Abstract
For almost three decades in many countries azathioprine has been used to treat relapsing-remitting multiple sclerosis. However its efficacy was usually considered marginal and following approval of β interferons for this indication it was no longer recommended as first line treatment, even if presently no conclusive direct β interferon-azathioprine comparison exists. To compare azathioprine efficacy versus the currently available β interferons in relapsing-remitting multiple sclerosis, a multicenter, randomized, controlled, single-blinded, non-inferiority trial was conducted in 30 Italian multiple sclerosis centers. Eligible patients (relapsing-remitting course; ≥2 relapses in the last 2 years) were randomly assigned to azathioprine or β interferons. The primary outcome was annualized relapse rate ratio (RR) over 2 years. Key secondary outcome was number of new brain MRI lesions. Patients (n = 150) were randomized in 2 groups (77 azathioprine, 73 β interferons). At 2 years, clinical evaluation was completed in 127 patients (62 azathioprine, 65 β interferons). Annualized relapse rate was 0.26 (95% Confidence Interval, CI, 0.19–0.37) in the azathioprine and 0.39 (95% CI 0.30–0.51) in the interferon group. Non-inferiority analysis showed that azathioprine was at least as effective as β interferons (relapse RRAZA/IFN 0.67, one-sided 95% CI 0.96; p<0.01). MRI outcomes were analyzed in 97 patients (50 azathioprine and 47 β interferons). Annualized new T2 lesion rate was 0.76 (95% CI 0.61–0.95) in the azathioprine and 0.69 (95% CI 0.54–0.88) in the interferon group. Treatment discontinuations due to adverse events were higher (20.3% vs. 7.8%, p = 0.03) in the azathioprine than in the interferon group, and concentrated within the first months of treatment, whereas in the interferon group discontinuations occurred mainly during the second year.
The results of this study indicate that efficacy of azathioprine is not inferior to that of β interferons for patients with relapsing-remitting multiple sclerosis. Considering also the convenience of the oral administration, and the low cost for health service providers, azathioprine may represent an alternative to interferon treatment, while the different side effect profiles of both medications have to be taken into account.
Trial Registration
EudraCT 2006-004937-13
Introduction
For almost three decades azathioprine (AZA) has been used in many countries to treat relapsing-remitting multiple sclerosis (MS) based on placebo controlled randomized clinical trials (RCTs) [1]–[4]. Efficacy however was usually considered marginal [5], [6], and following approval of β interferons (IFNs) AZA was no longer recommended as first-line therapy [7]. Lack of MRI evaluation, methodological weaknesses and the low power of the trials may have fostered perception of the poor efficacy of AZA, whereas consistently efficacious and safe IFN trials in MS [8]–[11] have made IFN a drug of choice for this indication [7]. However, meta-analyses [12]–[14], new comparative RCTs [15], [16], and MRI results [17], [18] suggest a similar effect size of AZA in relapsing-remitting MS. Presently no conclusive direct IFN-AZA comparison exists. This paper documents an independent multicenter RCT evaluating the non-inferiority of the efficacy of AZA vs. IFNs on clinical and MRI measures of disease activity in relapsing-remitting MS.
Materials and Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Protocol S1, Amendment S1, and Checklist S1.
Ethics statement
This study was approved by ethics committees in the coordinating center (Careggi University Hospital, Ethic Committee, Florence) and in each of the participating centers (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano; Clinica Neurologica, Novara; Università “La Sapienza”, Roma; Policlinico “G. Rodolico” Azienda Ospedaliero-Universitaria, Catania; Clinica Neurologica 2, Genova; Azienda Ospedaliera Universitaria Integrata, Verona; Ospedale Clinicizzato “Colle Dall'Ara”, Chieti; Università di Sassari, Sassari; Università di Napoli, Napoli; Ospedale S. Antonio, Padova; Ospedale Civile S. Agostino-Estense, Modena; Ospedale Santa Maria, Reggio Emilia; Policlinico Universitario Mater Domini, Catanzaro; Ospedale S. Gerardo, Monza; Azienda Ospedaliero-Universitaria S. Anna, Ferrara; Ospedali Riuniti, Ancona; Istituto S. Raffaele “G. Giglio”, Cefalù; Azienda Ospedaliero San Giovanni Battista, Università di Torino, Torino; Ospedale Sacro Cuore, Negrar; Ospedale Santa Chiara, Trento; Ospedale Regionale, Bolzano; Azienda Ospedaliero-Universitaria Senese, Policlinico “Le Scotte”, Siena; Ospedale “Misericordia e Dolce”, Prato; Università degli Studi di Pisa, Pisa; Policlinico “G. Martino”, Messina; Università degli Studi di Palermo, Palermo; Università Cattolica, Policlinico Gemelli, Roma; Dipartimento Neuroriabilitativo ASL CN1, Cuneo; Luigi Gonzaga Hospital, Orbassano Ethics Committees), adhered to Good Clinical Practice (GCP) guidelines and Declaration of Helsinki. The original trial was registered in 2006 in the EudraCT register (EUDRACT n.: 2006-004937-13) at a time that was prior to being accepted as a registry that fulfills the requirements by the International Committee of Medical Journal Editors (ICMJE) (http://www.icmje.org/faq_clinical.html). Since this registry was only considered to fulfill the requirements by the ICMJE since June 2011 and was not publicly available for several years after it was established, this precluded fulfilment of the requirements outlined by the ICMJE. We confirm that all ongoing and future trials are now registered.
Study design and patients
Designed as a multicenter, randomized, single-blinded, phase III clinical trial, the study assesses non-inferiority of AZA efficacy vs. IFNs over two years. Patients were recruited between February 2007 and March 2009 in 30 MS centers throughout Italy. Inclusion criteria were: age, 18–55 years; relapsing-remitting MS [19]; at least two clinical relapses in the preceding two years; a baseline Expanded Disability Status Scale (EDSS) [20] score from 1.0 to 5.5; effective female contraception and a signed informed consent. Exclusion criteria were: clinical relapses or steroid therapy 30 days prior to study entry; immunomodulatory or immunosuppressive treatments in the preceding year; concomitant diseases precluding IFN or AZA treatment; pregnancy or breastfeeding; cognitive decline preventing informed consent; pathological conditions interfering with MS evolution; non-steroidal anti-inflammatory drugs (NSAID) allergy or intolerance to AZA or IFNs.
The study was an independent academic initiative supported by the Italian Medicine Agency (Agenzia Italiana del Farmaco, AIFA) through a competitive Grant following a public call aimed to support independent Clinical Trials.
Randomization and blinding
Patients were selected for AZA or IFNs using a computer generated central randomization list (1∶1 ratio), in blocks of four and stratified by disability score (EDSS≤3.5 or >3.5). Patients were assessed by an unblinded treating and a blinded examining neurologist at their centers. Brain MRI images were centrally analyzed by two blinded independent experts at the Image Analysis Centre of the University of Florence (Italy).
Interventions
Treatment was prescribed free of charge by treating neurologists and self-administered within one month after screening and one week after randomization.
Standard treatment
The IFN-treated patients were either administered 250 µg of IFNβ-1b subcutaneously on alternate days (Betaferon), 30 µg of IFNβ-1a IM, weekly (Avonex); 22/44 µg of subcutaneous IFNβ-1a thrice weekly (Rebif). The type of IFNβ (Betaferon, Avonex or Rebif) was selected by the treating neurologist. The standard dose was titrated over the first four weeks.
Experimental treatment
The AZA-treated patients were given an oral target dose of 3 mg/kg/day, individually adjusted to their differential white cell counts. The initial 50 mg/day dose was subsequently titrated for the first six to eight weeks, increasing 50 mg every fortnight to the target dose.
Treatment adjustment and discontinuation criteria
For all medications, treatment adjustment criteria included: reaching grade two for adverse events (AEs) of Common Toxicity Criteria (CTC) [21], including n<800/µl lymphocyte count and n<3000/µl white blood cells. For AZA in case of grade two AEs, a 25/50 mg dose reduction was required. When the AE occurred during dose titration the higher dose was not prescribed. Returning to the target dose after reduction or increasing dose during titration was allowed for AEs occurring only once, otherwise the low dose was maintained. The treatment monitoring, including hemato-chemical tests (erythrocytes, hemoglobin, leukocytes with differential count, platelets, ALT, AST, GGT, ALP, and bilirubin), were performed quarterly. These tests were performed every fortnight during the first two months of treatment (one month for the IFNs) and when a grade two AE occurred. Treatment was discontinued for grade two AEs persistent at two subsequent controls after dose reduction. Other withdrawal criteria were: a grade three AE or AEs considered intolerable by patients or treating neurologists; treatment failure (i.e., more relapses during the study than in the previous two years, or an equal number of relapses and increase of at least one EDSS point confirmed after six months, or shift to a secondary progressive course); pregnancy; and consent withdrawal.
Co-interventions
Symptomatic treatments were allowed and 1 g of I.V. methylprednisolone was given for three-five days for relapses, as prescribed by the treating neurologist.
Procedures
The treating neurologist oversaw the overall medical management of patients, including drug prescription and self-administration instruction, scheduled (quarterly) and unscheduled (i.e., at the onset of new symptoms or complications) follow-up visits where he/she recorded symptoms, blood test results, clinical AEs and their management, and any treatment decision, including discontinuation. The examining neurologist was responsible for the neurological examination and EDSS scoring at scheduled (every six months) and unscheduled visits, that were requested by the treating neurologist to confirm relapses. These included the onset of new neurological symptom(s), or worsening of pre-existing ones from MS, determining worsening of at least one point in one or more functional system or at least 0.5 EDSS points. A new symptom was considered part of a new relapse if it lasted at least 48 hours with no fever, and if reported at least 30 days from the end of a previous relapse. To discontinue treatment a final visit was planned within 30 days from the last dose.
A Contract Research Organization (CRO) visited all centers before enrolment and every four months thereafter.
Outcomes
Clinical efficacy
The primary outcome was annualized relapse rate ratio (RR) over two years. Secondary clinical outcomes were: a) annualized relapse rate during the first and second year; b) proportion of patients with 0, 1, and ≥2 relapses during the first and second year; c) proportion of patients with corticosteroid-treated relapses; d) time to first relapse after randomization; e) proportion of patients with no confirmed disability progression, i.e., without an increase of at least one EDSS point confirmed after at least six months over two years; f) mean EDSS change from baseline to the end of follow-up; g) number of treatment failures; h) mean change of the MSQOL-54 scale [22] over two years.
Brain MRI
Brain lesions were evaluated through MRI scans performed over 30 days prior to treatment (baseline) and at two years (study completion). In the MRI study participated 23 Centers, all identified prior to the beginning of the study. The primary MRI outcome was the number of new T2 brain lesions, defined as new or enlarging lesions on T2-weighted scans. Secondary outcomes were: a) proportion of patients with 0, 1–2, ≥3 new T2 brain lesions; b) combined new and enhancing lesions (CE); c) mean and median Gadolinium contrast enhancing (Gd+) lesions on T1-weighted scans; d) proportion of patients with 0, 1–2, ≥3 Gd+ lesions. New lesion numbers were evaluated through dedicated software packages (Analyze 10.0), comparing each scan obtained at study completion with the corresponding baseline scan [see Methods S1 in File S1 for details].
Safety
Data was collected on: 1) AEs and serious AEs (SAEs); 2) patients with any AE; 3) patient withdrawal after any AE; 4) severity of any AE and their correlation with treatments as judged by the treating neurologist. Frequency and severity of AEs were actively assessed every three months or upon patient request. Severity was graded using the National Cancer Institute Common Terminology Criteria for AE [21]. SAE notification was sent to a specifically appointed Pharmacological Surveillance Unit (PSU).
Non-inferiority margin, power and sample size
Non-inferiority margin
To compare treatment relapse rates, a non-inferiority margin (M) was calculated following published guidelines [23]–[25], as a fraction of the mean effect of IFNs vs. placebo (EIFNvsPlacebo) on the same outcome measure in previous trials with the same inclusion criteria and follow-up period [8], [9], [11]. By next expressing the EIFNvsPlacebo as a relapse rate ratio, M was expressed as 50% of the excess to 1.0 of this rate ratio. Given the historical EIFNvsPlacebo of 1.46 ( = 2.55/1.75, corresponding to the relapse rate reduction through IFN treatment), M = 1.23 was therefore selected [8], [9]. The annualized new T2 lesion rate over two years was chosen as the primary MRI outcome, as this was the main MRI outcome available in the pivotal trial aimed at establishing the efficacy of IFNβ-1b vs. placebo and whose inclusion criteria and follow-up length were identical [8], [11], thereby enabling precise evaluation of the EIFNvsPlacebo on new T2 lesion rates, as their ratio was 2.67 ( = 6.4/2.4). Based on these data, a non-inferiority margin of M = 1.84 was established a priori, as 50% of the excess to 1.0 of the 2.67 historical ratio.
Power and sample size
Sample size was calculated to verify the non-inferiority of AZA against IFNs. With a power of 80%, α of 5% and under the hypothesis of no difference between the means of relapse rates (new T2 lesion rates for MRI), with an expected loss of 20% at follow-up, 360 patients (175/treatment arm) for relapse, and 192 patients (96/treatment arm) for MRI were needed. However, the sample size of the study was undermined by the revision of the Italian National Health System reimbursement criteria, that occurred during the recruitment period and allowed IFN therapy from the first MS attack, thus overcoming the required presence of at least two relapses during the previous two years, which was one of the inclusion criteria of this study. This change remarkably reduced the number of eligible patients and the recruitment slowed to such a low rate that the Steering Committee of the study judged the planned sample size not feasible any more. For this reason a protocol amendment, approved by the Independent Data and Safety Management Committee (IDSMC) and by the Ethic Committee of the Coordinating Center, recommended a 150 patient recruitment ceiling, accepting a power of 60–65% for relapses, and 80% for MRI outcome, under the hypothesis of no differences between the means of relapse/new T2 lesion rates [see Protocol S1 and Amendment S1 for details]. It is worth to note that the request of amendment was submitted by the Steering Committee exclusively on the basis of the observed accrual rate, when no data or codes were available.
Statistical analyses
Baseline characteristics
Baseline clinical and demographic characteristics were analyzed using χ2 test for categorical, and t-test (or Mann-Whitney test in the absence of Normal distribution) for continuous variables.
Clinical outcome measures
AZA efficacy was judged non-inferior to IFNs if the upper limit (UL) of the one-sided 95% confidence interval (95% CI) of the annualized relapse RRAZA/IFN over two years, calculated by Poisson regression, was <M = 1.23. Secondary outcomes were analyzed using χ2 test with one degree of freedom for rate comparison (based on Poisson regression); χ2 test with two degrees of freedom for number of relapsed patients; Kaplan-Meier curves, log-rank test and Cox proportional-hazards model for time to first relapse; Fisher's exact test for patients with no confirmed disability progression; and t-test for EDSS and MSQOL-54 score changes. For the annualized relapse rate, sensitivity analyses were performed adjusting for baseline covariates (number of relapses during the previous two years, baseline EDSS score, and disease duration from onset of symptoms), and excluding Avonex treated patients. An additional sensitivity analysis was performed to include in the analysis patients lost to follow-up, using two multiple imputation methods (monotone logistic regression and fully conditional specification [FCS] logistic regression method) [26]–[28], taking the randomized treatment as the covariate (i.e., incorporating possible different uncertainty due to different dropout rates between the two randomized treatment groups). All analyses were performed in the intention to treat (ITT) and per-protocol (PP, i.e. after excluding noncompliant patients and drop-outs) populations. In the analyses based on relapse rates and on proportion of patients with relapses or disability progression, patients lost to follow-up were excluded.
Brain lesions
AZAs were judged non-inferior to IFNs if the UL of the one-sided 95% CI of the annualized new T2 lesion rate ratio over two years, calculated by Poisson regression, was <M = 1.84. Secondary outcomes were analyzed through χ2 test with one degree of freedom for rate comparison (based on Poisson regression); χ2 test with two degrees of freedom for number of patients with lesions; and Mann-Whitney test for Gd+ lesion number. All analyses were performed in the ITT and PP populations.
Adverse Events
AEs were analyzed as rates, in terms of patients with AEs and overall number of AEs, using χ2 test based on Poisson regression for rate comparison, and χ2 test for categorical variable comparison for discontinued interventions after AEs, AE severity and correlation of AE with treatment. SAEs were described reporting their postulated correlation with treatment and any consequent discontinuation.
Data were reported following the CONSORT guidelines [29].
Results
Characteristics of participants
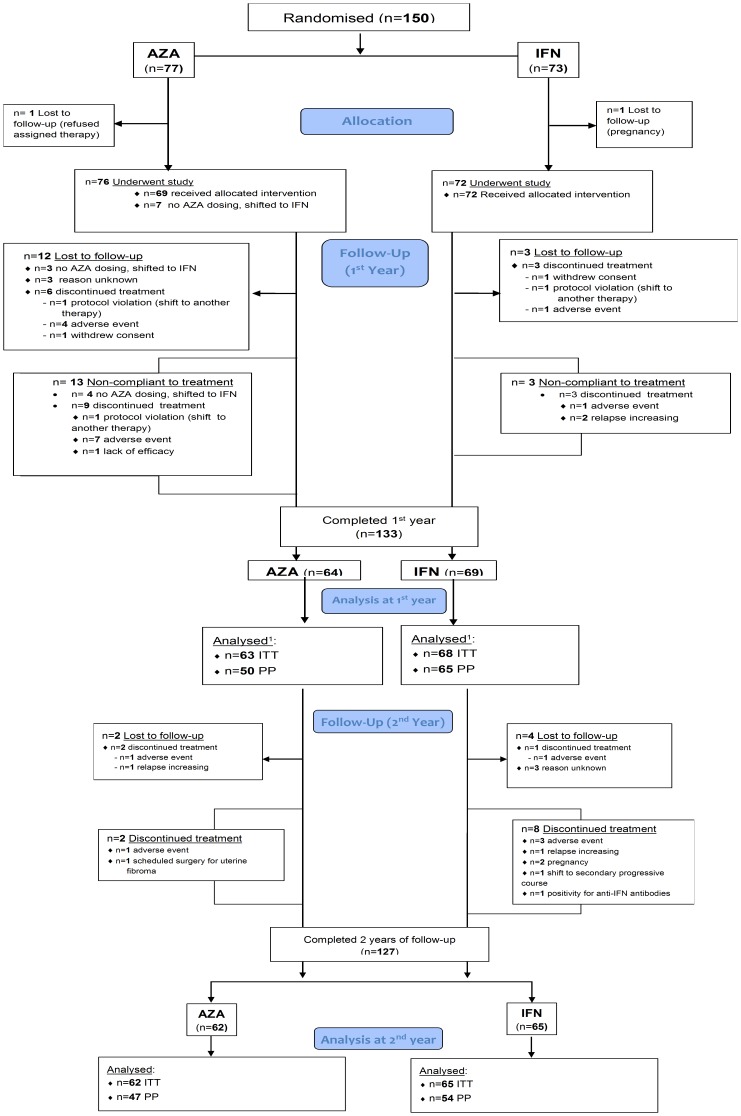
Figure 1 presents patient allocation and follow-up. Of the 150 randomized patients 77 and 73 were AZA- and IFN-assigned respectively. In the IFN group, 26 (36%) were assigned to Avonex, 5 (7%) to Betaferon, 35 (48%) to Rebif 22, and 7 (10%) to Rebif 44. Of the 150 patients screened at baseline, 127 completed the ITT follow-up: 62 (81%) in the AZA group, and 65 (89%) in the IFN group (overall 85%). Eight patients, initially randomized to AZA, refused consent and received IFN (out of these, four were lost to follow up). Including losses to follow up, treatment discontinuations were respectively 30 in the AZA group (39%; with the patients who refused to begin the treatment, n = 8) and 19 in the IFN group (26%). The majority of the discontinuations under AZA occurred in the first year (n = 26; 87%) whereas those under IFN occurred in the second year (n = 12; 63%). The discontinuations were 22 (32%) and 18 (25%) respectively, if only patients who began the treatments are included in the analysis of pharmacological compliance.
Figure 1. Flow-chart: patient allocation and follow-up.
Abbreviations: AZA, azathioprine; IFN, interferon; ITT, intention to treat; PP, per-protocol. 1One missing CRF at month 12.
Fourteen (47%) of 30 treatment discontinuations in the AZA group and 6 (32%) of 19 discontinuations in the IFN group were due to AEs; 2 (7%) of 30 patients in the AZA group and 3 (16%) of 19 patients in the IFN group discontinued for lack of efficacy. Demographic, clinical characteristics and MRI findings at baseline were highly comparable in both groups (Table 1), even considering the ITT (n = 127), the PP (n = 101), and the MRI (n = 97) populations who completed follow-up [data not shown]. Baseline characteristics were comparable even separately considering patients enrolled during the first and second year of recruitment [data not shown].
Table 1. Baseline characteristics of the patients.
| Characteristic | AZA (N = 77) | IFN (N = 73) | p-value1 |
| Demographic characteristics | |||
| Female – No. (%) | 49 (63.6%) | 50 (68.5%) | p = 0.53 |
| Age - Years | |||
| Mean ± SD | 38.1±8.9 | 36.6±8.8 | p = 0.31 |
| Median (range) | 37.9 (21.3–56.5) | 37.6 (19.1–58.8) | |
| Clinical characteristics | |||
| Duration of disease from onset of symptoms - Years | |||
| Mean ± SD | 6.8±7.1 | 5.7±5.7 | |
| Median (range) | 3.4 (0.5–25.3) | 3.4 (0.3–24.8) | p = 0.53 |
| Relapses in previous 2 years | |||
| Mean ± SD | 2.38±0.78 | 2.41±0.89 | |
| Median (range) | 2 (0–5) | 2 (0–6) | p = 0.91 |
| No. patients with relapses in previous 2 years - No. (%) | |||
| 0–12 | 3 (3.9%) | 2 (2.7%) | |
| 2 | 48 (62.3%) | 47 (64.4%) | p = 0.91 |
| ≥3 | 26 (33.8%) | 24 (32.9%) | |
| No. patients with previous histories of … - No. (%) | |||
| AZA treatment | 1 (1.3%) | 1 (1.4%) | p = 0.95 |
| IFN treatment | 4 (5.2%) | 3 (4.1%) | |
| EDSS score3 | |||
| Mean ± SD | 1.9±0.9 | 1.9±0.9 | |
| Median (range) | 1.5 (1.0–5.5) | 1.5 (0.0–5.0) | p = 0.86 |
| Patients with concomitant diseases – No. (%)4 | 5 (6.9%) | 4 (5.8%) | p = 0.80 |
Abbreviations: AZA, azathioprine; EDSS, Expanded Disability Status Scale; IFN, interferon; SD, standard deviation.
P-values for AZA vs. IFN comparison were obtained through: χ2 test with one or two degrees of freedom for sex, number of patients with previous histories of AZA/IFN treatment, number of patients with relapses with concomitant disease and with Gd+ lesions; t-test for age; Mann-Whitney test for duration of disease, number of relapses, EDSS score, number of Gd+ lesions and T2 lesion load.
Protocol violations.
Scores on the EDSS range from 0 to 10, with higher scores indicating greater degree of disability.
The sum does not add up to the total because of some missing values.
Efficacy - clinical outcomes
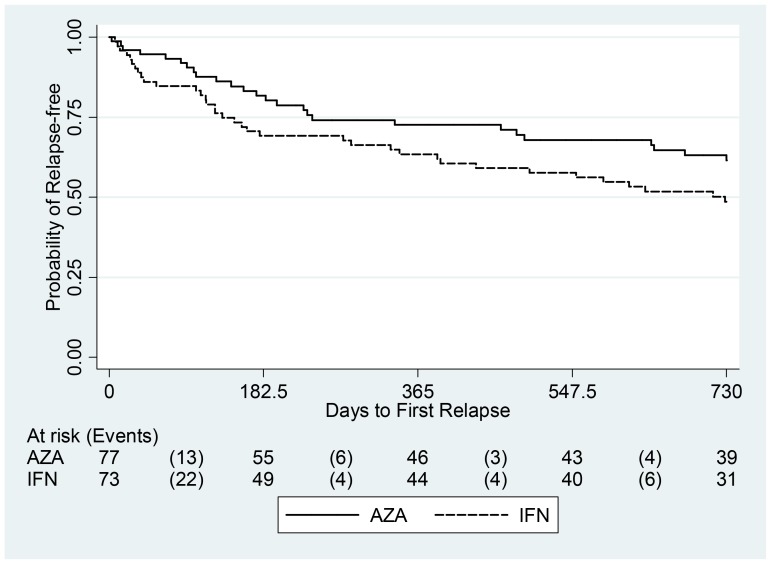
From the primary efficacy analysis, AZA emerges as significantly non-inferior to IFN (Fig. 2), as the upper limit (UL) of the one-sided 95% CI for the annualized relapse RRAZA/IFN was 0.96, i.e., below the non-inferiority margin M ( = 1.23; p<0.01). This UL is also significantly (p = 0.03) below a more stringent non-inferiority margin M1 = 1.0, corresponding to 100% of the effect of IFNs vs. placebo. The UL of the one-sided 99% CI for the RRAZA/IFN (i.e., 1.12), corresponding to the 75% of the IFN effect vs. placebo, was also significantly below the non-inferiority margin of M = 1.23 (p<0.01). The annualized relapse rates observed over two years among the AZA and the IFN treated subjects were 0.26 and 0.39, respectively (p = 0.07, adjusted p = 0.06; Table 2). The corresponding RRAZA/IFN was 0.67 (95% CI, 0.43–1.03) based on the 127 patients who completed follow-up, 0.67 (95% CI, 0.40–1.12) based on 150 randomized patients and using the monotone logistic regression multiple imputation method, and 0.69 (95% CI, 0.43–1.10) using the FCS logistic regression multiple imputation method [data not shown]. Adjusted analysis gave similar results (Table 2), confirming the robustness of the findings. In addition, comparable results were obtained in a sensitivity analysis excluding the Avonex treated patients (the annualized relapse rate over two years among Betaferon or Rebif treated patients was 0.37, with a corresponding RRAZA/IFN of 0.70, 95% CI, 0.43–1.15) [data not shown]. No significant difference was noted between AZA and IFN in the proportion of patients with 0, 1, 2, ≥3 relapses over two years and separately in the first or the second year, the proportion of patients with corticosteroid-treated relapses, and the proportion of patients with no confirmed disability progression over two years. (Table 2). There were six treatment failures in the AZA group and five in the IFN group. For QOL, no difference was observed between the treatments, for both physical and mental-QOL (p = 0.94 and 0.93, respectively) [data not shown]. Figure 3 shows Kaplan-Meier curves of the time to first relapse: no significant difference was observed in terms of log-rank (p = 0.11) or Cox proportional-hazards model results, with a hazard ratio of 0.66 (95% CI, 0.40–1.10). Similar results were obtained in sensitivity analyses excluding Avonex treated patients (log-rank p = 0.15) [data not shown]. The analyses performed in the PP population yielded similar findings [data not shown].
Figure 2. Primary clinical outcome over 2 years: non-inferiority of effect of AZA vs. IFN, represented as annualized relapse rate ratio (RRAZA/IFN) compared with the pre-established non-inferiority margin M ( = 1.23) and with a margin M1 = 1.0.
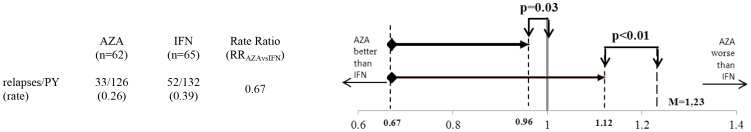
One-sided 99% CI of the 0.67 ratio (upper-limit, UL = 1.12), represents an effect of AZA vs. IFNs equivalent to at least 75% of the effect of IFNs vs. Placebo. One-sided 95% CI of the same ratio (UL = 0.96), represents an effect of AZA vs. IFNs equivalent to at least 100% of the effect of IFNs vs. Placebo. Abbreviations: AZA, azathioprine; IFN, interferon; PY, person-years; RR, rate ratio.
Table 2. Secondary clinical outcomes.
| Outcome | 1st Year | 2nd Year | Overall (2 years of follow-up) | ||||||
| AZA (N = 63) | IFN (N = 68) | p-value1 | AZA (N = 62) | IFN (N = 65) | p-value1 | AZA (N = 62) | IFN (N = 65) | p-value1 | |
| Relapses | |||||||||
| Annualised relapse rate (95% CI) | 0.37 (0.25–0.56) | 0.47 (0.34–0.67) | p = 0.37 | 0.18 (0.10–0.32) | 0.29 (0.18–0.45) | p = 0.19 | 0.26 (0.19–0.37) | 0.39 (0.30–0.51) | p = 0.07 |
| Adjusted annualised relapse rate (95% CI)2 | - | - | - | - | - | - | 0.27 (0.19–0.38) | 0.41 (0.31–0.54) | p = 0.06 |
| No. of patients with relapse - No. (%) | |||||||||
| 0 | 45 (71.4%) | 44 (64.7%) | p = 0.63 | 52 (83.9%) | 49 (75.4%) | p = 0.42 | 39 (62.9%) | 31 (47.7%) | p = 0.22 |
| 1 | 14 (22.2%) | 17 (25.0%) | 9 (14.5%) | 13 (20.0%) | 15 (24.2%) | 23 (35.4%) | |||
| ≥2 | 4 (6.4%) | 7 (10.3%) | 1 (1.6%) | 3 (4.6%) | 8 (12.9%) | 11 (16.9%) | |||
| No. of patients with relapses treated with corticosteroids – No. (%) | |||||||||
| 0 | - | - | - | - | - | - | 40 (64.5%) | 34 (52.3%) | p = 0.22 |
| 1 | - | - | - | - | - | - | 16 (25.8%) | 22 (33.9%) | |
| ≥2 | - | - | - | - | - | - | 6 (9.7%) | 9 (13.9%) | |
| Disability 3 | |||||||||
| Patients with no confirmed disability progression - % (95% CI)4 | - | - | - | - | - | - | 98.2 (91.5–99.9) | 92.0 (81.8–97.4) | p = 0.19 |
| Change from baseline in EDSS score – Mean (95% CI)5 | - | - | - | - | - | - | −0.08 (−0.31; 0.16) | 0.22 (−0.03; 0.47) | p = 0.08 |
Abbreviations: AZA, azathioprine; IFN, interferon.
P-values for AZA vs. IFN comparison were obtained through χ2 test with one degree of freedom for rate comparison, χ2 test with two degrees of freedom for number of patients with relapses, Fisher's exact test for patients with no confirmed disability progression, and t-test for change in EDSS score.
The analyses were adjusted for number of relapses during the previous two years, baseline EDSS score, and duration of disease from symptom onset.
The analyses were based on 56 AZA and 50 IFN patients respectively, because of some missing values.
A confirmed disability progression was defined as an increase of no less than one point of the EDSS score confirmed at least after six months; 95% CI were estimated through the exact method. All the patients, with the exception of two (who did not report a disability progression), had a baseline EDSS score between 1 and 5.
Adjusted for baseline EDSS score.
Figure 3. Time to first relapse.
Beneath the plot patients at risk and number of events (in brackets) by treatment were reported for each interval of 6 months. Abbreviations: AZA, azathioprine; IFN, interferon.
Efficacy - MRI outcomes
Of the 122 patients given baseline MRI (61 per group), 97 completed the ITT follow-up: 50 (82%) in the AZA group, and 47 (77%) in the IFN group. The ratio of annualized new T2 lesion rates of AZA vs. IFNs was 1.10 (Fig. 4). The corresponding UL of the 95% one-sided CI was 1.45, below the non-inferiority margin M = 1.84, indicating an AZA vs. IFN effect equivalent to at least 73% of the IFNs vs. placebo effect. Moreover, the UL of the one-sided 99% CI for the new T2 lesion RRAZA/IFN (i.e., 1.63) was also significantly below the non-inferiority margin of M = 1.84 (p<0.01). Table 3 summarizes the MRI outcomes: no significant difference was noted between AZA and IFNs for new T2, new CE, and Gd+ lesions. The annualized new T2 lesion rate was 0.69 (95% CI, 0.54–0.88) in the IFN and 0.76 (95% CI, 0.61–0.95) in the AZA patients (p = 0.75). Adjustments for inflammatory activity at baseline, expressed by the Gd+ lesion number confirmed these findings. Analyses performed in the PP population (81 patients: 40 in the AZA and 41 in the IFN group) confirmed these results [data not shown].
Figure 4. Non-inferiority of the effect AZA vs. IFN on new T2 lesions over 2 years.
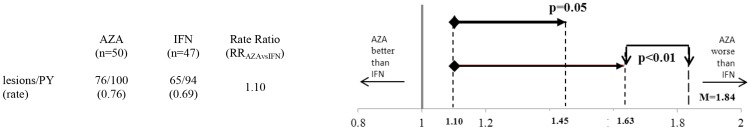
One-sided 99% CI (upper-limit, UL = 1.63), and one-sided 95% CI (UL = 1.45), of the effect of AZA vs. IFNs as for annualized new T2 lesion rate ratio (RRAZA/IFN), compared with the pre-established non-inferiority margin (M = 1.84), representing an effect of AZA vs. IFNs equivalent to the 73% of the effect of IFNs vs placebo. Abbreviations: AZA, azathioprine; IFN, interferon; PY, person-years; RR, rate ratio.
Table 3. MRI outcomes. New brain lesions.
| Outcome | Overall (2 years of follow-up) | ||
| AZA (N = 50) | IFN (N = 47) | p-value1 | |
| New T2 lesions | |||
| Annualised new T2 lesion rate (95% CI) | 0.76 (0.61–0.95) | 0.69 (0.54–0.88) | p = 0.75 |
| No. of patients with new T2 lesions - No. (%) | |||
| 0 | 27 (54.0%) | 21 (45.0%) | |
| 1–2 | 11 (22.0%) | 18 (38.0%) | p = 0.41 |
| ≥3 | 12 (24.0%) | 8 (17.0%) | |
| New Combined Unique (CE) lesions | |||
| Annualised new CE lesion rate (95% CI) | 0.78 (0.63–0.98) | 0.70 (0.55–0.90) | p = 0.53 |
| Gd+ lesions | |||
| Gd+ lesion number | |||
| Mean ± SD | 0.20±0.50 | 0.40±1.35 | |
| Median (range) | 0 (0–2) | 0 (0–5) | p = 0.52 |
| No. patients with Gd+ lesions - No. (%) | |||
| 0 | 41 (84.0%) | 43 (91.5%) | |
| 1–2 | 8 (16.0%) | 1 (2.0%) | p = 0.39 |
| ≥3 | 0 (0.0%) | 3(6.5%) | |
| Missing data | 1 | 0 | |
Abbreviations: AZA, azathioprine; IFN, interferon.
P-values for AZA vs. IFN comparison were obtained through χ2 test with one degree of freedom for rate comparison, χ2 test with two degrees of freedom for number of patients with lesions, and Mann-Whitney test for Gd+ lesion number.
Safety comparison
The rate of patients with at least one AE was not different between the two groups (p = 0.28), however the rate of AEs was higher in the AZA group (p<0.01) (Table 4). The most frequently reported AEs were flu-like symptoms, more frequent in IFNs (p<0.01), nausea/vomiting and abnormal blood count more frequent in AZA-treated patients (p<0.01). AE-related discontinued interventions were more frequent among AZA (20.3%) than IFN (7.8%) patients (p = 0.03). SAEs and other AEs are described in Tables S1 and S2 in File S1.
Table 4. Adverse Events.
| Event | AZA | IFN | p-value1 |
| (Npatients = 69, Nevents = 308, PY = 108) | (Npatients = 77, Nevents = 241, PY = 136) | ||
| All AEs 2 | |||
| Patients – No./PY and rate (95%CI) | 65/108 | 68/136 | p = 0.28 |
| 0.60 (0.47–0.77) | 0.50 (0.40–0.64) | ||
| AEs - No./PY and rate (95%CI) | 308/108 | 241/136 | p<0.01 |
| 2.85 (2.54–3.19) | 1.77 (1.56–2.01) | ||
| Most frequently reported AEs 2 | |||
| Influenza-like illness | |||
| Patients – No./PY and rate (95%CI) | 3/108 | 39/136 | p<0.01 |
| 0.03 (0.01–0.08) | 0.29 (0.20–0.39) | ||
| AEs - No./PY and rate (95%CI) | 3/108 | 41/136 | p<0.01 |
| 0.03 (0.01–0.08) | 0.30 (0.22–0.41) | ||
| Fever | |||
| Patients – No./PY and rate (95%CI) | 2/108 | 19/136 | p<0.01 |
| 0.02 (0.00–0.07) | 0.14 (0.08–0.22) | ||
| AEs - No./PY and rate (95%CI) | 2/108 | 20/136 | p = 0.01 |
| 0.02 (0.00–0.07) | 0.15 (0.09–0.23) | ||
| Local allergic reaction | |||
| Patients – No./PY and rate (95%CI) | 0/108 | 13/136 | - |
| 0.10 (0.05–0.16) | |||
| AEs - No./PY and rate (95%CI) | 0/108 | 14/136 | - |
| 0.10 (0.06–0.17) | |||
| Systemic allergic reaction | |||
| Patients – No./PY and rate (95%CI) | 3/108 | 0/136 | - |
| 0.03 (0.01–0.08) | |||
| AEs - No./PY and rate (95%CI) | 3/108 | 0/136 | - |
| 0.03 (0.01–0.08) | |||
| Nausea/vomiting | |||
| Patients – No./PY and rate (95%CI) | 30/108 | 1/136 | p<0.01 |
| 0.28 (0.19–0.40) | 0.01 (0.00–0.04) | ||
| AEs - No./PY and rate (95%CI) | 35/108 | 1/136 | p<0.01 |
| 0.32 (0.23–0.45) | 0.01 (0.00–0.04) | ||
| Abnormal blood count | |||
| Patients – No./PY and rate (95%CI) | 46/108 | 24/136 | p<0.01 |
| 0.43 (0.31–0.57) | 0.18 (0.11–0.26) | ||
| AEs - No./PY and rate (95%CI) | 106/108 | 39/136 | p<0.01 |
| 0.98 (0.80–1.19) | 0.29 (0.20–0.39) | ||
| Other abnormal blood tests3 | |||
| Patients – No./PY and rate (95%CI) | 24/108 | 37/136 | p = 0.44 |
| 0.22 (0.14–0.33) | 0.27 (0.19–0.37) | ||
| AEs - No./PY and rate (95%CI) | 46/108 | 54/136 | p = 0.72 |
| 0.43 (0.31–0.57) | 0.40 (0.30–0.52) | ||
| Other AE | |||
| Patients – No./PY and rate (95%CI) | 51/108 | 47/136 | p = 0.12 |
| 0.47 (0.35–0.62) | 0.35 (0.25–0.46) | ||
| AEs - No./PY and rate (95%CI) | 70/108 | 54/136 | p<0.01 |
| 0.65 (0.51–0.82) | 0.40 (0.30–0.52) | ||
| Discontinued interventions due to AEs | |||
| No. of patients with discontinued interventions due to AEs (%) | 14 (20.3%) | 6 (7.8%) | p = 0.03 |
| Seriousness of AE 5 | |||
| No. of events (%)4 | |||
| Minor/Moderate | 291 (96.0%) | 236 (98.3%) | p = 0.12 |
| Major/Serious | 12 (4.0%) | 4 (1.7%) | |
| Correlation with study treatment | |||
| No. of events (%)4 | |||
| Non-correlated/Unlikely | 63 (20.7%) | 49 (20.4%) | p = 0.95 |
| Possible/Likely | 242 (79.3%) | 191 (79.6%) |
Abbreviations: AZA, azathioprine; IFN, interferon; PY, person-years.
P-values for AZA vs. IFN comparison were obtained through χ2 test with one degree of freedom for rate comparison, discontinued interventions due to adverse events, seriousness of adverse event, and correlation of event with treatment.
All 95% CI were estimated using the exact method.
Liver enzymes, thyroid function and bilirubin level.
The sum does not add up to the total because of some missing values.
Discussion
Principal findings
This study directly compared AZA and IFN efficacy on clinical and MRI outcomes in relapsing-remitting MS patients. The results indicated that AZA was non-inferior to IFNs in reducing relapses and new brain lesions over two years. The effect size on the primary end point (annualized relapse rate ratio) was 0.67, with the upper CIs indicating that in the worst case scenario efficacy of AZA vs. placebo can be estimated as at least 100% (95% CI) or as at least 75% (99% CI) of that of IFNs, according to the CIs level selected. The effect size on new brain lesions (the main secondary outcome measure) was 1.1 with the upper CI levels (95%) indicating that in the worst case scenario efficacy of AZA vs. placebo could be estimated as at least 73% of that of the IFNs. The direct comparison of AZA and IFN efficacy therefore indicated a similar effect size, in reducing both relapses and new brain lesions. Both treatments were similarly efficacious in time to the first relapse, in slowing disability accumulation, and in the other secondary clinical and MRI outcome measures examined. Both medications showed better efficacy in the second year, probably for a delay in fully exerting their activity during the first months of treatment, at least in part determined by the initial dose titration. The observed lag of efficacy was similar for both treatments.
Similar efficacy of AZA and IFNs was observed both in the ITT and in the PP analysis and in the different sensitivity analyses performed. As in this study the comparator treatment included all the IFNs as a group, a sensitivity analysis excluding Avonex treated patients (probably the less efficacious of the IFNs [30]) confirmed the results of the main analysis.
AZA was compared to all the IFNs as a group because a centralized choice of one specific IFN could have raised allegation of conflict of interests, as in this academically driven independent study the medications were prescribed and charged to the NHS. In addition, under these experimental conditions, a centralized selection of a specific IFN could have reduced and distorted patient accrual in the participating centers.
The remarkable internal consistency between clinical and MRI data, between the ITT and the PP analysis and among the different sensitivity analyses, supported the robustness of the results. It must be pointed out that consistency between ITT and PP analysis is a critical requirement for reliability of non-inferiority studies [23]–[25].
The present study strengthens previous results of AZA vs. placebo [1]–[4] or vs. IFN [14]–[16], and expands previous available data as for the first time MRI was included as an outcome of AZA efficacy, thus allowing contemporary assessment of relapses and brain lesions accumulation. The previous MRI studies [17]–[18] indeed were informative for supporting the hypothesis of AZA efficacy on brain lesions, but were not aimed to assess clinical outcomes and were based on retrospective or open label designs [17]–[18].
It must be noted that the results of the present study were obtained administering AZA at the target dose of 3 mg/Kg/day, adjusted according to leuko/lymphocyte count. This approach was similar to that of the trials that also showed the most remarkable reduction in relapse rates induced by AZA [2]–[4], [15], suggesting that appropriate dosage represents an important variable administering this treatment.
No unknown AEs occurred. Overall similar numbers of patients developed at least one AE. Leuko/lymphopenia in the AZA group was not associated with a higher incidence of infections and should be considered part of the desired mechanism of action. However, treatment discontinuations after AEs were significantly higher in the AZA group, mainly occurring during the first months of treatment. Most of the discontinuations for IFNs were in the second year, confirming already known different temporal AE profile of each treatment.
Strengths and weaknesses of the study
The main limit of the study was probably the sample size, which resulted smaller than planned. This was due to difficulties in recruiting and retaining patients in the trial, particularly following the change in the Italian NHS reimbursement criteria that occurred during the recruitment period. Indeed, rational basis of a direct comparison and randomization between an old generic medication and a new approved drug were sometimes hard to explain both to neurologists and patients and contributed to these difficulties.
However, the sample size affected only the initial power estimate based on the conservative hypothesis of no difference between the means of the relapse rates. Indeed, the data obtained during the study, showing a difference favoring AZA, allowed a power sufficient to establish non-inferiority at statistically robust levels of significance. Moreover, as documented by Schulz and Grimes [31] trials with low sample size might be acceptable if investigators use methodological rigor to eliminate bias and properly report to avoid misinterpretation.
Another possible limitation could be related to patient knowledge of the treatment. Indeed, out of the patients who refused the assigned treatment, all had been randomized to AZA. As this occurred before the first dose of AZA was administered, it was necessarily due to a different perception by the patients of this therapy with respect to the IFNs, which were specifically approved for MS. Successfully blinding of patients seemed unrealistic given the profoundly different side effects of AZA and IFNs of which the patients had been informed in detail. Indeed, analysis of blinding in previous studies revealed a strong tendency to treatment awareness in patients receiving IFNs [10], [32].
Dropout rates was another possible issue in this study. Although the overall number of patients who withdrew the study was only 15%, a higher number of patients were lost to follow up in the AZA than in the IFN group, mainly during the first year. As this event may have diluted true differences between treatments, sensitivity analyses, based on two multiple imputation methods, were performed and no difference in the RRAZA/IFN estimate was observed, thus confirming the results obtained in the analysis of patients who completed the follow-up.
Finally, the different number of treatment discontinuations observed between the two groups (i.e., 39% of patients on AZA and 26% on IFN) could have impacted the study effect size. However, if only patients who began the treatment according to the study protocol are considered, a similar number of patients discontinued (32% on AZA and 25% on IFNs), suggesting similar compliance of the two medications over two years. The clear difference was that treatment interruptions were more frequent in the first year in the AZA group and in the second year in the IFN group.
Implications for clinical practice
The present study was the first independent RCT that directly compared efficacy of a generic medication (AZA) to a drug specifically approved for MS (IFN) using a non-inferiority design. The authors believe that the results of this study are robust, clinically meaningful and relevant for clinical practice, supporting AZA as a rational and effective alternative to IFNs in relapsing-remitting MS, particularly considering the convenience of oral administration and the cost, lower than the other available treatments. Nevertheless, the different side effect profiles of both medications have to be taken into account.
Supporting Information
CONSORT checklist.
(DOC)
Trial protocol.
(PDF)
Amendment to the protocol.
(PDF)
Methods S1, Outcomes. Brain MRI: Scan acquisition specifications. Table S1, Serious Adverse Events (SAEs). Table S2, AEs – subtypes.
(DOCX)
Acknowledgments
The authors wish to thank: The Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) for the financial support; the Interdipartimental Center for Magnetic Resonance Imaging of University of Florence for the support in the MRI analysis; Paul Bowerbank for his help in reviewing the English of the manuscript.
Group information
The Multicenter Azathioprine Interferon-ß Non-Inferiority (M.A.I.N.) Trial Group and investigators are as follows: Steering committee: L Massacesi (Dipartimento di Neuroscienze, Psicologia, Farmaco e Salute del Bambino Università di Firenze, Italy; Neurologia 2, Azienda Ospedaliero-Universitaria Careggi, Firenze, Italy.), G Filippini, C Milanese, A Solari (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano), L La Mantia (Unità di Neurologia - Multiple Sclerosis Center, I.R.C.C.S. Santa Maria Nascente Fondazione Don Gnocchi, Milano), MD Benedetti (Dipartimento Universitario di Neurologia, Azienda Ospedaliera Universitaria Integrata, Verona), S Amoroso (Dipartimento di Neuroscienze, Sezione di Farmacologia, Università Politecnica delle Marche, Ancona), G Mancardi (Dipartimento Neuroscienze, Università di Genova, Genova), D Orrico (Divisione di Neurologia, Ospedale Civile Santa Chiara, Trento), G Tedeschi (Clinica Neurologica, Università di Napoli), M Battaglia (AISM, FISM, Genova), MG Valsecchi (Centro di Biostatistica per l'Epidemiologia Clinica, Università Milano-Bicocca, Monza). Study coordinators C Milanese, L Massacesi. Randomization centre A Solari. Data Coordination and Analysis: G Filippini, I Tramacere (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano). Image Analysis Centre: L Massacesi, L Vuolo (Dipartimento di Neuroscienze, Azienda Ospedaliero-Universitaria Careggi, Firenze). Independent data safety management committee (IDSMC) G Tognoni (Istituto Mario Negri, Milano), R D'Alessandro (Clinica Neurologica, Università di Bologna), L Provinciali (Clinica Neurologica, Ospedali Riuniti, Ancona). Pharmacologic surveillance Unit S Amoroso (Dipartimento di Neuroscienze, Sezione di Farmacologia, Università Politecnica delle Marche, Ancona). Study sites and hospitals (PI = Principal investigator) Dipartimento di Neuroscienze, Psicologia, Farmaco e Salute del Bambino Università di Firenze and Neurologia 2, Azienda Ospedaliero-Universitaria Careggi, Firenze; L Massacesi (PI), A Repice, A Barilaro, L Vuolo. Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano; C Milanese (PI), P Confalonieri. Unità di Neurologia - Multiple Sclerosis Center, I.R.C.C.S. Santa Maria Nascente Fondazione Don Gnocchi, Milano; L La Mantia. Clinica Neurologica, Novara; M Leone (PI), S Ruggerone, P Naldi. Dipartimento di Scienze Neurologiche “La Sapienza”, Roma; C Pozzilli (PI), F De Angelis. Policlinico “G. Rodolico”, Azienda Ospedaliero-Universitaria, Catania; F Patti (PI), S Messina. Dipartimento di Neuroscienze, Clinica Neurologica 2, Genova; G Mancardi (PI), E Capello. Dipartimento Universitario di Neurologia, Azienda Ospedaliera Universitaria Integrata, Verona; MD Benedetti (PI), A Gajofatto. Centro Sclerosi Multipla, Clinica Neurologica, Ospedale Clinicizzato “Colle Dall'Ara”, Chieti; A Lugaresi (PI), G De Luca. Clinica Neurologica, Università di Sassari; G Rosati (PI), M Pugliatti. Clinica Neurologica, Università di Napoli; G Tedeschi (PI), S. Bonavita. UO Neurologia, Ospedale S. Antonio, Padova; B Tavolato (PI). Dipartimento di Neuroscienze, Clinica Neurologica, Modena; P Sola (PI). Ospedale Santa Maria, Reggio Emilia; L Motti (PI). Clinica Neurologica, Policlinico Universitario Mater Domini, Catanzaro; A Quattrone (PI). Clinica Neurologica, Ospedale S. Gerardo, Monza; M Frigo (PI). Clinica Neurologica, Azienda Ospedaliero-Universitaria S. Anna, Ferrara; MR Tola (PI). Clinica Neurologica, Ospedali Riuniti, Ancona; M Danni (PI). UO Neurologia, Istituto S. Raffaele “G. Giglio”, Cefalù; L Grimaldi (PI). Dipartimento di Neuroscienze, Azienda Ospedaliero San Giovanni Battista, Università di Torino, Torino; P Cavalla (PI). UO Neurologia, Ospedale Sacro Cuore, Negrar; F Marchioretto (PI), M Pellegrini. Divisione Neurologia, Ospedale Santa Chiara, Trento; D Orrico (PI). Divisione di Neurologia, Ospedale Regionale, Bolzano; R Schoenhuber (PI). Azienda Ospedaliero-Universitaria Senese, Policlinico “Le Scotte”, Siena; M Ulivelli (PI). UO Neurologia, Ospedale “Misericordia e Dolce”, Prato; M Falcini (PI). Dipartimento di Neuroscienze, Sezione di Neurologia, Pisa; A Iudice (PI). UOC Neurologia, Policlinico “G. Martino”, Messina; C Messina (PI). Dipartimento di Neuroscienze, Clinica Neurologica, Palermo; G Savettieri (PI). Dipartimento di Neuroscienze, Università Cattolica, Policlinico Gemelli, Roma; AP Batocchi (PI). Dipartimento Neuroriabilitativo ASL CN1, Cuneo; F Perla (PI). Ospedale S. Luigi Gonzagal, Orbassano; A Bertolotto (PI).
Funding Statement
The present study was funded by AIFA (Agenzia Italiana del Farmaco, www.agenziafarmaco.gov.it). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. The British, Dutch MSATG (1988) Double-masked trial of azathioprine in multiple sclerosis. british and dutch multiple sclerosis azathioprine trial group. Lancet 2: 179–183. [PubMed] [Google Scholar]
- 2. Ellison GW, Myers LW, Mickey MR, Graves MC, Tourtellotte WW, et al. (1989) A placebo-controlled, randomized, double-masked, variable dosage, clinical trial of azathioprine with and without methylprednisolone in multiple sclerosis. Neurology 39: 1018–1026. [DOI] [PubMed] [Google Scholar]
- 3. Goodkin DE, Bailly RC, Teetzen ML, Hertsgaard D, Beatty WW (1991) The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology 41: 20–25. [DOI] [PubMed] [Google Scholar]
- 4. Milanese C, La Mantia L, Salmaggi A, Eoli M (1993) A double blind study on azathioprine efficacy in multiple sclerosis: Final report. J Neurol 240: 295–298. [DOI] [PubMed] [Google Scholar]
- 5. Clegg A, Bryant J, Milne R (2000) Disease-modifying drugs for multiple sclerosis: A rapid and systematic review. Health Technol Assess 4: i–101, i-iv, 1-101. [PubMed] [Google Scholar]
- 6. Yudkin PL, Ellison GW, Ghezzi A, Goodkin DE, Hughes RA, et al. (1991) Overview of azathioprine treatment in multiple sclerosis. Lancet 338: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 7. Goodin DS, Frohman EM, Garmany GP Jr, Halper J, Likosky WH, et al. (2002) Disease modifying therapies in multiple sclerosis: Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology and the MS council for clinical practice guidelines. Neurology 58: 169–178. [DOI] [PubMed] [Google Scholar]
- 8. IFNB MSG (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. the IFNB multiple sclerosis study group. Neurology 43: 655–661. [DOI] [PubMed] [Google Scholar]
- 9. PRISMS (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (prevention of relapses and disability by interferon beta-1a subcutaneously in multiple sclerosis) study group. Lancet 352: 1498–1504. [PubMed] [Google Scholar]
- 10. Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. the multiple sclerosis collaborative research group (MSCRG). Ann Neurol 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 11. Paty DW, Li DK (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI study group and the IFNB multiple sclerosis study group. Neurology 43: 662–667. [DOI] [PubMed] [Google Scholar]
- 12. Casetta I, Iuliano G, Filippini G (2007) Azathioprine for multiple sclerosis. Cochrane Database Syst Rev (4) CD003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Filippini G, Munari L, Incorvaia B, Ebers GC, Polman C, et al. (2003) Interferons in relapsing remitting multiple sclerosis: A systematic review. Lancet 361: 545–552. [DOI] [PubMed] [Google Scholar]
- 14. Palace J, Rothwell P (1997) New treatments and azathioprine in multiple sclerosis. Lancet 350: 261. [DOI] [PubMed] [Google Scholar]
- 15. Etemadifar M, Janghorbani M, Shaygannejad V (2007) Comparison of interferon beta products and azathioprine in the treatment of relapsing-remitting multiple sclerosis. J Neurol 254: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 16. Milanese C, La Mantia L, Salmaggi A, Caputo D (2001) Azathioprine and interferon beta-1b treatment in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 70: 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cavazzuti M, Merelli E, Tassone G, Mavilla L (1997) Lesion load quantification in serial MR of early relapsing multiple sclerosis patients in azathioprine treatment. A retrospective study. Eur Neurol 38: 284–290. [DOI] [PubMed] [Google Scholar]
- 18. Massacesi L, Parigi A, Barilaro A, Repice AM, Pellicano G, et al. (2005) Efficacy of azathioprine on multiple sclerosis new brain lesions evaluated using magnetic resonance imaging. Arch Neurol 62: 1843–1847. [DOI] [PubMed] [Google Scholar]
- 19. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, et al. (2001) Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 20. Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 21.CTC (2003). Cancer therapy evaluation program, common terminology criteria for adverse event, version 3.0, DCTD, NCI, NIH, DHHS.
- 22. Solari A, Filippini G, Mendozzi L, Ghezzi A, Cifani S, et al. (1999) Validation of Italian multiple sclerosis quality of life 54 questionnaire. J Neurol Neurosurg Psychiatry 67: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee for medicinal product for human use (CHMP) (2005). Guideline on the choise of the non-inferiority margin. doc. ref. EMEA/CPMP/EWP/2158/99.
- 24. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ, et al. (2006) Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA 295: 1152–1160. [DOI] [PubMed] [Google Scholar]
- 25. Sackett DL (2004) Superiority trials, noninferiority trials, and prisoners of the 2-sided null hypothesis. ACP J Club 140: A11. [PubMed] [Google Scholar]
- 26.Rubin DB. (1987) Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons. [Google Scholar]
- 27.Brand JPL. (1999) Development, implementation and evaluation of multiple imputation strategies for the statistical analysis of incomplete data sets, ph.D. thesis, erasmus university, rotterdam.
- 28. van Buuren S, Boshuizen HC, Knook DL (1999) Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 18: 681–694. [DOI] [PubMed] [Google Scholar]
- 29. Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG (2012) CONSORT Group (2012) Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 308: 2594–2604. [DOI] [PubMed] [Google Scholar]
- 30. Filippini G, Del Giovane C, Vacchi L, D'Amico R, Di Pietrantonj C, et al. (2013) Immunomodulators and immunosuppressants for multiple sclerosis: A network meta-analysis. Cochrane Database Syst Rev 6: CD008933. [DOI] [PubMed] [Google Scholar]
- 31. Schulz KF, Grimes DA (2005) Sample size calculations in randomised trials: Mandatory and mystical. Lancet 365: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 32. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group (1995) Interferon beta-1b in the treatment of multiple sclerosis: Final outcome of the randomized controlled trial. Neurology 45: 1277–1285. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist.
(DOC)
Trial protocol.
(PDF)
Amendment to the protocol.
(PDF)
Methods S1, Outcomes. Brain MRI: Scan acquisition specifications. Table S1, Serious Adverse Events (SAEs). Table S2, AEs – subtypes.
(DOCX)