weights of WT ob/ob and WT/TG ob/ob mice from 3–24 wk of age, males (A), n ≥ 6 and females (B), n ≥ 5. (C) Body composition of male mice at 24 wk, n as in A. (D) Liver weight in WT/TG ob/ob mice compared with WT ob/ob mice as a total weight or % body weight, n ≥ 6 males. (E) H&E staining of representative liver sections from fasted male (four, Left) and female (four, Right) mice at 24 wk at 200× magnification. TAG deposition appears as circular “empty” droplets. Values are mean ± SEM, *P < 0.05 by Mann–Whitney U test.
Leptin is known to inhibit hepatic lipogenesis by suppression of Srepb1c, a transcriptional regulator of lipogenic genes (30). Alleviation of this Srebp1c suppression, in concert with hyperinsulinemia in ob/ob mice causes hepatosteatosis (31), which we observed in livers from WT ob/ob mice. Surprisingly, WT/TG mice did not develop hepatosteatosis. Instead, WT/TG ob/ob livers were less enlarged (Fig. 3D) and exhibited much reduced lipid deposition than WT ob/ob littermates. Furthermore, WT/TG ob/ob livers seemed healthy, with normal zonation, and we observed no evidence of fibrosis (Fig. 3E).
Elevated DLK1 Protects Against High Fat Diet-Induced Steatosis by Decreasing Hepatic Lipogenesis.
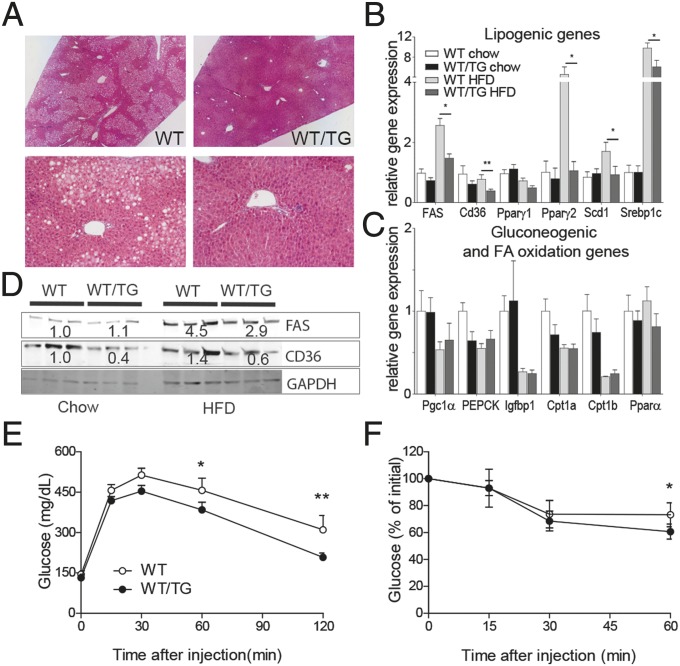
To explore the protective role of DLK1 in hepatosteatosis further, male TG mice and WT littermates were fed a 45% fat diet [high-fat diet (HFD)] from 4 wk to 24 wk. At 6 mo of age, WT/TG mice were significantly smaller than WT due to a reduction in both lean and adipose mass (Table S4). The HFD regimen led to fasting hyperglycemia, hyperinsulinemia (Table S5), and marked steatosis in the livers of WT mice (Fig. 4A), with associated elevation of lipogenic gene expression compared with chow-fed mice (Fig. 4B). Although the WT/TG mice on the HFD developed similarly raised levels of fasting glucose and insulin (Table S5), the liver was devoid of large lipid droplets (Fig. 4A), and the total TAG content was significantly reduced (WT, 210 ± 50 mg/g tissue; WT/TG, 89 ± 12 mg/g tissue, n = 7 per genotype, P = 0.026, Mann–Whitney U test). Serum levels of alanine aminotransferase (ALT), a marker of hepatocyte damage (Table S5), in WT/TG were low compared with the high levels observed in WT animals. WT/TG mice on a HFD had reduced expression of the lipid transporter Cd36 and of lipogenic pathway genes. Consistently, levels of CD36 and FAS protein were also reduced (Fig. 4C). Importantly, Pparγ2 was refractory to induction by the HFD (Fig. 4B), indicating that DLK1 inhibits hepatic triglyceride deposition by preventing induction of the de novo lipogenesis pathway.
Fig. 4.
Elevated DLK1 protects against diet-induced steatosis by decreasing hepatic lipogenesis. (A) H&E staining of representative sections from fasted male mice at 24 wk fed on a HFD, at 40× and 200× magnification. (B and C) RT-qPCR of liver genes from WT and WT/TG animals fed a chow or HFD at 24 wk (males, fasted for 16 h), relative to WT chow = 1.0, mean ± SEM, n > 7 per genotype. *P < 0.05, **P < 0.01 by Mann–Whitney U test. (D) Western blotting of liver lysates from mice in B. Mean normalized values are shown as text. GTT (E) and ITT (F) on 6-mo-old male mice on a HFD. WT/TG was compared with WT littermates by one-way ANOVA with Bonferroni’s post hoc test. WT, n = 9; WT/TG, n = 8. *P < 0.05, **P < 0.01. Error bars show SEM.
The effect of DLK1 overexpression was specific to the lipogenic transcriptional response because we did not see a difference between WT and WT/TG animals in the expression levels of genes in the gluconeogenic (Pgc1α, PEPCK, and Igfbp1) or lipid oxidation pathways (Cpt1α and -1β, Pparα). Furthermore, these genes responded appropriately to elevated insulin in both genotypes (Fig. 4D). The proximal insulin-signaling pathway did not seem to be affected by elevated DLK1 because insulin stimulation led to equal levels of AKT phosphorylation in both WT and WT/TG livers (Fig. S3). Finally, elevated DLK1 was associated with improved glucose tolerance and whole-body insulin sensitivity on a HFD (Fig. 4 E and F), which was not secondary to alterations in circulating adipokine levels (Table S5). Together these findings indicate that DLK1 acts specifically on the lipogenesis pathway without impairment of hepatic insulin sensitivity or altered adipokine production.
Increasing DLK1 Dosage Causes Increased Pituitary GH Secretion.
Hepatic GH signaling can protect the liver from steatosis (3–6). Depletion of hepatic GH signaling is thought to cause accumulation of triglycerides in the liver in two ways. Firstly, ablation of GH signaling in the liver itself prevents suppression of the fatty acid translocase Cd36, and Pparγ, resulting in increased FA import and biosynthesis (3–5). Secondly, ablation of GH-stimulated hepatic IGF1 release causes elevated circulating GH that induces expression of adipose tissue lipases, adipose tissue lipid mobilization, and lipid flux to the liver (5).
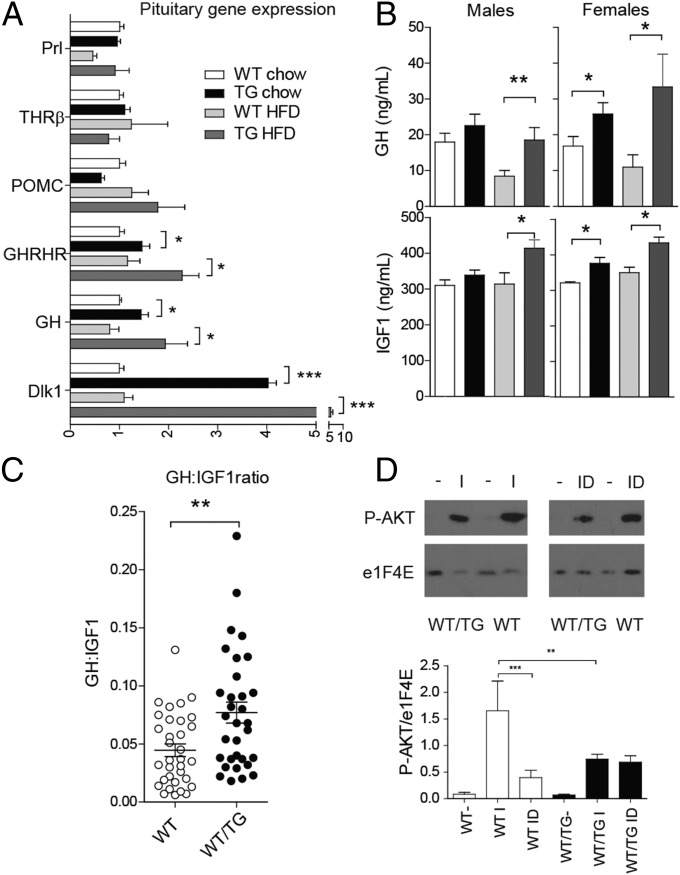
Because Dlk1 is expressed at very high levels in pituitary somatotrophs (Fig. 1) (24), we asked whether the GH axis was modified in WT/TG mice. In WT/TG mice, GH gene expression was elevated in whole anterior glands from chow-fed mice and those fed a HFD (Fig. 5A). Another somatotroph marker, the growth hormone-releasing hormone receptor (GHRHR) also had elevated expression, but no consistent change in markers for other pituitary cell types, including thyrotrophs [thyroid hormone receptor beta (THRβ)], lactotrophs (Prolactin), or corticotrophs [(Pro-opiomelanocortin (POMC)], was observed (Fig. 5A). HFD-fed WT/TG male mice also had elevated serum GH, with a trend for increased circulating GH in chow-fed males, and serum GH was elevated in females (Fig. 5B). Other neuroendocrine axes were not perturbed in WT/TG mice under unstimulated conditions (Table S6).
Fig. 5.
Dlk1-overexpressing mice have elevated pituitary growth hormone secretion as a result of local resistance to IGF1 feedback. (A) RT-qPCR of anterior pituitary genes in WT and WT/TG animals fed a chow or HFD at 24 wk (fasted for 16 h, 70C mice), relative to WT chow = 1.0. WT and WT/TG, n = 7 per genotype. Prl, prolactin. (B) Serum GH (Upper) and IGF1 (Lower) in 16-h fasted chow and HFD-fed mice, measured between 1000 hours and 1200 hours, n = 6–15 per sex per genotype. *P < 0.05, **P < 0.01 by Student's t-test. (C) Ratio of GH:IGF1 levels from all WT and WT/TG mice above (n = 33 WT and 32 WT/TG, males and females combined because no significant difference was found between them). **P < 0.01 by Mann–Whitney U test. (D) Western blot of AKT phosphorylation after IGF (I) or IGF1 plus DLK1 (ID) stimulation of pituitary explants from WT and WT/TG mice (Upper). The level of phosphorylation was normalised to total protein levels and charted (Lower), compared by one-way ANOVA with Bonferroni’s multiple comparison test, **P < 0.01, ***P < 0.001.
Circulating IGF1 levels tracked GH serum levels (Fig. 5B), indicating that the liver responded appropriately to elevated GH by increasing IGF1 secretion. The protection from hepatosteatosis observed in WT/TG mice is therefore consistent with the elevated circulating GH acting on the liver to suppress fatty-acid translocation and lipogenesis.
Raised IGF1 normally feeds back centrally to inhibit GH release, maintaining homeostasis of the GH axis. To test whether DLK1 overexpression was causing resistance to IGF1 feedback, we generated a proxy measure of IGF1 sensitivity by dividing circulating GH values by circulating IGF1 levels. We found that WT/TG mice had an elevated GH:IGF1 ratio, consistent with IGF1 resistance (Fig. 5C). To investigate this mechanism further, we examined the proximal signaling events in the IGF1 pathway. Whole pituitary explants were isolated from adult mice, incubated without serum, and then stimulated with IGF1. In WT mice, IGF1 stimulation caused the expected robust phosphorylation of AKT, which was inhibited when the explants were also treated with the ectodomain of DLK1 (Fig. 5D). In pituitary explants derived from WT/TG mice, which express high levels of membrane-bound DLK1 (Fig. 1D), IGF1 stimulation caused an attenuated AKT phosphorylation signal, and the addition of the DLK1 ectodomain did not suppress signal transduction further (Fig. 5D). These data demonstrate that both local and secreted DLK1 are able to inhibit IGF1 signaling and cause elevated GH expression due to a defect in negative feedback control.
Muscle Fuel Use and Energy Expenditure Are Altered in Dlk1-Overexpressing Mice.
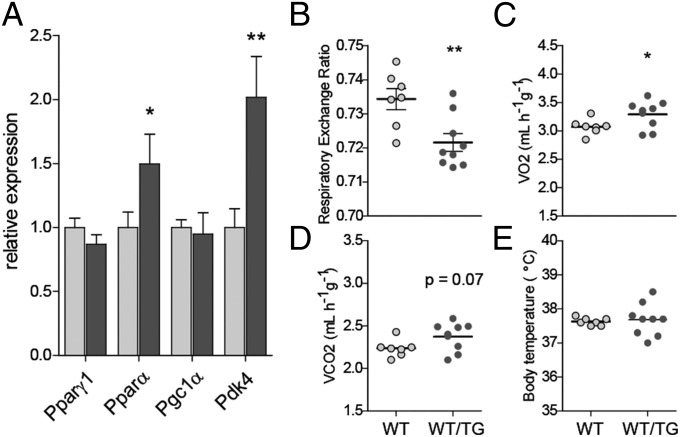
During periods of fasting, FFAs are used as fuel particularly by the muscles. Elevated circulating GH levels in adults are promoted by fasting and cause a switch of fuel use in peripheral tissues from carbohydrates to fats (2), in part by regulating Pdk4 (22, 32). Pdk4 encodes pyruvate dehydrogenase complex (PDC) kinase, a negative regulator of PDC that regulates the entry of glycolytic products into the tricarboxylic acid cycle. We found that transcription of Pdk4 was elevated in WT/TG muscle, consistent with increased GH and elevated skeletal FA oxidation (Fig. 6A). Consistently, when HFD mice were evaluated by indirect calorimetry after an overnight fast, WT/TG mice had the expected decrease in respiratory exchange ratio (Fig. 6B), indicating an increase in lipid oxidation for energy production. We also observed increased oxygen consumption (Fig. 6C), indicative of increased energy expenditure. We saw no differences in body temperature between the two groups (Fig. 6E), and UCP1 expression in brown adipose tissue (BAT) was not significantly altered between the two genotypes (Fig. S4). We concluded that the phenotype of DLK1-overexpressing mice was consistent with a shift in fuel use toward muscle fatty acid oxidation, due to elevated circulating GH.
Fig. 6.
Muscle fuel use and energy expenditure are altered in Dlk1-overexpressing mice. (A) RT qPCR of muscle genes in WT and WT/TG animals fed a HFD at 24 wk, measured by WT/TG (70C) male mice fasted for 16 h, n = 7 per genotype, on mixed soleus/gastrocnemius muscle. (B–D) Indirect calorimetry on mice fed a HFD, average respiratory exchange ratio (RER) (B), oxygen consumption (C), and CO2 production (D) after a 16-h fast in WT and WT/TG mice, presented per gram body mass. n = 7 WT and 9 WT/TG. (E) Body temperature evaluation performed on mice in B–D. Values are mean ± SEM. All comparisons between WT and WT/TG (A–E) were performed by Mann-Whitney U test, *P < 0.05, **P < 0.01.
Discussion
In this study, we report for the first time, to our knowledge, that overexpression of Dlk1 from endogenous control elements can prevent hepatosteatosis by modulation of the growth hormone pathway. By generating an experimental model in which the normal expression pattern of the gene is preserved, but expression dosage was increased, we were able to demonstrate previously unidentified effects of this protein on whole-body energy homeostasis.
In Dlk1-TG mice, we did not observe a defect in adult adipocyte expansion in response to positive energy balance. This result was surprising because, in cell culture models, preadipocytes of both embryonic and adult origin are inhibited by treatment with the DLK1 ectodomain (12, 33). In addition, transgenic mice overexpressing soluble DLK1 from ectopic promoters have defective WAT differentiation, leading to a failure of WAT expansion, elevated serum lipids, and impaired glucose tolerance with insulin resistance (13, 14).
The 70-kb BAC transgenic mice differ from these previous models because the transgene recapitulates the endogenous spatiotemporal expression pattern of Dlk1, as well as causing overexpression of both ectodomain and membrane-tethered forms of the protein. Finally, quantification of the amount of circulating DLK1 was not reported for the previous models whereas we have shown that serum DLK1 levels are at four to seven times WT levels in our three independent transgenic lines. Comparison of the phenotypes of mice with elevated serum DLK1 (13, 14) to entopic overexpression of all isoforms (this study) further reinforces recent findings that correct localization and dosage of both membrane-tethered and soluble DLK1 are necessary for this important protein to produce its biological effects (16, 17).
Our results indicate that, in the context of positive energy balance, the dosage of DLK1 modifies well-established pathways of physiological adaptation that abrogate the toxic effects of excess nutrients. The protection from steatosis conferred by elevated DLK1 was apparent in both a dietary obesity model, and also after genetic ablation of leptin (ob/ob mice). The reduction in steatosis was not secondary to an alteration in WAT because leptin-deficient mice with or without the Dlk1-transgene had identical body composition, but TAG deposition in the liver was substantially reduced in the WT/TG ob/ob. Elevated DLK1 was protective against hepatosteatosis by reducing expression of hepatic lipid import and synthesis genes. In addition, muscles from Dlk1 transgenic animals had elevated expression of genes that promote fatty-acid oxidation. Together, these gene-expression changes caused a shift in whole body fuel utilization away from glucose and toward fatty acids via elevated GH signaling.
We found that elevated DLK1 caused increased expression of GH mRNA in the adult pituitary gland and that this rise was accompanied by increased circulating GH levels. Experiments in rat primary pituitary cell cultures conducted over 25 y ago demonstrated that IGF1 suppresses GH production by reducing its rate of transcription (34). More recently, direct evidence for IGF1 feedback to the pituitary was provided by a genetic model of ablated IGF1 signaling in somatotrophs (the SIGFKO mouse) (35). In this model, lack of IGF1 receptors in somatotrophs resulted in a two- to threefold increase in circulating GH (35). Consistent with these data, we demonstrated that isolated pituitary explants from DLK1-overexpressing mice were refractory to IGF1 signaling. The mechanism by which IGF1 signaling feeds back to GH transcription is not fully understood but is known to be dependent on the binding of POUF1/CREB binding protein (CBP) to the GH promoter (36). IGF1 treatment of pituitary cells reduces Pit1 transcription, thus reducing the bioavailability of POUF1 (37), and reduces CREB binding to the GH promoter by posttranscriptional mechanisms (36).
Several previous studies have implicated DLK1 in GH regulation (38–41), and also conversely GH in the regulation of DLK1 (42–44). Notably, a recent study reported a reduction in GH secretion after Dlk1 deletion (40). However, a somatotroph-specific deletion of Dlk1 did not alter steady-state GH transcription, consistent with extrapituitary signaling mechanisms being involved (39). Because we were able to demonstrate IGF1 resistance in isolated pituitary explants in the presence of elevated DLK1, we propose that it is under normal circumstances of high DLK1 dosage that this molecule is exerting its biological effect.
Increased GH production in Dlk1-TG mice caused some, but not all, of the expected phenotypes of enhanced GH signaling; as predicted, Dlk1-TG mice had reduced adipose tissue accumulation (45), had elevated expression of genes in the muscle that promote FA oxidation (22), and were protected from hepatosteatosis by reducing the expression of genes controlling lipid import (CD36) and synthesis (Srebp1c and Pparγ) (4). In contrast, the Dlk1-TG mice displayed moderately improved insulin sensitivity rather than the insulin resistance predicted by multiple models of GH signaling ablation (45). In addition, linear growth was not affected in our model. Recent experiments using conditional targeting of the GH receptor have highlighted that its concerted actions on multiple tissues are necessary for the full spectrum of GH action, and that timings of GH action are also crucial, especially to mediate its growth effects (46). Importantly, loss of GH signaling in adipose tissue affects body composition without alterations to glucose homeostasis or growth (46); and hepatic steatosis can occur in the absence of alterations to whole-body glucose homeostasis (4). We speculate that the moderate increase in GH in the Dlk1-TG mice is sufficient to modify fuel utilization by peripheral tissues without large changes to glucose homeostasis or growth. In support of this interpretation of our data, the reported phenotype of SIGFRKO mice is remarkably similar to that of the DLK1-overexpression model described here: they exhibit moderately elevated expression of GH and GHRHR mRNA, as well as circulating GH levels, and increased serum IGF1. Moreover, SIGFRKO mice have normal linear growth but reduced WAT deposition, and no impairment in glucose homeostasis (35). However, it is possible that DLK1 may have additional actions on fuel homeostasis that modify the diabetogenic effects of GH.
Our work highlights the possibility that selective pressures may be acting on Dlk1 to regulate its dosage during life-history periods when enhanced peripheral lipid oxidation is beneficial for survival: during embryogenesis, during suckling, and in the mother during pregnancy. These are all periods of life when lipogenesis may be inhibited and tissue FA oxidation promoted to spare scarce glucose for growth (47). We propose that the normal function of DLK1 is to modulate the GH axis to shift the metabolic mode of the organism toward peripheral FA oxidation and away from lipid storage. Therefore, disruption of DLK1 dosage may have important consequences for energy homeostasis and metabolic disease.
Experimental Procedures
Breeding of Transgenic Animals.
All experiments involving mice were carried out in accordance with UK Government Home Office licensing procedures. The generation of the Dlk1-TG BAC transgenic lines (70A, 70B, and 70C) was described in ref. 18. We crossed 70C hemizygous transgenic mice with heterozygous ob/+ (Lepob/Lep+) mice on a C57BL/6 background (29) to obtain mice hemizygous for the 70C transgene and homozygous for the leptin point mutation (+/70C_Lepob/Lepob), along with control littermates (+/+_Lepob/Lepob). Animals were housed at a density of three to four per cage in a temperature-controlled room (20–22 °C) with a 12-h light/dark cycle. Chow diet provided 11% energy from fats (SDS RM3, LBS), and the HFD provided 45% (D12451; Research Diets Inc.). Food and water were available ad libitum unless otherwise noted.
Body Composition, Indirect Calorimetry, and Body Temperature.
Dual-energy X-ray absorptiometry (DEXA; Lunar Corporation, www.lunarcorp.com) was used to measure body composition. Oxygen consumption was measured in 24-wk-old transgenic and WT mice from the HFD cohort after an overnight fast, by an OXYMAX System 4.93 indirect calorimeter (Columbus Instruments) as described previously (48). Body temperature values represent the mean of four rectal temperature measurements over a 2-wk interval between 1400 hours and 1600 hours with a TES-319 digital thermometer (TES Electronic Corp.).
Pituitary Explant Culture and Stimulations.
Whole pituitaries were dissected from 6-mo-old female mice into HBSS, cut in half, and then transferred to serum-free DMEM and incubated at 37 °C for 4 h with 5% CO2 in 48-well dishes. The medium was changed to fresh serum-free DMEM, and then IGF1 (50 nM; Sigma-Aldrich) alone or IGF1 plus DLK1 (100 ng/mL; R&D Systems) was added to one half of each pituitary. After 10 min, the explants were rinsed and snap frozen. Before gel electrophoresis for Western blotting, explants were completely homogenized in Laemmli buffer with DTT.
Statistical Analysis.
All statistical tests were performed using the GraphPad Prism Software, version 4.00 for Windows (GraphPad Software, www.graphpad.com).
Supplementary Material
Acknowledgments
We thank Jenny Corish, Mary Cleaton, Chris Angiolini, Maggie Dinsdale, and Helen Westby for technical assistance. Work was supported by grants from the Wellcome Trust; European Commission FP7, Epigenesis and Epihealth (to A.C.F.-S.); and UK Medical Research Council (to A.C.F.-S. and M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406119111/-/DCSupplemental.
References
- 1.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34(6 Pt 2):643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 2.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937–19944. doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barclay JL, et al. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152(1):181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]
- 5.Sos BC, et al. 2011. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J Clin Invest 121(4):1412–1423, and erratum (2011) 121:3360.
- 6.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148(6):2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 7.Takada S, et al. Delta-like and gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10(18):1135–1138. doi: 10.1016/s0960-9822(00)00704-1. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman SM. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14(16):1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 9.Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993;268(6):3817–3820. [PubMed] [Google Scholar]
- 10.Jensen CH, et al. Studies on the isolation, structural analysis and tissue localization of fetal antigen 1 and its relation to a human adrenal-specific cDNA, pG2. Hum Reprod. 1993;8(4):635–641. doi: 10.1093/oxfordjournals.humrep.a138110. [DOI] [PubMed] [Google Scholar]
- 11.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73(4):725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 12.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17(2):977–988. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111(4):453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villena JA, et al. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): A new model of partial lipodystrophy. Diabetes. 2008;57(12):3258–3266. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcés C, Ruiz-Hidalgo MJ, Bonvini E, Goldstein J, Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation. 1999;64(2):103–114. doi: 10.1046/j.1432-0436.1999.6420103.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrón SR, et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475(7356):381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortensen SB, et al. Membrane-tethered delta-like 1 homolog (DLK1) restricts adipose tissue size by inhibiting preadipocyte proliferation. Diabetes. 2012;61(11):2814–2822. doi: 10.2337/db12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rocha ST, et al. Gene dosage effects of the imprinted delta-like homologue 1 (dlk1/pref1) in development: Implications for the evolution of imprinting. PLoS Genet. 2009;5(2):e1000392. doi: 10.1371/journal.pgen.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Rocha ST, et al. Restricted co-expression of Dlk1 and the reciprocally imprinted non-coding RNA, Gtl2: Implications for cis-acting control. Dev Biol. 2007;306(2):810–823. doi: 10.1016/j.ydbio.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann E, Krogh TN, Højrup P, Skjødt K, Teisner B. Mouse fetal antigen 1 (mFA1), the circulating gene product of mdlk, pref-1 and SCP-1: Isolation, characterization and biology. J Reprod Fertil. 1996;107(2):279–285. doi: 10.1530/jrf.0.1070279. [DOI] [PubMed] [Google Scholar]
- 21.Charalambous M, et al. Imprinted gene dosage is critical for the transition to independent life. Cell Metab. 2012;15(2):209–221. doi: 10.1016/j.cmet.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijayakumar A, et al. Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS ONE. 2012;7(9):e44777. doi: 10.1371/journal.pone.0044777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halder SK, et al. Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology. 1998;139(7):3316–3328. doi: 10.1210/endo.139.7.6081. [DOI] [PubMed] [Google Scholar]
- 24.Larsen JB, et al. Fetal antigen 1 and growth hormone in pituitary somatotroph cells. Lancet. 1996;347(8995):191. doi: 10.1016/s0140-6736(96)90374-8. [DOI] [PubMed] [Google Scholar]
- 25.Jensen CH, et al. Neurons in the monoaminergic nuclei of the rat and human central nervous system express FA1/dlk. Neuroreport. 2001;12(18):3959–3963. doi: 10.1097/00001756-200112210-00021. [DOI] [PubMed] [Google Scholar]
- 26.Andersen DC, Jensen L, Schrøder HD, Jensen CH. “The preadipocyte factor” DLK1 marks adult mouse adipose tissue residing vascular cells that lack in vitro adipogenic differentiation potential. FEBS Lett. 2009;583(17):2947–2953. doi: 10.1016/j.febslet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Andersen DC, et al. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009;27(4):898–908. doi: 10.1634/stemcells.2008-0826. [DOI] [PubMed] [Google Scholar]
- 28.Floridon C, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66(1):49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 29.Medina-Gomez G, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3(4):e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakuma T, et al. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97(15):8536–8541. doi: 10.1073/pnas.97.15.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yahagi N, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277(22):19353–19357. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]
- 32.Jeong JY, Jeoung NH, Park KG, Lee IK. Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab J. 2012;36(5):328–335. doi: 10.4093/dmj.2012.36.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing K, et al. Expression regulation and function of Pref-1 during adipogenesis of human mesenchymal stem cells (MSCs) Biochim Biophys Acta. 2009;1791(8):816–826. doi: 10.1016/j.bbalip.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita S, Melmed S. Insulinlike growth factor I regulation of growth hormone gene transcription in primary rat pituitary cells. J Clin Invest. 1987;79(2):449–452. doi: 10.1172/JCI112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero CJ, et al. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol Endocrinol. 2010;24(5):1077–1089. doi: 10.1210/me.2009-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero CJ, et al. Insulin-like growth factor 1 mediates negative feedback to somatotroph GH expression via POU1F1/CREB binding protein interactions. Mol Cell Biol. 2012;32(21):4258–4269. doi: 10.1128/MCB.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo AI, Aranda A. Differential regulation of pituitary-specific gene expression by insulin-like growth factor 1 in rat pituitary GH4C1 and GH3 cells. Endocrinology. 1997;138(12):5442–5451. doi: 10.1210/endo.138.12.5585. [DOI] [PubMed] [Google Scholar]
- 38.Puertas-Avendaño RA, et al. Role of the non-canonical notch ligand delta-like protein 1 in hormone-producing cells of the adult male mouse pituitary. J Neuroendocrinol. 2011;23(9):849–859. doi: 10.1111/j.1365-2826.2011.02189.x. [DOI] [PubMed] [Google Scholar]
- 39.Appelbe OK, Yevtodiyenko A, Muniz-Talavera H, Schmidt JV. Conditional deletions refine the embryonic requirement for Dlk1. Mech Dev. 2013;130(2-3):143–159. doi: 10.1016/j.mod.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung LY, Rizzoti K, Lovell-Badge R, Le Tissier PR. Pituitary phenotypes of mice lacking the notch signalling ligand delta-like 1 homologue. J Neuroendocrinol. 2013;25(4):391–401. doi: 10.1111/jne.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansell PJ, et al. Regulation of growth hormone expression by Delta-like protein 1 (Dlk1) Mol Cell Endocrinol. 2007;271(1-2):55–63. doi: 10.1016/j.mce.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlsson C, et al. Growth hormone and prolactin stimulate the expression of rat preadipocyte factor-1/delta-like protein in pancreatic islets: Molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology. 1997;138(9):3940–3948. doi: 10.1210/endo.138.9.5408. [DOI] [PubMed] [Google Scholar]
- 43.Wolfrum C, et al. Role of Foxa-2 in adipocyte metabolism and differentiation. J Clin Invest. 2003;112(3):345–356. doi: 10.1172/JCI18698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdallah BM, et al. Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007;148(7):3111–3121. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 45.List EO, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32(3):356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.List EO, et al. The role of GH in adipose tissue: Lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000;16(3):202–210. doi: 10.1002/1520-7560(200005/06)16:3<202::aid-dmrr116>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Medina-Gomez G, et al. The link between nutritional status and insulin sensitivity is dependent on the adipocyte-specific peroxisome proliferator-activated receptor-gamma2 isoform. Diabetes. 2005;54(6):1706–1716. doi: 10.2337/diabetes.54.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.