Abstract
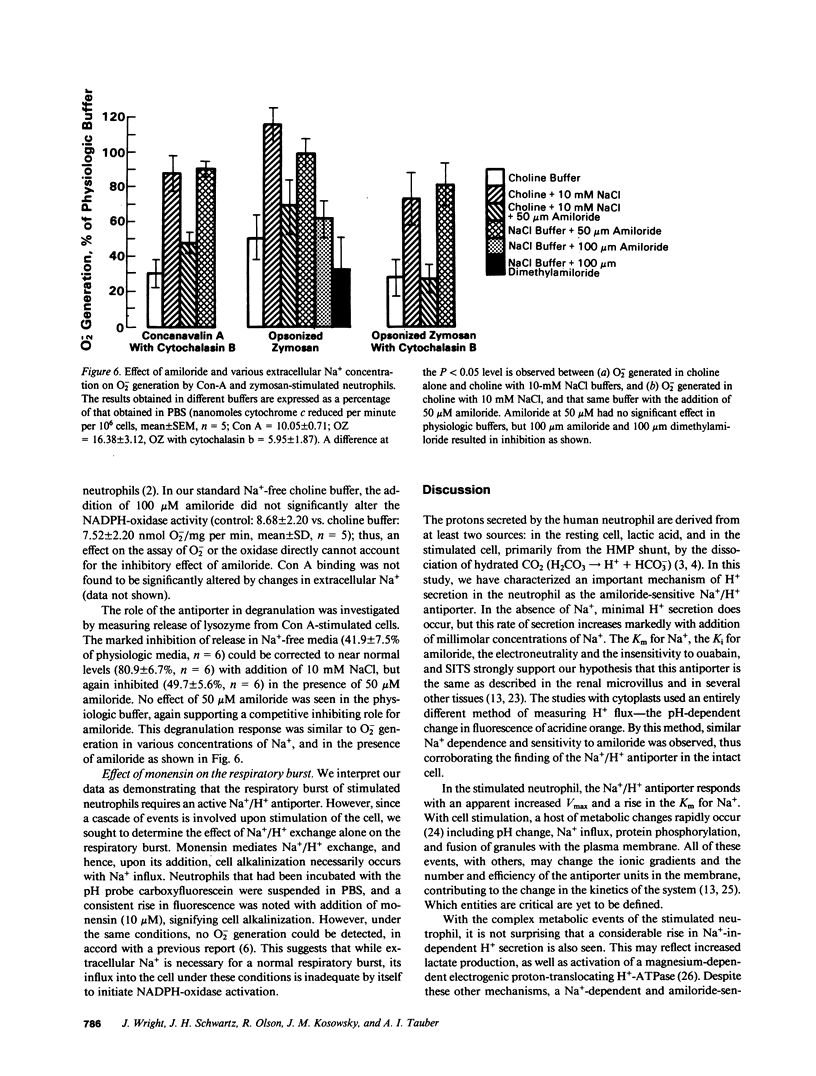
The reducing equivalents used by the human neutrophil respiratory burst oxidase are derived from NADPH generated by the hexose monophosphate shunt. The CO2 generated by the HMP shunt is spontaneously hydrated and the protons (H+) are secreted upon the dissociation of carbonic acid. The mechanism and significance of H+ secretion by the resting and stimulated neutrophil was investigated. A basal rate of H+ secretion by resting neutrophils observed in a choline buffer was augmented with the addition of sodium (Na+) (Km for Na+ was 3.22 +/- 0.32 mM). Amiloride, a Na+/H+ antiporter inhibitor, reduced H+ secretion in Na+-containing buffers with a Ki = 1.02 microM. This Na+/H+ exchange mechanism was also operative in cells stimulated with a variety of agonists, and an increased H+ flux, relative to resting cells, was observed at higher Na+ concentrations. Cytoplasts incorporating acridine orange were also used to assess Na+-H+ flux. Cytoplasts were used to avoid alteration of the fluorescent pH probe by HOCl formed in intact neutrophils. Alkalinization of the cytoplasm was dependent on extracellular Na+ in concentrations similar to that found to augment H+ secretion in intact cells. Also, amiloride competitively inhibited H+ secretion by the cytoplasts. Both superoxide (O2-) production and lysozyme release in cells stimulated with opsonized zymosan or concanavalin A was significantly inhibited in the absence of Na+, restored to normal with the addition of Na+ in low concentrations, and inhibited again in the presence of amiloride. A Na+/H+ antiporter similar to that found in other cell types is present in the human neutrophil and appears linked to activation of the respiratory burst and degranulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol. 1983 Dec;245(6):F647–F659. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Cuatrecasas P. Phorbol esters rapidly stimulate amiloride-sensitive Na+/H+ exchange in a human leukemic cell line. J Cell Biol. 1984 Jul;99(1 Pt 1):340–343. doi: 10.1083/jcb.99.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Busa W. B., Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984 Apr;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- De Togni P., Della Bianca V., Bellavite P., Grzeskowiak M., Rossi F. Studies on stimulus-response coupling in human neutrophils. II. Relationships between the effects of changes of external ionic composition on the properties of N-formylmethionylleucylphenylalanine receptors and on the respiratory and secretory responses. Biochim Biophys Acta. 1983 Feb 22;755(3):506–513. doi: 10.1016/0304-4165(83)90256-8. [DOI] [PubMed] [Google Scholar]
- Della Bianca V., Bellavite P., De Togni P., Fumarulo R., Rossi F. Studies on stimulus-response coupling in human neutrophils. I. Role of monovalent cations in the respiratory and secretory response to N-formylmethionylleucylphenylalanine. Biochim Biophys Acta. 1983 Feb 22;755(3):497–505. doi: 10.1016/0304-4165(83)90255-6. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Gomperts B. D. Ionophore monensin induces Na+-dependent secretion from rabbit neutrophils. requirement for intracellular Ca2+ stores. Biochim Biophys Acta. 1983 Oct 25;763(3):292–298. doi: 10.1016/0167-4889(83)90137-4. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Amiloride-sensitive Na+/H+ exchange in human neutrophils: mechanism of activation by chemotactic factors. Biochem Biophys Res Commun. 1984 Jul 31;122(2):755–762. doi: 10.1016/s0006-291x(84)80098-4. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Goetz J. D., Rothstein A. 22Na+ fluxes in thymic lymphocytes. II. Amiloride-sensitive Na+/H+ exchange pathway; reversibility of transport and asymmetry of the modifier site. J Gen Physiol. 1984 Oct;84(4):585–600. doi: 10.1085/jgp.84.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J. K., Albrich J. M., Green T. R., Rosen H., Klebanoff S. Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J Biol Chem. 1984 Apr 25;259(8):4812–4821. [PubMed] [Google Scholar]
- Ives H. E., Rector F. C., Jr Proton transport and cell function. J Clin Invest. 1984 Feb;73(2):285–290. doi: 10.1172/JCI111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass H. J., Hopkins J., Neale G., Peters T. J. The estimation of serum lysozyme: a comparison of four assay methods. Biochem Med. 1977 Aug;18(1):52–57. doi: 10.1016/0006-2944(77)90048-5. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Stimulus-response coupling in the human neutrophil. Transmembrane potential and the role of extracellular Na+. Biochim Biophys Acta. 1980 Sep 2;601(1):180–194. doi: 10.1016/0005-2736(80)90523-4. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- Mollinedo F., Schneider D. L. Subcellular localization of cytochrome b and ubiquinone in a tertiary granule of resting human neutrophils and evidence for a proton pump ATPase. J Biol Chem. 1984 Jun 10;259(11):7143–7150. [PubMed] [Google Scholar]
- Roos D., Voetman A. A., Meerhof L. J. Functional activity of enucleated human polymorphonuclear leukocytes. J Cell Biol. 1983 Aug;97(2):368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Anderson L., Swanson P. D. Inhibition of Na+-Ca2+ exchange in rat brain by amiloride. Mol Pharmacol. 1983 Sep;24(2):251–258. [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F., Naccache P. H. Chemotactic factors activate differentiable permeation pathways for sodium and calcium in rabbit neutrophils. Effect of amiloride. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1271–1276. doi: 10.1016/0006-291x(81)90757-9. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I., De Weer P. Sodium and potassium fluxes and membrane potential of human neutrophils: evidence for an electrogenic sodium pump. J Gen Physiol. 1982 Mar;79(3):453–479. doi: 10.1085/jgp.79.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Macara I. G., Levenson R., Housman D., Cantley L. Evidence that a Na+/Ca2+ antiport system regulates murine erythroleukemia cell differentiation. J Biol Chem. 1982 Jan 25;257(2):773–780. [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Vicentini L. M., Miller R. J., Villereal M. L. Evidence for a role of phospholipase activity in the serum stimulation of Na+ influx in human fibroblasts. J Biol Chem. 1984 Jun 10;259(11):6912–6919. [PubMed] [Google Scholar]
- Zerbe G. O., Archer P. G., Banchero N., Lechner A. J. On comparing regression lines with unequal slopes. Am J Physiol. 1982 Mar;242(3):R178–R180. doi: 10.1152/ajpregu.1982.242.3.R178. [DOI] [PubMed] [Google Scholar]
- van Zwieten R., Wever R., Hamers M. N., Weening R. S., Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981 Jul;68(1):310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]


