Abstract
TCR-mediated specific recognition of antigenic peptides in the context of classical MHC molecules is a cornerstone of adaptive immunity of jawed vertebrate. Ancillary to these interactions, the T cell repertoire also includes unconventional T cells that recognize endogenous and/or exogenous antigens in a classical MHC-unrestricted manner. Among these, the mammalian nonclassical MHC class I-restricted invariant T cell (iT) subsets, such as iNKT and MAIT cells, are now believed to be integral to immune response initiation as well as in orchestrating subsequent adaptive immunity. Until recently the evolutionary origins of these cells were unknown. Here we review our current understanding of a nonclassical MHC class I-restricted iT cell population in the amphibian Xenopus laevis. Parallels with the mammalian iNKT and MAIT cells underline the crucial biological roles of these evolutionarily ancient immune subsets.
Keywords: Evolution of immunity, Nonclassical MHC class I, Xenopus, iVα6T, Invariant T cells, XNC, MAIT, iNKT, Comparative immunology, Innate T cells, Tadpole, Viral immunity
Introduction
Major histocompatibility complex (MHC) class I genes encode a variety of multifaceted antigen presenting molecules critically involved in both innate and adaptive immune responses. Classical MHC class Ia (class Ia) genes encode highly polymorphic and broadly expressed molecules that are essential for the differentiation and function of adaptive CD8+ T cells. These conventional CD8+ T cells express highly diversified somatically rearranged T cell antigen receptors (TCRs) that specifically recognize antigenic peptides in the context of class Ia molecules. The structurally similar but functionally disparate nonclassical MHC class I genes (class Ib and class I-like) encode a heterogeneous group of molecules that, in general, are oligomorphic and have a more limited tissue distribution. In a manner more akin to innate immune receptor interactions, many of these nonclassical MHC class I molecules bind and present a selective range of conserved molecular or structural patterns to distinct effector cell populations (reviewed in [1–3]). Indeed, whereas class Ia genes are pivotal during adaptive immune responses, nonclassical MHC class I molecules are mostly appreciated as regulators of the innate immune response through their interaction with various germline-encoded receptors expressed on natural killer (NK) cells. In addition, it is well-established that certain nonclassical MHC class I molecules act as ligands for innate-like αβT cells, termed invariant T (iT) cells. In mammals, two distinct subsets of nonclassical MHC class I-restricted iT-cell with different antigen specificities and effector properties have been described: the CD1d restricted iNKT cells and the MHC molecule-related 1 (MR1)-restricted mucosal-associated invariant T (MAIT) cells [4–7]. Compared to conventional naïve T lymphocytes, these iT cells display memory/effector-like phenotypes and are rapidly activated following primary stimulation [8]. This antigen specific activation of a relatively large lymphocyte subpopulation that bypasses the need for clonal expansion facilitates rapid recognition of invading microbes thereby highlighting the importance of iT cells during the early phases of antimicrobial defenses. Current evidence shows that distinct iT subsets exhibit a remarkable degree of functional plasticity and, upon activation, can secrete either pro-inflammatory or immunosuppressive cytokines that subsequently influence different effector cells including NK cells, conventional T cells, macrophages, neutrophils and dendritic cells [9–13].
The innate immune-like functional characteristics and their use of semi-invariant TCRs, in many ways akin to germ line-encoded receptors suggest that iT cells represent a primordial T cell compartment with disparate recognition/activation mechanisms compared to conventional T cells [14]. Recently, we identified, in the amphibian Xenopus laevis, a distinct nonclassical MHC class I-restricted iT cell population. These Xenopus nonclassical 10 (XNC10)-restricted iVα6T cells appear to be critically involved in tadpole immunity [15]. This discovery provides evidence of an evolutionarily ancient origin of iT cells. Furthermore, this indicates that despite the indeterminate evolution and general lack of nonclassical MHC class I orthology, physiologically important functions of nonclassical MHC class I molecules in the development and functional regulation of specialized innate-like unconventional T cells has been evolutionarily retained across vertebrates.
Unlike mammals, the Xenopus immune system, and in particular, T cell differentiation is subject to a major developmental remodeling during metamorphosis. Although both tadpoles and adult frogs are immunocompetent and have conventional CD8+ T cells, the tadpole thymus lacks significant class Ia protein expression until metamorphosis [16–18]. However, several distinct nonclassical class I genes are expressed in the tadpole thymus suggesting a prominent involvement of these genes in T cell development at a stage when class Ia function is suboptimal [19, 20].
In this review, we highlight the functional and evolutionarily conserved roles of key nonclassical MHC class I molecules as restricting elements in iT cell biology in light of the recently identified X. laevis XNC10-restricted iT cell subset. We also discuss the presence of distinct unconventional T cell subsets in non-mammalian vertebrates and address the plausible key roles of these populations during immune system development and initiation of immune responses. Mammalian CD1d restricted-iNKT and MR1 restricted-MAIT cells have been reviewed in detail, most recently in [21] and [22, 23]. Thus, the focus of this review lies in discerning the biological analogies and differences between these mammalian iT cells and the evolutionarily antecedent Xenopus unconventional T cells.
Specialized roles of jawed vertebrate nonclassical MHC class I genes
Evolution of nonclassical MHC class I genes
Nonclassical MHC class I genes are present in varying numbers in all taxa of jawed vertebrates, from chondrichthyes to mammals; this underlines the biological importance of these molecules. However, the evolutionary history of nonclassical MHC class I genes has been dynamic resulting in multiple diversifications and species-specific adaptations (reviewed in [1]). Indeed, even among closely related species, nonclassical MHC genes typically display extensive intra-species variation in gene composition, numbers and genomic organization [1, 24, 25]. This has been partly attributed to the “birth and death” model of evolution in which new genes arise via gene duplication [26]. While some of these duplicated genes are maintained in the genome others undergo neofunctionalization or degradation [27, 28]. To date, phylogenetic relationships among various nonclassical MHC class I genes are not fully understood and only few unambiguous orthologous or even homologous have been described across different vertebrate orders and families.
Phylogenetic analysis of the human and murine nonclassical MHC genes indicates a loose grouping where genes encoding nonclassical class I peptide-presenting molecules typically cluster more closely with class Ia genes of their respective species [25] (and reviewed in [1]). This indicates an evolutionarily recent species-specific divergence. In fact, these nonclassical MHC class I genes, which include the human HLA-G and HLA-F as well as the murine Qa and Q families, are thought to have diverged as recently as ~5–20 million years ago (MYA) from the class Ia of their respective linages [2]. In general, these nonclassical MHC class I molecules have retained many of the features of a class Ia molecule including presentation of peptide antigens. However, the possible peptide repertoire of these nonclassical MHC class I molecules is probably more limited than the class Ia peptide repertoire [29–32]. In addition, many of these nonclassical MHC class I genes display tissue-specific expression and perform functions different from those of class Ia molecules [33–36]. In humans and mice, these nonclassical class I genes are located within the MHC locus proper in relative close proximity to the class Ia genes. In contrast, other nonclassical class I genes are phylogenetically divergent from class Ia molecules of the same species and more related to the corresponding gene of another species. Many of these nonclassical MHC genes have diverged to perform disparate functions such as ligands for various NK receptors, γδT cells and iT cells. The best studied amongst these nonclassical class I genes are the CD1 family and MR1 [37, 38]. Compared to class Ia, CD1 and MR1 have distinct antigen binding grooves that are optimized to bind and present unique non-peptide antigens to distinct T cell subsets [39–41]. In both humans and mice, genes of MR1 and CD1 families are linked and located on a different chromosome from the one housing the MHC locus [42]. However, in human, genomic analysis has established that the genomic segment with MR1–CD1 genes is paralogous to the segment encoding the HLA class Ia genes [43–45]. The ancient origin of the CD1 family is further supported by the identification of two divergent CD1 genes in chickens, suggesting that this gene was present prior to the mammalian/avian split and thus, has been maintained for ~310 million years of evolution [46, 47]. To date, MR1 has only been described in mammals. However, the chicken MHC class I-like molecule YF1*7.1 that shares 38 % sequence identity with, and is structurally similar to, human MR1, may represent an avian MR1-like gene [41, 48]. Based on these studies, it has been hypothesized that both MR1 and CD1 are phylogenetically old and, possibly via different evolutionary paths, might have evolved from a duplication of the MHC class I locus in a primordial reptilian ancestor. It has been further inferred that this duplication facilitated the divergence of the MR1 and CD1 genes. Thus, while retaining a general role as antigen presenting molecules, these nonclassical MHC class I genes have become specialized in presenting non-peptide antigens to distinct subsets of iT cells.
While several ectothermic vertebrate genomes have been fully sequenced and annotated, to date no CD1 or MR1 homologs have been identified. In fact, although the syntenic regions containing CD1 have been located in the genome of X. tropicalis and X. laevis, no gene models with CD1 or MR1 sequence similarity have been found (Y. Ohta, personal communication). More generally, to date, no clear orthologous relationships have been established for any nonclassical MHC class I genes between endothermic and ectothermic vertebrates. Nevertheless, comparative analysis has identified a nonclassical MHC class I gene in the amphibian X. laevis, termed XNC10, which is required for the function and development of a distinct subpopulation of iT cells. This provides evidence for the existence of a T cell subset functionally similar to the mammalian CD1- and MR1-restricted iT cells in amphibians. Further, it emphasizes the integral and evolutionary conserved roles of nonclassical class I molecules in the development and function of specialized T cell populations.
Nonclassical MHC class I molecules as restricting elements for mammalian iT cells
Members of the CD1 gene family comprise five distinct isoforms (CD1a–e) that display a typical MHC class I structure, a limited polymorphism, and a highly hydrophobic antigen-binding groove optimized for the presentation of lipids and glycolipids. Each CD1 isoform has unique structural features, particularly within the peptide-binding groove, and is adapted to present a specific, albeit overlapping repertoire of lipid structures. Whereas CD1 genes are ubiquitously expressed in mammals and most likely in avian [47], the total number of genes and expression profiles of the different CD1 isoforms are disparate among different species (reviewed in [37]). CD1d homologs have been described in humans, primates, rodents and ruminants [37]. In human and mice, CD1d has been shown to be the restricting element for NKT cells (reviewed in [49, 50]). NKT cells are subdivided into two groups. Type I or invariant NKT (iNKT) cells express a semi-invariant TCR composed of an evolutionary conserved invariant TCRα-chain (Vα24-Jα18 in humans/Vα14-Jα18 in mice [51]) associated with a limited TCRβ-chain repertoire. Based on robust gene expression of a homologous iTCRα chain, type I iNKT cells are also inferred to exist in rhesus macaques [52] and rats [53]. In contrast, type II NKT cells are CD1d restricted but do not express the canonical invariant TCRα chain and display a broader TCRαβ repertoire. Type I iNKT cells are functionally defined as being effectively activated by the CD1d ligand glycolipid α-galactoceramide (αGalCer) originally derived from the marine sponge Agelas mauritanus [54]. In contrast, type II NKT cells do not recognize αGalCer. More recently, iNKT cells were also demonstrated to recognize, in the context of CD1d, endogenous antigens and microbial glycolipids derived from different microorganisms. These glycolipids include: α-glucosyldiacylglycerols from Streptococcus pneumoniae [55]; α-glucuronosylceramides and α-galacturonosyl-ceramide from Sphingomonas spp. [56]; and α-galactosyldiacylglycerols from Borrelia burgdorferi [57]. Although these different microbial ligands typically share the α-glycoside linkage, their structures are quite diverse. It is of note that the interaction between the semi-invariant TCR on iNKT cells and the lipid–CD1d complex is primarily mediated by the invariant CDR3α loop and to a lesser extent the CDR2β loop; this differs from conventional TCR–peptide–class Ia interactions [58] (reviewed in [49, 59]). Furthermore, the interaction pattern between the semi-invariant TCR and the antigen–CD1d complex is similar irrespective of antigens (i.e., the iTCR is capable of recognizing different antigens [60]). Thus, iNKT cells can bind a range of antigens through the recognition of conserved structural motifs. Interestingly, the pattern of CD1d–antigen–iTCR interaction is almost identical between humans and mice [61]. This intra-species cross-reactivity between human and mouse CD1d-iNKT is in stark contrast to class Ia–peptide–TCR interactions, indicating that semi-invariant TCRs of iNKT cells function as pattern recognition receptors [49, 62, 63]. In addition to this direct iTCR-mediated recognition of microbial ligands, a second pathway of iNKT cell activation that is independent of microbial lipid recognition has been described (reviewed in [64]). This type of activation relies on the binding by CD1d of endogenous or self-ligands and the sensing endogenous lipids overexpressed as a result of microbial infection [65]. Thus, iNKT cell activation during infection can be driven by both TCR-mediated recognition of microbial products (direct) or by self-ligand/cytokine-mediated (indirect).
The second nonclassical MHC class I molecule involved in the regulation and function of iT cells in mammals is MR1. The gene encoding MR1 originally described in humans has now been identified in a number of different mammals including non-human primates, rodents, cattle, sheep, and more recently, in placental mammals [38, 66]. An MR1-like gene has also been described in chickens [48]. The mammalian MR1s are monogenic and display between 81 and 89 % sequence identity in the putative peptide binding domains. Thus, the MR1 gene is the most highly conserved mammalian nonclassical MHC class I gene [66]. Whereas MR1 mRNA transcript levels are ubiquitously expressed, the MR1 protein accumulates in the ER; and only low levels of MR1 are detected on the cell surface [67]. This suggests that MR1 surface expression is inducible and possibly subject to limiting factors. Furthermore, MR1 exists in both a folded and unfolded conformation but only the properly folded conformation can activate MAIT cells [68]. MR1-restricted MAIT cells have many similarities with iNKT cells, but clearly represent a unique and functionally distinct iT cell subpopulation. Indeed, MAIT cells express an evolutionarily conserved invariant TCRα (Vα7.2-Jα33 in humans/Vα19-Jα33 in mice), distinct from that of iNKT cells (reviewed in [69]). In humans, MAIT cells are abundant in peripheral blood and liver where they are as much as 1–8 and 20–45 % of the total T cell pools, respectively. Additionally, these cells localize in the gastrointestinal tract and associated organs such as the mesenteric lymph nodes. Human MAIT cells express high levels of chemokine receptors involved in cell trafficking to the intestine and liver (CCR6 and CXCR6); they do not express CCR7 that is required for migration into lymph nodes [70]. Both human and mouse MAIT cells mediate antimicrobial responses in a MR1-dependent manner [69]. MR1-dependent MAIT cell activation has been demonstrated in the context of several different microbial challenges, including gram-positive, gram-negative bacteria and yeast, but not viruses [69]. More recently, a direct cytotoxic effect of MAIT cells was also demonstrated against epithelial cells infected with Shigella flexneri but not Salmonella enterica typhimurium bacteria [71]. These studies underline the important role of MAIT cells in antimicrobial immunity. A selection of MR1 ligands have recently been elucidated as pterin analogs, which are derived from vitamin B metabolism as well as lumazine compounds [41]. As many vitamin biosynthetic pathways are unique to and present in most (but not all) bacteria and yeast, this provides a way for MAIT cells to detect microbial infections [41].
Nonclassical MHC class I genes in ectothermic vertebrates
Like endothermic vertebrates, amphibians, teleostean and cartilaginous fish species possess varying numbers of divergent MHC nonclassical class I genes that are heterogeneous, display lower polymorphism and have a more tissue restricted expression pattern when compared to their class Ia counterparts. However, the evolutionary history of class Ib genes in ectothermic vertebrates remains largely unknown and the phylogenetic relationships of these genes are difficult to establish. With regard to amphibians, both X. laevis and X. (Siluriana) tropicalis possess a single class Ia gene per genome and a large number of Xenopus nonclassical MHC class I (XNCs) genes, which, in contrast to mammals, display an unusual degree of inter-species conservation [72, 73] (these genes are discussed in detail in “Genetic and evolutionary conservation of nonclassical MHC class I genes in divergent Xenopus species”). In comparison, a large number of both putative class Ia and nonclassical class I genes have been identified in the urodele amphibian Ambystoma mexicanum, although none of these genes are orthologous to any of the XNC/SNC genes identified to date [74]. Furthermore, Southern blot analysis suggests that nonclassical, class Ia and MHC class II genes are linked in A. mexicanium, which is unlike Xenopus where most nonclassical class I genes identified to date form a single linkage group outside the MHC region [74]. Variable numbers of highly divergent species-specific MHC nonclassical class I genes have also been described in elasmobranchs [75, 76]. Similarly, teleost species encode a number of divergent class I genes that based on evolutionary relationships have been grouped into distinct lineages (U-, Z/ZE-, L- and S-lineage) and are differentially distributed among species [77]. For example, genes belonging to the U-lineage, (containing both putative class Ia and nonclassical class I genes) are broadly represented among divergent species, whereas to date, the L-lineage that consists of highly divergent nonclassical class I genes has only been identified in salmonids and cyprinids [78]. Notably, the Atlantic cod (Gadhus morhua) has lost MHC class II genes coinciding with a large expansion (more than 100 genes) of their U-lineage class I genes that segregate into two distinct phylogenetic clades [79]. These different class I clades display distinct ratios of non-synonymous to synonymous mutations and differences in the average nucleotide diversity per site, suggesting possible neofunctionalization for this MHC class I lineage [79]. To date, despite the presence of nonclassical MHC class I genes in all ectothermic vertebrates, no conserved orthologs have been identified across distant species and the functional roles of ectothermic nonclassical MHC class I genes remains largely unknown. However, the fact that these genes are present in all taxa of jawed vertebrates attests to their evolutionary primordial origins.
Genetic and evolutionary conservation of nonclassical MHC class I genes in divergent Xenopus species
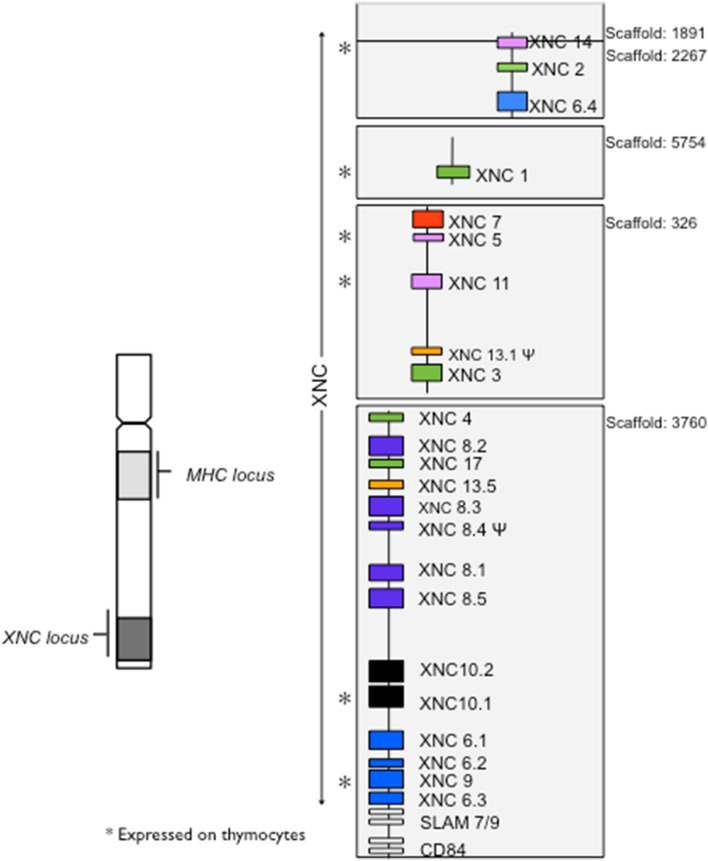
Many Xenopus nonclassical MHC class I (XNC) genes display an unusually high degree of inter-species conservation both in their primary sequence as well as in their genomic organization [73]. Indeed, multiple distinct XNC lineage orthologs have been reported among species of the anuran subfamily Xenopodinae. Notably, this high degree of conservation is apparent in XNC genes identified in both X. laevis and X. tropicalis despite the fact that these species are estimated to have diverged more than 65 MYA [80, 81], which roughly corresponds in time to the split between rodent and primate ancestors. Both X. laevis and X. tropicalis possess a large number of nonclassical MHC class I genes (XNC for Xenopus and SNC for Silurana). Currently, 23 XNC and 29 unique SNC genes (including pseudogenes) have been identified. The genomic organization, based on the current genome using the http://xenopus.lab.nig.ac.jp, XenVis 2.0 assembly, of the XNC loci in X. laevis is depicted in Fig. 1. Based on sequence similarities in the α1 and α2 domains, these genes have been grouped into 17 distinct subfamilies. Among these 17 subfamilies, 11 have clear orthologous relationships between X. laevis and X. tropicalis sharing between 70 and 85 % amino acid identities in their putative ligand binding domains. This degree of conservation of multiple nonclassical class I genes is in contrast to that observed across mammals (with the exception of CD1 and MR1), and as such, suggests evolutionarily disparate paths for amphibian nonclassical MHC class I genes and those from other vertebrates. Collectively, all XNC/SNC genes form an independent phylogenetic clade, distinct from other vertebrate nonclassical MHC class I genes as well as amphibian class Ia sequences, suggest that XNC genes are all derived from a common ancestor [73]. The phylogenetic relationships among XNC/SNC genes also indicate that many of these gene subfamilies have undergone either species-specific lineage expansions or contractions [73, 82]. Each XNC/SNC subfamily member is distinct, particularly in the putative ligand binding domains, suggesting that various XNC/SNC gene lineages have evolved to perform distinct functions. To date the functional roles of most XNCs remains unknown. However, since their discovery by Flajnik et al. [72], a wealth of information has been garnered. Many XNC/SNC genes display highly tissue-specific expression patterns and developmental stages-specific expression [19, 20]. Some XNC genes are differentially regulated in response to infections by ranavirus (see below) and bacteria such as Mycobacterim marinum (Edholm and Robert, unpublished observations). Certain XNC gene products are also implicated in tumor immunity. Specifically, overall silencing of XNC expression in the 15/0 lymphoid tumor using RNA interference results in increased tumorigenicity in vivo [83]. Additionally, with the recent generation of an XNC10-tetramer that specifically binds distinct XNC10-restricted unconventional T cell subsets, putative biological roles and evolutionary functional origins of nonclassical class I molecules are now emerging [15].
Fig. 1.
Genomic organization of X. laevis nonclassical MHC class I genes. XNCs are located distally from the MHC locus on the same chromosome with the MHC in the middle of the long arm and the XNC locus at the tip of the same arm [84]. Organization of the 23 XNC genes including pseudogenes, indicated by Ψ. XNC genes are located on five different scaffolds based on the Xenopus laevis Genome project http://xenopus.lab.nig.ac.jp, XenVis 2.0 assembly. The number of each scaffold is indicated on the right. XNC genes expressed on tadpole thymocytes are indicated by an asterisk. Orthologous relationships defined by multiple sequence alignments and neighbor-joining phylogenetic analysis of the α1/α2 domains of XNC genes are indicated by colors with each color representing a phylogenetic grouping based on a bootstrap value of >95. Flanking unrelated genes are shown as white boxes
XNC10
The XNC10 gene was originally identified from the X. laevis thymic lymphoid tumor cell line 15/0 [20] and subsequently shown to be expressed at varying levels in several independently derived X. laevis lymphoid tumor cell lines [20]. The genomic structure of XNC10 is similar to that of a typical MHC class I gene with three extracellular immunoglobulin domains, a transmembrane and a cytoplasmic region. In the genome, XNC10 is located distally from the class Ia gene [84] within the XNC locus (Fig. 1). The XNC10 gene is primarily expressed in the thymus, specifically by immature thymocytes located in the thymic cortex [20]. During ontogeny, XNC10 expression is detected from the onset of thymic organogenesis, before significant detection of class Ia protein. Additionally, low levels of XNC10 expression are detected in the spleen of premetamorphic tadpoles and adults. Based on co-immunoprecipitation experiments using transfection of tagged β2-microglobulin (b2m) into 15/0 tumor cells, XNC10 molecule is most likely expressed on the surface as a heterodimer associated with b2m [83]. Interestingly, tagged recombinant XNC10 proteins expressed in cell lines are mostly retained intracellularly leading to only a small fraction expressed at the cell surface; this is similar to MR1 (Edholm and Robert, unpublished data). In vivo expression of the XNC10 protein appears to be complex and may include extensive post-translational modifications. A recently generated panel of anti-XNC10 antibodies raised against different parts of the molecule will permit us to elucidate this aspect of XNC10 biology [107].
A XNC10 ortholog termed SNC10 has been identified in X. tropicalis. SNC10 shares 54 and 62 % sequence identity to XNC10 in the α1 and α2 domains, respectively [73]. This is comparable to the degree of conservation of CD1d between humans and mice (~60 % identity) whose divergence in evolutionary history, similar to that of X. laevis and X. tropicalis is ~65 MYA. Phylogenetic analysis indicates that XNC/SNC10 represents a unique clade intermediate between X. laevis and X. tropicalis class Ia genes and other XNC genes. This suggests that the XNC/SNC10 gene lineage was one of the first to diverge from a common XNC/SNC ancestor. The expression pattern of SNC10 in X. tropicalis mirrors that of XNC10 in X. laevis including primary expression on thymocytes from early development when class Ia protein expression is naturally low. This suggestion of a conserved function of XNC/SNC10 homologs is further supported by examination of additional Xenopus species [82]. Of interest, species from the Xenopodinae subfamily exemplifies a rare example of speciation in vertebrates by genome duplication via allopolyploidization [80]. Indeed, the Xenopus genus represents an exceptional case among jawed vertebrates comprising of a collection of species with different degrees of natural polyploidy. There are true diploid species such as, X. (Siluriana) tropicalis; a series of teraploid species, including X. laevis, (4n; X. gilli, X. clivii, X. largeni, X. fraseri, X. pygmaeus, X. borealis, X. mulleri and S. epitropicalis), octoplod (8n; X. wittei, X. vestitus, X. andrei, X. boumbaensi and X. amieti) and at least two dodecaploid species (12n; X. longipes and X. ruwenzoriensis). A single functional, monomorphic gene encoding XNC10 has been identified in all ten Xenopodinae species studied to date regardless of genomic ploidy. These results are consistent with the idea that certain nonclassical MHC class I genes are evolutionarily primordial and possibly even as ancient as class Ia.
Unconventional T cell subsets
T cell subset heterogeneity in ectothermic vertebrates
T cell-mediated immune responses of ectothermic vertebrates share many similarities with those described in mammals and birds. For example, allo-antigen specific T cell-mediated cytotoxicity as well as T helper cell-like functions have been demonstrated in several teleostean [85–87] and amphibian species (reviewed in [88]). Furthermore, all four types of TCR genes have been identified in amphibian, reptiles, teleosts and elasmobranchs indicating that γδT cells and αβT cells are present across jawed vertebrates. Additionally, a plethora of classical TCR signaling components, co-stimulatory/inhibitory receptor as well as CD4, CD8α and CD8β co-receptor homologs have been identified in many ectothermic vertebrate species. These findings are consistent with a phylogenetically conserved and evolutionary ancient T cell sub division into CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T helper cell subsets. However, whereas a single CD4 gene structurally similar to its mammalian counterpart has been described in X. laevis [88], two types of CD4 co-receptors have been isolated from several teleost species: a CD4 molecule with 4 Ig domains and a CD4-like molecule with 2–3 Ig domains [87, 90–92]. Furthermore, in addition to the loss of MHC class II and invariant chain genes, the Atlantic cod appears to also lack genes encoding CD4 [79]. Based on the recent genome sequencing of the elephant shark (Callorhinchus milii), key elements associated to the CD8+ T cell linage including class Ia, cytokines and transcription factors are conserved in elasmobranch [93]. Notably, with the exception of the Th1 lineage many transcription factors and cytokines typically associated with CD4+ T helper cell lineages diversification in mammals appears to be absent or very divergent in the C. milii genome suggesting the presence of a limited T helper cell subset diversification [93]. In teleosts, although the functional roles and sub-compartmentalizations of T helper cells remain poorly understood, gene homologs encoding transcription factors and cytokines defining T helper subsets such as Th1, Th2, Th17, Treg have been identified [94–96].
Identification of unconventional T cell subsets in Xenopus
Studies conducted with Xenopus during the past three decades have revealed the presence of NK-like lymphocytes with MHC class Ia-unrestricted cytotoxicity [97] as well as distinct T cell sub-populations with varying MHC class I restriction and effector functions. These T cell subsets are summarized in Table 1 and include (1) conventional class Ia-restricted CD8+ T cells [98]; (2) unconventional class Ia unrestricted CD8+ T [99, 100]; (3) NKT cells [101]; and (4) class Ib restricted invariant T cells [15]. Gene expression analysis also suggests the presence of a CD4+ T helper subset in Xenopus but these cells have yet to be phenotypically and functionally characterized [89]. Xenopus cell-mediated antigen-specific class Ia restricted cytotoxicity against both major and minor H-antigens has been characterized using total splenocytes and antibody purified CD8+ T cell populations. Adoptive cell transfer of primed CD8+ T cells has further showed that these cells proliferate and accumulate in the spleen of isogeneic recipients in response to immunization with MHC (minor H-antigens) disparate antigens. Collectively, these studies unequivocally demonstrated that Xenopus can generate typical class Ia restricted CD8+ CTL responses.
Table 1.
Overview of distinct T cell subsets in Xenopus laevis compared to human and mouse nonclassical MHC class I-restricted iT cell populations
| Xenopus | Human and mouse | |||||
|---|---|---|---|---|---|---|
| CTL | CCU-CTL | NKT | iT | Type I iNKT | MAIT | |
| TCRα | Broad repertoire | Broad repertoire | ND | Type I invariant: Vα6-Jα1.43/Type II restricted | Invariant: hVα24/m14-Jα18 | Invariant: hVα7.2/m19-Jα33 |
| TCRβ | Broad repertoire | Broad repertoire | Broad repertoire | Type I invariant: Vβ6/Vβ13 and Jβ13/Jβ3/Type II restricted | mVβ2,7,8/hVβ11 | mVβ6,8/hVβ2,13 |
| Phenotype | CD8+ | CD8+ | CD8+ | DN, CD8ααdim | CD8+, CD4+, DN | CD8+, DN (CD4+) |
| NK 1F8 marker | − | − | + | − | NA | NA |
| MHC restriction | Class Ia | XNC | ND | XNC10 | CD1d | MR1 |
| Ligand | Peptides? | ND | ND | ND | Lipids | Vitamin B metabolites |
| Function | Anti-viral response | Class Ia negative tumor rejection | ND | Tadpole anti-viral immunity 15/0 tumor rejection | Tumor immunity, viral immunity and autoimmunity | Anti-microbial |
ND not determined, NA not applicable
In addition to conventional CD8+ CTLs, the description of a subpopulation of CD8+ T cells with class Ia unrestricted cytotoxicity has provided the first evidence for unconventional CTLs in amphibians. These cells, termed classical class Ia-unrestricted CTLs (CCU-CTLs) specifically recognize and kill the highly tumorigenic class Ia negative thymic lymphoid tumor 15/0 [99]. Since the 15/0 tumor cell line express several nonclassical MHC class I molecules, in particular XNC6, XNC10 and XNC11, as well as β2-microglobulin, it was postulated that CCU-CTLs interact with distinct XNC molecules [20]. This hypothesis was supported by the findings that 15/0 tumor transfectants expressing short hairpin RNA (shRNAs) targeted to globally knock down XNC expression display increased resistance to CD8+ CCU-CTL-mediated killing in vitro and increased tumorigenicity in vivo [83]. These findings are in concordance with the involvement of XNC molecules in unconventional T cell-mediated cytotoxicity. Notably, the indiscriminate down-regulation of the total XNC expression on the 15/0 tumor cells also resulted in increased susceptibility to NK-cell like mediated killing in vitro [83] suggesting that different XNC subfamilies interact with distinct, non overlapping effector populations.
NK cells were characterized in adult Xenopus using the anti-NK cell mAb 1F8 [97]. In addition to NK cells, a small subset of CD8+ T cells co-expressing the NK cell-associated marker recognized by the 1F8 mAb was identified, suggesting the existence of NKT cells in amphibians. These CD8+ NKT cells constitute ~4 % of the total T cell population in the spleen and express fully rearranged TCRβ transcripts bearing Vβ segments derived from at least three different Vβ families [101]. To date, the TCRαβ repertoire diversity of these NKT cells have yet to be characterized and it is still unclear whether there is any bias in their TCRα usage or target specificity. Although the functional roles of these NKT cells remains to be elucidated, in vitro stimulation of CD8+ T cells using transient low level mitogen stimulation resulted in induced surface expression of the 1F8-reactive NK cell-associated molecules in about 30 % of CD8+ T cells [101]. Furthermore, up-regulation of NK-associated markers correlated with decreased cytotoxicity of CD8+ T cells against MHC mismatched tumor targets in vitro [101] suggesting that the role of NK cell-associated molecules in regulation of CD8+ T cell activity is phylogenetically conserved.
Xenopus nonclassical MHC class I-restricted iT cell subsets
In addition to X. laevis conventional and unconventional CD8+ CTLs expressing broad TCRαβ repertoires, we recently identified two distinct subsets of nonclassical MHC class I (XNC10)-restricted unconventional αβT cells with limited TCRαβ repertoires: type I and type II [15]. Both subsets were isolated based on reactivity to XNC10-tetramers and subsequently subdivided based on CD8 surface expression and TCRαβ repertoire. Type I cells, coined iVα6 T cells, are CD8−/CD4− double negative (no CD8 transcript detected by RT-PCR) and express a semi-invariant TCR consisting of an invariant TCRα chain (Vα6-Jα1.43) paired with a limited TCRβ chain repertoire. The invariant Vα6-Jα1.43 chain is the product of a single Vα-Jα rearrangement with a non-variable strictly germ-line encoded CDR3α junctional region (i.e., no detectable n-nucleotide diversifications). Moreover, no CDR3α variability was observed among Vα6-Jα1.43 rearrangements isolated from X. laevis with different genetic backgrounds. Comparably, the iVα6 T cell TCRβ repertoire is more diverse, yet very limited in comparison to conventional CD8+ T cells with predominant usage of the Vβ6/Vβ13 and Jβ13/Jβ3 gene segments [15].
Type II XNC10-tetramer+ cells express surface CD8, albeit at lower levels than conventional T cells; presumably, it is expressed as a homodimeric CD8αα receptor, since only the CD8α gene is expressed. Type II cells have a more diverse TCRαβ repertoire compared to type I cells, although the invariant Vα6-Jα1.43 is predominant representing 58 % of the total TCRα rearrangements [15]. Since the subdivision of XNC10-tetramer+ type I and type II cells is based on CD8 surface staining, it is possible that under certain conditions, type I iVα6 T cells induce CD8 expression. This would account for the overrepresentation of the iVα6-Jα1.43 rearrangement in type II XNC10-tetramer+ cells. To date the relationship and putative functional disparity between these two types of XNC10-T+ cells remains unclear. However, it is noteworthy that this subdivision is reminiscent of mammalian type I and type II NKT cells. In mammals, although both type I and type II NKT cells recognize glycolipids presented by CD1d, only type I iNKT cells are strongly reactive against CD1d tetramers loaded with the glycolipid αGalCer, thereby suggesting distinct antigen specificities between the two subsets [102]. Recently, it was shown that a significant portion of type II NKT cells are potently activated by β-d-glucopyranosylceramide (β-GlcCer). Although type I and type II NKT cells produce the same profiles of major cytokines following activation through their TCRs, they have distinct cytokine-producing capacities, indicating non-overlapping functional roles [103].
Functional roles of Xenopus XNC10-restricted iT cell subsets
Both type I and type II XNC10-tetramer cells are abundant in the spleen of adult frogs. Type I typically represent ~3.5 % and type II ~2.5 % of the total lymphocyte population. In addition, type I cells represent a significant fraction of peripheral blood leukocytes (0.5–1.5 %). As in adults two distinct XNC10-tetramer populations, either CD8− or CD8dim, were identified among the splenocytes of premetamorphic tadpoles (2–3 weeks of age, developmental stage 55; [104]). Thus, XNC10-restricted T cells constitute a prominent fraction of splenic T cells during early ontogeny when class Ia protein expression is suboptimal and after metamorphosis in class Ia competent adults. Functional characterization of XNC10-restricted iT cells has rapidly progressed by assessing their roles in antiviral and tumor immunity of natural class Ia-deficient tadpoles. The critical involvement of iT cells during viral infection was first inferred from the observation that at an early developmental stages XNC10-deficient transgenic tadpoles (expressing an anti-XNC10 shRNA) had a greater than two-fold increase in susceptibility to infection with the ranavirus Frog virus 3 (FV3). FV3 is a highly prevalent, large double stranded DNA virus that causes extensive disease and mortalities of wild and cultured amphibian species. FV3 and FV3-like viruses have gained considerable attention due to the alarming rate with which these pathogens are spreading to new ectothermic hosts (i.e., from amphibians, to fish and reptiles; reviewed in [105]). Although, Xenopus tadpoles are more susceptible to FV3 infection than adults, they do mount anti-viral immune responses, and typically it takes 4–6 weeks for them to succumb to FV3 infection [106]. In contrast, a significant fraction of XNC10-deficient tadpoles die within the first 2 weeks following infection. Moreover, this accelerated mortality in transgenic tadpoles correlated with increased viral loads and increased viral dissemination from kidneys (the primary site of viral replication) to other organs (manuscript in preparation, Edholm and Robert). These findings provide evidence that XNC10-restricted iT cells are critically involved in tadpole antiviral immunity.
To further explore the functional significance of tadpole iT cells, we took advantage of the X. laevis transplantable 15/0 thymic tumor model. The 15/0 tumor is highly tumorigenic when transplanted into LG-15 syngeneic tadpoles and adults. Importantly, this tumor is class Ia negative but expresses several XNC molecules including XNC10. Using this model we recently showed that iT cells accumulate in the tadpole peritoneal cavity following intraperitoneal transplantation with 15/0 tumor cell line [107]. Interestingly, whereas XNC10-tetramer+ cells are rare in the peritoneal cavity of naïve tadpoles, these cells make up a significant fraction (10–20 %) of peritoneal leucocytes 7 days following transplantation with the 15/0 tumor. These XNC10-tetramer+ cells were almost exclusively of the CD8− type I phenotype suggesting that these different XNC10-tetramer reactive subsets indeed have distinct functional roles [107].
The presently unknown identity of the XNC10 ligand?
In mammals, the class Ia protein fold is made up of the two membrane distal domains, α1 and α2, and consists of a platform made up of eight antiparallel β-strands, topped by two semi parallel α-helixes oriented away from the cell membrane, thereby creating a shallow groove at the top of the molecular structure [108]. This groove is closed at both ends and highly adapted to present either self or non-self peptides 8–10 amino acids in length. Class Ia peptide binding occurs through side chain independent recognition of the peptide main backbone via nine invariant, highly evolutionarily conserved amino acid residues as well as through MHC allele-specific peptide anchoring residues. The invariant residues are clustered in two shallow pockets, A and F, located at both ends of the groove and form hydrogen bonds with the amino and carboxyl terminal ends of the peptide, respectively [109]. With the exception of these residues, the class Ia peptide-binding domain is highly polymorphic and under strong positive selection (i.e., variation rather than conservation of residues is selected) [110]. Compared to class Ia, many nonclassical MHC class I molecules bind a narrower range of antigens, each with its own distinct molecular and/or structural pattern. Crystallography data have revealed that although the basic MHC class I fold architecture is maintained, the antigen binding grooves of most nonclassical MHC class I molecules studied to date display distinct structural and chemical compositions [1]. These differences determine the nature and repertoire of the antigen presented by these molecules. For example, whereas some nonclassical MHC class I molecules present a relatively diverse array of peptides in a manner highly reminiscent of class Ia, the binding groove of other nonclassical MHC class I molecules has evolved to either preferentially present specific peptides (HLA-E and Qa-1 [111, 112]), modified proteins (M3 [113]), lipids (CD1 [39]), vitamin metabolites (MR1; [114]) or to be empty (T22, MIC-A [1]). Thus, insights into the type of ligands bound and subsequently clues to the functions of different class Ib molecules can, to a certain extent, be inferred from the primary and tertiary composition of the putative peptide binding grooves.
In Xenopus, the ligand presented by XNC10 is unknown. However, several lines of evidence indicate that XNC10 presents a conserved ligand. Based on its deduced amino acid sequence, XNC10 displays a MHC class I-like structure with a putative peptide-binding groove that could potentially accommodate a ligand. Furthermore, the putative XNC10 ligand binding residues, identified based on sequence alignments with human HLA-A invariant residues [108, 115], do not conform with either class Ia or any of the other XNC genes [73]. Of the nine invariant residues in the XNC10 sequence five residues are distinct from class Ia and other XNC proteins suggesting that XNC10 present a non-peptide ligand [73]. Comparably, these putative XNC10 ligand-binding residues, especially in the C-terminus, are highly conserved among XNC10 homologs from different species within the Xenopodinae sub-family including those of the X. tropicalis homolog SNC10 again suggesting that XNC10 binds a conserved antigenic motif [73]. Furthermore, despite relatively high mRNA expression, XNC10 protein is barely detectable on the cell surface of both splenocytes and tumor cells and remains predominantly intracellular in transfected cell lines (Edholm and Robert, unpublished observations). This suggests that XNC10 requires a ligand to be translocated and/or remain stably at the cell surface. Moreover, the tissue-specific expression pattern of XNC10, with predominant expression in thymocytes, might limit the overall repertoire of available ligands. Finally, given that the CDR3α region of the invariant Vα-chain is exclusively germline encoded, with no n-nucleotide additions and identical among animals of different genetic backgrounds, it is likely that the resulting TCR binds a conserved motif.
Xenopus XNC-mediated iT cell ontogeny
Xenopus T cell development
Intra-thymic T cell development follows a series of defined stages characterized by molecularly programmed events that result in cellular selection and specific T cell lineage differentiation (reviewed in [116, 117]). In mammals, besides conventional adaptive T cells, the thymus also supports the development of unconventional T cells such as iT cells. However, the criteria governing ontogeny and lineage differentiation of iT cell subsets are distinct from those of conventional T cells. In fact, it has been suggested that many of the distinct phenotypical and functional properties attributed to iT cells are due to the different ways in which they respond to thymic positive and negative selection signals [118, 119]. In Xenopus, thymus ontogeny share many similarities with that of mammals (reviewed in [88]) including successive waves of immature T cell precursors moving into the thymus during embryogenesis where they expand, differentiate, undergo selection and subsequently exit as mature T cells [120, 121]. However, unlike mammals, the Xenopus immune system in general and T cell differentiation in particular, is subject to an additional developmental program during metamorphosis. The transition from tadpoles to adults during metamorphosis is accompanied by a drastic reduction in the total numbers of thymocytes and splenic lymphocytes and leads to the differential expression of many adult-specific proteins. Following metamorphosis, T cell differentiates from new wave of precursors that differentiate and undergo selection in a distinct adult type environment. One particular intriguing feature affecting T cell selection in Xenopus is the differential regulation of MHC class I and II genes during tadpole and adult life stages. In adults, MHC class II molecules are ubiquitously expressed on all lymphocytes. By contrast, in tadpoles, class II surface expression is only observed on B cells, macrophages and various epithelial surfaces including the thymic epithelium, but not on thymocytes or peripheral T cells [122]. Similarly, although both MHC class Ia and β2-microglobulin gene expression are detectable by RT-PCR in the thymus of tadpoles as early as 3 days post fertilization (corresponding to developmental stage 39; Goyos and Robert, unpublished), class Ia protein surface expression is sub-optimal in tadpoles [16]. Indeed, consistent class Ia surface expression is first detected (using multiple anti-class Ia mAbs), during the pre-metamorphic developmental stage 56–58 and subsequently increases during metamorphosis [18, 123]. Moreover, the tadpole thymus lacks significant expression of the LMP7 gene, a component of the immunoproteasome subunit, until the onset of metamorphosis [19]. Collectively, these data suggest that T cell selection is differentially regulated during the two life stages and that MHC class Ia-mediated T cell selection is likely to be suboptimal in tadpoles. It is noteworthy that whereas in mammals experimental impairment of class Ia expression results in severe immunodeficiency and/or death, Xenopus tadpoles, despite their sup-optimal class Ia expression, are immunocompetent and have circulating CD8+ T cells. This suggests that tadpoles have a specialized functional T cell compartment following a differentiation program distinct from that of adult, a program that may be more dependent on nonclassical MHC class I selected T cells. Our current understanding of tadpole and adult T cell development is outlined in Fig. 2. Given the limitation in the absolute number of lymphocytes in tadpoles (10–50,000 splenic T cells at developmental stage 55), differentiation of T cells with a limited TCR repertoire selected to recognize conserved pathogen determinants would be advantageous. In support of this possibility, multiple XNC genes are expressed in the thymocytes in young tadpoles at the onset of thymic organogenesis that could participate in early T cell development [20]. Specifically, in addition to XNC10, 6 distinct XNC genes display preferential expression on immature radiosensitive thymocytes; XNC1, 4, 5, 9, 11 and 14 (Edholm and Robert, unpublished observations).
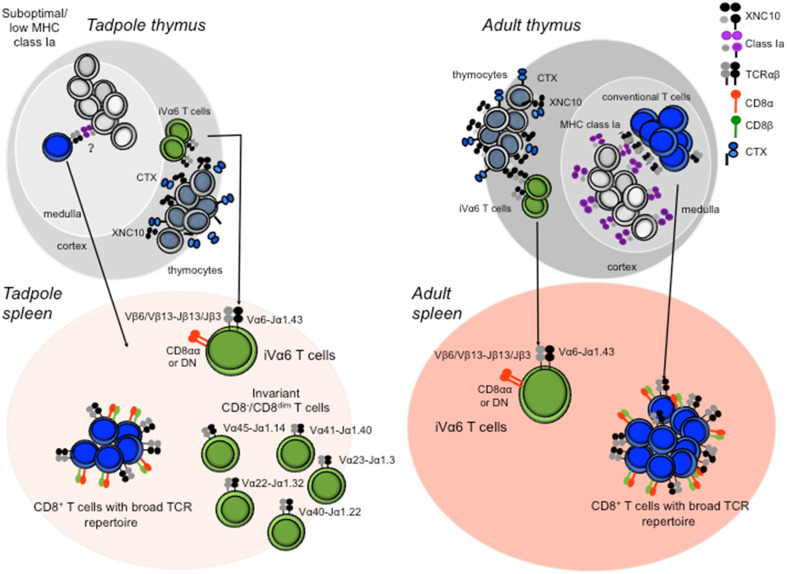
Fig. 2.
Differential development of conventional and unconventional αβT cell in X. laevis tadpole and adults. Conventional αβT cells as well as unconventional iT cells arise from the thymus. During tadpole development the suboptimal surface expression of classical MHC class I molecules is possibly compensated for by expression of various XNC molecules that select for distinct populations of invariant T cells. Specifically, iVα6 T cells are most likely selected by XNC10 expressed on immature CTX+ thymocytes and subsequently migrate to the spleen where they make up ~2–4 % of the total lymphocyte population. The tadpole spleen is also populated by five additional distinct iT cell subsets, each expressing a unique invariant TCRα rearrangement. Collectively, these iT cells make up the majority of CD8− and CD8dim T cells in the tadpole spleen. This suggests that Xenopus rely more extensively on XNC restricted iT cells during early developmental stage when tadpoles have a limited number of T cell and suboptimal classical MHC expression. Despite the suboptimal classical MHC protein expression the tadpole also posses a minor fraction of circulating CD8+ T cells expression a broad unrestricted TCRα repertoire. During metamorphosis and the transition from tadpoles to adults, a drastic reduction in the total number of thymocytes and splenic lymphocytes is observed accompanied by consistent surface expression of classical MHC in the thymic medulla. Thus, in the adult type thymus, the key element for conventional CD8+ T cell selection is present. The adult thymus also express XNC10 on thymocytes located in the thymic cortex, which is most likely required for selection of iVα6 T cells. Following selection both conventional CD8+ T cells and iVα6 T cells migrate to the spleen where they typically constitute 15–20 and 1–4 % of the total lymphocyte population respectively
Xenopus tadpole nonclassical MHC class I-restricted iT cell subsets
The suboptimal expression of class Ia protein, especially in the thymus, the concomitant expression by thymocytes of at least seven different XNC genes and the constraint in number of peripheral lymphocytes, all suggest that T cell differentiation in tadpoles is unusual. Using a global approach based on 5′RACE-PCR and next generation 454 pyrosequencing to determine the putative TCRα repertoire in tadpoles, we recently reported that the overall TCRα repertoire in the CD8− and CD8dim T cell populations in the spleen of tadpoles at early developmental stages (e.g., stage 50) is highly biased towards six distinct invariant TCRVα-Jα rearrangements [15]. We identified three highly dominant invariant Vα rearrangements in the CD8dim+ population (Vα6-Jα1.43, Vα45-Jα1.14 and Vα23-Jα1.3). Two of these rearrangements, Vα6-Jα1.43 and Vα23-Jα1.3, were also over-represented in the CD8− population together with three additional invariant Vα rearrangements (Vα22-Jα1.32, Vα40-Jα1.22 and Vα41-Jα1.40). Moreover, none of these invariant TCRα chains exhibited any evidence of n-nucleotide diversification in their junctional regions [15]. Each TCRα chain was unique and displayed distinct CDR1α, CDR2α and CDR3α regions. The various Vα segments utilized belong to different V-families that are dispersed throughout the TCRα loci, suggesting that the preferential usage is a result of a selection process rather than a spatially directed preferential rearrangement during development. Importantly, although the specific silencing of XNC10 resulted in an ablated expression of the canonical Vα6-Jα1.43 rearrangement, the relative expression of the five other unique invariant TCRα rearrangements was not affected (Edholm and Robert, unpublished observations). This is intriguing because it implies the presence of other distinct iT cell subsets. In light of this, it is tempting to speculate that there are multiple XNC restricted invariant or semi-invariant T cell lineages in tadpoles. This would allow Xenopus tadpoles to preferentially generate a pool of innate-like T cells that are capable of rapidly, albeit less specifically, mounting immune effector functions. This is particularly relevant since Xenopus tadpoles (like other ectothermic vertebrates) hatch in the surrounding antigen-rich water and develop free of maternal influences. As such, within 2 weeks (developmental stage 50), the Xenopus immune system is under pressure to produce a TCR repertoire capable of recognizing a broad array of antigens on the basis of relatively few lymphocytes (15–20,000 T cells). Since the potential TCR repertoire in Xenopus far exceeds the number of its T lymphocytes, it is probable that additional mechanisms have evolved to produce a functional but more limited lymphocyte repertoire during early ontogeny.
Comparisons of Xenopus and mammalian iT cell population ontogeny
In addition to impairing antiviral immunity, XNC10 gene loss-of-function established in transgenic Xenopus by RNA interference completely abolishes the development of XNC10-iT cells and the occurrence of the canonical iVα6-Jα1.43 rearrangement [15]. However, the ontogeny of XNC10-restricted iT cells is still poorly understood and the specific criteria governing iT cells lineage divergence from conventional T cells remains to be elucidated. In mammals, conventional T cells, iNKT and MAIT all arise from a common uncommitted thymocyte precursor. Specific iT cell lineage differentiation occurs following progression into the CD4/CD8 double positive (DP) stage, which is concurrent with rearrangements of the TCRα chain and subsequent expression of TCRαβ heterodimers on the cell surface. The likely mechanism governing this lineage divergence is positive selection mediated by specific MHC restricting elements [124–126]. Conventional αβT cell selection is dependent on heterotypic interaction between the TCR on DP thymocytes and either classical MHC class Ia or class II/peptide complexes expressed on the thymic epithelia [127]. In contrast, iNKT and MAIT cell development is dependent on selection on CD1d [4, 128] or MR1 [8], respectively, expressed on immature cortical thymocytes and thus requires homotypic interaction between DP thymocytes.
Currently, the specific cell type mediating XNC10-dependent iVα6 selection in Xenopus has not been directly identified. However, multiple indirect lines of evidence suggest that iVα6 T cells, similar to mammalian iT cells, are selected by hematopoietically derived immature thymocytes. Distinct stages of thymocyte differentiation have been characterized in Xenopus where immature and mature thymocytes are defined based on changes in surface expression of the cortical thymocyte-specific Xenopus cell surface marker (CTX; [129–131]). CTXhigh thymocytes most likely represent a developmental stage equivalent to that of mammalian DP thymocytes. CTX is a type I transmembrane glycoprotein comprised of a variable-like and a constant immunoglobulin domains. CTX is highly expressed on undifferentiated cortical thymocytes and appears to serve as an adhesion and/or signaling molecule during Xenopus thymocyte differentiation. In tadpoles, XNC10 is preferentially expressed on immature (CD5−/CTXhigh) and to a lesser extent mature (CD5+/CTXlow) CD8+ thymocytes. In fact, XNC10 RNA can be detected from the onset of early thymic organogenesis prior to the occurrence of fully differentiated mature thymocytes [20]. Moreover, XNC10 expression becomes undetectable following sublethal γ-irradiation that has been shown to preferentially deplete thymocytes [20, 129, 132]. Likewise, XNC10 expression pattern is mirrored in X. tropicalis where the XNC10 ortholog SNC10 is preferentially expressed on hematopoietically-derived thymocytes from early onset thymic organogenesis [73]. Collectively, these data imply that XNC10 is preferentially expressed on thymic hematopoietic cells, suggesting that similar to mammalian iT cells, Xenopus iT cells are selected by CTX+ DP thymocytes rather than by the thymic stroma.
Aside from the requirement for XNC10, it is unknown what other components are involved in Xenopus iT cell lineage-specific expansion and differentiation. Interestingly, whereas in vivo CTX protein is expressed almost exclusively by immature thymocytes co-expressing XNC10, CTX is also highly expressed on several Xenopus thymic lymphoid tumor cell lines. Activation of these tumor cells through anti-CTX mAb cross-linking induces abnormal cell division resulting in accumulation of cells in the G2/M phase [133]. This suggests that CTX, through control of the cell cycle, may play a role in selection and differentiation of T cells. In addition to CD1d-induced positive selection, mammalian iNKT (but not MAIT) lineage expansion and differentiation requires homotypic interaction between members of the signaling lymphocytic activation molecules (SLAM) family [134], specifically SLAMF1 and SLAMF6 receptors [135]. These signals lead to downstream recruitments of the SLAM-associated protein SAP and the Scr-family kinase Fyn and activation of NF-κB. Following CD1d and SLAM receptor-mediated initial selection, iNKT cell precursors undergo a series of intra-thymic differentiation steps after which they either exit the thymus for the periphery where they mature and express NK1.1 (CD161 in humans), or alternatively they undergo a proliferative burst and remain as long-term residents in the thymus [4, 136] and reviewed in [137]. Comparably, using XNC10-tetramers, we showed that while iVα6 T cells are abundant in the spleen, these cells are rare in tadpole and adult thymus, where they typically represent less than 0.05 % of the total thymocytes. This suggests that iVα6 T cells undergo rapid egress from the thymus following XNC10-mediated selection; then they accumulate and possibly expand and undergo additional maturation in peripheral organs such as the spleen. This would be reminiscent of MAIT cell development. In contrast to iNKT cells that are abundant in the thymus, MAIT cells are very rare and display a naive phenotype in both thymus and cord blood. They have been shown to exit the thymus as immature cells [8, 23, 69]. Furthermore, MAIT cells undergo what has been described as a stepwise developmental process that involves B cell-independent MR1 restricted intra-thymic selection followed by B cell-dependent clonal expansion and maturation in the periphery [7, 139]. This peripheral maturation step also appears to be dependent on commensal flora, as MAIT cells are absent in germ-free mice [139]. MAIT cells are partially expanded after re-colonization of the bacterial flora in germ-free mice [139] suggesting a complex relationship between MAIT cells and mucosal microbes. It is also, hypothesized, that microbial products within the gut flora may regulates MR1 surface expression.
Another important aspect possibly influencing iT cell lineage development is how these cells respond to a strong agonistic signal. Following positive selection and prior to thymic egress, conventional T cells undergo a defined developmental stage during which T cell clones expressing a TCR that bind the MHC/self peptide complex with strong avidity are deleted, thereby removing potentially auto-reactive cells from the peripheral T pool. In mouse, negative selection is primarily mediated by dendritic cells located in and around the thymic cortex/medulla interface [140]. The precise role and importance of negative selection for mammalian iT cell development is not fully elucidated. It has been suggested, that at least with respect to iNKT cells, there is a defined developmental stage during which these cells have an increased susceptibility to negative selection (reviewed in [124] and [137]). Two studies using in vitro fetal thymic organ cultures (FTOC) have shown that, consistent with negative selection, NKT cell development is abrogated in the presence of α-GalCer, a strong NKT cell agonist [138, 141]. Importantly, these studies demonstrated that while early addition of α-GalCer leads to fewer NKT cells, a delayed introduction of the compound, in vivo or in vitro did not affect NKT cell development [138, 141]. Additionally, CD1d over expression on dendritic cells in CD1d transgenic mice and bone marrow chimeras reduces but does not abrogate the number of NKT cells as compared to wild type mice [138]. These data suggest that a strong TCR engagement during a defined developmental window is also detrimental for NKT cells, which is consistent with the idea that potentially self reactive NKT cells are sensitive to negative selection. However, while fewer NKT cells were generated in the presence of a strong agonist, neither of these studies demonstrated deletion of a pre-formed NKT-cell subset in response to negative selection.
Concluding remarks
Nonclassical MHC class I-restricted iT cells have gained considerable attention in recent years because of their potential as regulators of immune function. However, until recently the evolutionary history of these T cell subsets was unclear, as they had only been unequivocally identified in humans and mice. The recent identification of nonclassical MHC class I-restricted iT cell subsets in the amphibian X. laevis provides evidence that these cell types are not limited to mammals. Moreover, comparative studies underline the biological importance of iT cells. With this in mind the existence of functional analogs to iT cells in ectothermic vertebrates is intriguing. Xenopus iVα6T cells share many characteristics with mammalian iNKT and MAIT cells. Taken together, the suboptimal thymic class Ia protein expression, the expression of several distinct XNC genes on thymocytes from early thymic organogenesis, and the highly biased TCRα repertoire, suggest that the tadpole immune system relies more heavily on distinct populations of XNC restricted iT cells than that of the adult frog. Also the phylogenetic analysis and lack of sequence identity among XNC subfamilies suggest that different XNC genes have distinct antigen presentation properties. Therefore, it is possible that the different iT cells, each restricted by a specific XNC molecule, and as such recognize distinct antigens and have different effector functions. This developmental strategy would permit the Xenopus tadpole immune system to rapidly, albeit less specifically, recognize and respond to conserved pathogenic motifs. This is of particular biological relevance as tadpoles have to generate lymphocyte receptor repertoires with a limited number of T cells within 2 weeks following hatching into antigen-rich surrounding waters. Given the vast numbers and diversities of nonclassical class I genes seen across ectothermic vertebrates, it is tempting to further speculate that unconventional nonclassical MHC class I-restricted T cell populations are more common than previously thought.
Acknowledgments
We thank Dr. Nicolas Cohen for helpful discussions and critical reading of the manuscript. This research was supported by Grants R24-AI-059830 from National Institute of Allergy and Infectious Diseases (NIH/NIAID).
Abbreviations
- MHC
Major histocompatibility complex
- XNC
Xenopus non-classical
- SNC
Siluriana non-classical
- MR1
Major histocompatibility molecule related 1
- TCR
T cell receptor
- MAIT
MR1 associated invariant T cells
- iNKT
Invariant natural killer T cells
- iT cells
Invariant T cells
- FV3
Frog virus 3
- MYA
Million years ago
- CTL
Cytotoxic T lymphocytes
- CCU-CTL
Classical class Ia-unrestricted CTLs
- NKT
Natural killer T cells
- αGalCer
Glycolipid α-galactoceramide
- DP
Double positive
- DN
Double negative
- SLAM
Signaling lymphocytic activation molecules
- CTX
Cortical thymocyte-specific Xenopus
References
- 1.Adams EJ, Luoma AM. The adaptable major histocompatibility complex (MHC) fold: structure and function of nonclassical and MHC class I-like molecules. Annu Rev Immunol. 2013;31:529–561. doi: 10.1146/annurev-immunol-032712-095912. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5(6):459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 3.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8(6):563–568. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182(6):2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 6.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 8.Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, Streeter PR, Lewinsohn DA, Lewinsohn DM. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6(1):35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat Rev Immunol. 2003;3(3):211–222. doi: 10.1038/nri1028. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23(9):529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 11.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 12.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Gabriel CL, Parekh VV, Van Kaer L. Invariant natural killer T cells: innate-like T cells with potent immunomodulatory activities. Tissue Antigens. 2009;73(6):535–545. doi: 10.1111/j.1399-0039.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- 14.Gold MC, Lewinsohn DM. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol. 2013;11(1):14–19. doi: 10.1038/nrmicro2918. [DOI] [PubMed] [Google Scholar]
- 15.Edholm ES, Albertorio Saez LM, Gill AL, Gill SR, Grayfer L, Haynes N, Myers JR, Robert J. Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Nat Acad Sci USA. 2013;110(35):14342–14347. doi: 10.1073/pnas.1309840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137(12):3891–3899. [PubMed] [Google Scholar]
- 17.Du Pasquier L, Weiss N. The thymus during the ontogeny of the toad Xenopus laevis: growth, membrane-bound immunoglobulins and mixed lymphocyte reaction. Eur J Immunol. 1973;3(12):773–777. doi: 10.1002/eji.1830031207. [DOI] [PubMed] [Google Scholar]
- 18.Rollins-Smith LA, Flajnik MF, Blair PJ, Davis AT, Green WF. Involvement of thyroid hormones in the expression of MHC class I antigens during ontogeny in Xenopus . Dev Immunol. 1997;5(2):133–144. doi: 10.1155/1997/38464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salter-Cid L, Nonaka M, Flajnik MF. Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol. 1998;160(6):2853–2861. [PubMed] [Google Scholar]
- 20.Goyos A, Ohta Y, Guselnikov S, Robert J. Novel nonclassical MHC class Ib genes associated with CD8 T cell development and thymic tumors. Mol Immunol. 2009;46(8–9):1775–1786. doi: 10.1016/j.molimm.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 22.Birkinshaw RW, Kjer-Nielsen L, Eckle SB, McCluskey J, Rossjohn J. MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol. 2014;26C:7–13. doi: 10.1016/j.coi.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Le Bourhis L, Mburu YK, Lantz O. MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol. 2013;25(2):174–180. doi: 10.1016/j.coi.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Piontkivska H, Nei M. Birth-and-death evolution in primate MHC class I genes: divergence time estimates. Mol Biol Evol. 2003;20(4):601–609. doi: 10.1093/molbev/msg064. [DOI] [PubMed] [Google Scholar]
- 25.Adams EJ, Parham P. Species-specific evolution of MHC class I genes in the higher primates. Immunol Rev. 2001;183:41–64. doi: 10.1034/j.1600-065x.2001.1830104.x. [DOI] [PubMed] [Google Scholar]
- 26.Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci USA. 1997;94(15):7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes AL, Nei M. Evolution of the major histocompatibility complex: independent origin of nonclassical class I genes in different groups of mammals. Mol Biol Evol. 1989;6(6):559–579. doi: 10.1093/oxfordjournals.molbev.a040573. [DOI] [PubMed] [Google Scholar]
- 28.Rogers JH. Mouse histocompatibility-related genes are not conserved in other mammals. EMBO J. 1985;4(3):749–753. doi: 10.1002/j.1460-2075.1985.tb03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joyce S, Tabaczewski P, Angeletti RH, Nathenson SG, Stroynowski I. A nonpolymorphic major histocompatibility complex class Ib molecule binds a large array of diverse self-peptides. J Exp Med. 1994;179(2):579–588. doi: 10.1084/jem.179.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotzschke O, Falk K, Stevanovic S, Grahovac B, Soloski MJ, Jung G, Rammensee HG. Qa-2 molecules are peptide receptors of higher stringency than ordinary class I molecules. Nature. 1993;361(6413):642–644. doi: 10.1038/361642a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee N, Malacko AR, Ishitani A, Chen MC, Bajorath J, Marquardt H, Geraghty DE. The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity. 1995;3(5):591–600. doi: 10.1016/1074-7613(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 32.Diehl M, Munz C, Keilholz W, Stevanovic S, Holmes N, Loke YW, Rammensee HG. Nonclassical HLA-G molecules are classical peptide presenters. Curr Biol. 1996;6(3):305–314. doi: 10.1016/s0960-9822(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 33.Fuzzi B, Rizzo R, Criscuoli L, Noci I, Melchiorri L, Scarselli B, Bencini E, Menicucci A, Baricordi OR. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol. 2002;32(2):311–315. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Marine JB, Shirakata Y, Wadsworth SA, Hooley JJ, Handy DE, Coligan JE. Role of the Q10 class I regulatory element region 1 in controlling tissue-specific expression in vivo. J Immunol. 1993;151(4):1989–1997. [PubMed] [Google Scholar]
- 35.Ellis SA, Palmer MS, McMichael AJ. Human trophoblast and the choriocarcinoma cell line BeWo express a truncated HLA Class I molecule. J Immunol. 1990;144(2):731–735. [PubMed] [Google Scholar]
- 36.Loke YW, King A, Burrows T, Gardner L, Bowen M, Hiby S, Howlett S, Holmes N, Jacobs D. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50(2):135–146. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- 37.Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. doi: 10.1007/978-3-540-69511-0_1. [DOI] [PubMed] [Google Scholar]
- 38.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci USA. 2009;106(20):8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277(5324):339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 40.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6(8):819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 41.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi H, Kurosawa Y, Hashimoto K. Expanded genomic organization of conserved mammalian MHC class I-related genes, human MR1 and its murine ortholog. Biochem Biophys Res Commun. 1998;250(3):558–564. doi: 10.1006/bbrc.1998.9353. [DOI] [PubMed] [Google Scholar]
- 43.Shiina T, Ando A, Suto Y, Kasai F, Shigenari A, Takishima N, Kikkawa E, Iwata K, Kuwano Y, Kitamura Y, Matsuzawa Y, Sano K, Nogami M, Kawata H, Li S, Fukuzumi Y, Yamazaki M, Tashiro H, Tamiya G, Kohda A, Okumura K, Ikemura T, Soeda E, Mizuki N, Kimura M, Bahram S, Inoko H. Genomic anatomy of a premier major histocompatibility complex paralogous region on chromosome 1q21-q22. Genome Res. 2001;11(5):789–802. doi: 10.1101/gr.175801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiina T, Inoko H. Comparative genome analysis of MHC region. Tanpakushitsu kakusan koso protein, nucleic acid, enzyme. 2001;46(16 Suppl):2246–2253. [PubMed] [Google Scholar]
- 45.Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15(3):351–362. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- 46.Salomonsen J, Sorensen MR, Marston DA, Rogers SL, Collen T, van Hateren A, Smith AL, Beal RK, Skjodt K, Kaufman J. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci USA. 2005;102(24):8668–8673. doi: 10.1073/pnas.0409213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MM, Wang C, Parisini E, Coletta RD, Goto RM, Lee SY, Barral DC, Townes M, Roura-Mir C, Ford HL, Brenner MB, Dascher CC. Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc Natl Acad Sci USA. 2005;102(24):8674–8679. doi: 10.1073/pnas.0500105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hee CS, Gao S, Loll B, Miller MM, Uchanska-Ziegler B, Daumke O, Ziegler A. Structure of a classical MHC class I molecule that binds “non-classical” ligands. PLoS Biol. 2010;8(12):e1000557. doi: 10.1371/journal.pbio.1000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair S. Whats in a name? J Maxillofac Oral Surg. 2011;10(3):183–184. doi: 10.1007/s12663-011-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8− T cells in mice and humans. J Exp Med. 1994;180(3):1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kashiwase K, Kikuchi A, Ando Y, Nicol A, Porcelli SA, Tokunaga K, Omine M, Satake M, Juji T, Nieda M, Koezuka Y. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics. 2003;54(11):776–781. doi: 10.1007/s00251-002-0527-8. [DOI] [PubMed] [Google Scholar]
- 53.Matsuura A, Kinebuchi M, Chen HZ, Katabami S, Shimizu T, Hashimoto Y, Kikuchi K, Sato N. NKT cells in the rat: organ-specific distribution of NK T cells expressing distinct V alpha 14 chains. J Immunol. 2000;164(6):3140–3148. doi: 10.4049/jimmunol.164.6.3140. [DOI] [PubMed] [Google Scholar]
- 54.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 55.Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, Miyazaki Y, Gomez-Velasco A, Rogers P, Dahesh S, Uchiyama S, Khurana A, Kawahara K, Yesilkaya H, Andrew PW, Wong CH, Kawakami K, Nizet V, Besra GS, Tsuji M, Zajonc DM, Kronenberg M. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12(10):966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35(6):1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 57.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 58.Burrows SR, Chen Z, Archbold JK, Tynan FE, Beddoe T, Kjer-Nielsen L, Miles JJ, Khanna R, Moss DJ, Liu YC, Gras S, Kostenko L, Brennan RM, Clements CS, Brooks AG, Purcell AW, McCluskey J, Rossjohn J. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proc Natl Acad Sci USA. 2010;107(23):10608–10613. doi: 10.1073/pnas.1004926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 60.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 61.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31(1):47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8(10):1105–1113. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 63.Florence WC, Xia C, Gordy LE, Chen W, Zhang Y, Scott-Browne J, Kinjo Y, Yu KO, Keshipeddy S, Pellicci DG, Patel O, Kjer-Nielsen L, McCluskey J, Godfrey DI, Rossjohn J, Richardson SK, Porcelli SA, Howell AR, Hayakawa K, Gapin L, Zajonc DM, Wang PG, Joyce S. Adaptability of the semi-invariant natural killer T-cell receptor towards structurally diverse CD1d-restricted ligands. EMBO J. 2009;28(23):3781. doi: 10.1038/emboj.2009.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J Exp Med. 2011;208(6):1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010;22(2):68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65(2):115–124. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- 67.Lion J, Debuysscher V, Wlodarczyk A, Hodroge A, Serriari NE, Choteau L, Ouled-haddou H, Plistat M, Lassoued K, Lantz O, Treiner E. MR1B, a natural spliced isoform of the MHC-related 1 protein, is expressed as homodimers at the cell surface and activates MAIT cells. Eur J Immunol. 2013;43(5):1363–1373. doi: 10.1002/eji.201242461. [DOI] [PubMed] [Google Scholar]
- 68.Reantragoon R, Kjer-Nielsen L, Patel O, Chen Z, Illing PT, Bhati M, Kostenko L, Bharadwaj M, Meehan B, Hansen TH, Godfrey DI, Rossjohn J, McCluskey J. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J Exp Med. 2012;209(4):761–774. doi: 10.1084/jem.20112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32(5):212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 71.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Core M, Sleurs D, Serriari NE, Treiner E, Hivroz C, Sansonetti P, Gougeon ML, Soudais C, Lantz O. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9(10):e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flajnik MF, Kasahara M, Shum BP, Salter-Cid L, Taylor E, Du Pasquier L. A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus . EMBO J. 1993;12(11):4385–4396. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goyos A, Sowa J, Ohta Y, Robert J. Remarkable conservation of distinct nonclassical MHC class I lineages in divergent amphibian species. J Immunol. 2011;186(1):372–381. doi: 10.4049/jimmunol.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sammut B, Du Pasquier L, Ducoroy P, Laurens V, Marcuz A, Tournefier A. Axolotl MHC architecture and polymorphism. Eur J Immunol. 1999;29(9):2897–2907. doi: 10.1002/(SICI)1521-4141(199909)29:09<2897::AID-IMMU2897>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 75.Bartl S, Baish MA, Flajnik MF, Ohta Y. Identification of class I genes in cartilaginous fish, the most ancient group of vertebrates displaying an adaptive immune response. J Immunol. 1997;159(12):6097–6104. [PubMed] [Google Scholar]
- 76.Wang C, Perera TV, Ford HL, Dascher CC. Characterization of a divergent non-classical MHC class I gene in sharks. Immunogenetics. 2003;55(1):57–61. doi: 10.1007/s00251-003-0542-4. [DOI] [PubMed] [Google Scholar]
- 77.Lukacs MF, Harstad H, Bakke HG, Beetz-Sargent M, McKinnel L, Lubieniecki KP, Koop BF, Grimholt U. Comprehensive analysis of MHC class I genes from the U-, S-, and Z-lineages in Atlantic salmon. BMC Genom. 2010;11:154. doi: 10.1186/1471-2164-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dijkstra JM, Katagiri T, Hosomichi K, Yanagiya K, Inoko H, Ototake M, Aoki T, Hashimoto K, Shiina T. A third broad lineage of major histocompatibility complex (MHC) class I in teleost fish; MHC class II linkage and processed genes. Immunogenetics. 2007;59(4):305–321. doi: 10.1007/s00251-007-0198-6. [DOI] [PubMed] [Google Scholar]
- 79.Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrom M, Gregers TF, Rounge TB, Paulsen J, Solbakken MH, Sharma A, Wetten OF, Lanzen A, Winer R, Knight J, Vogel JH, Aken B, Andersen O, Lagesen K, Tooming-Klunderud A, Edvardsen RB, Tina KG, Espelund M, Nepal C, Previti C, Karlsen BO, Moum T, Skage M, Berg PR, Gjoen T, Kuhl H, Thorsen J, Malde K, Reinhardt R, Du L, Johansen SD, Searle S, Lien S, Nilsen F, Jonassen I, Omholt SW, Stenseth NC, Jakobsen KS. The genome sequence of Atlantic cod reveals a unique immune system. Nature. 2011;477(7363):207–210. doi: 10.1038/nature10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans BJ. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana) Front Biosci. 2008;13:4687–4706. doi: 10.2741/3033. [DOI] [PubMed] [Google Scholar]
- 81.Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol. 2004;33(1):197–213. doi: 10.1016/j.ympev.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 82.Edholm ESAG, Taran J, DeJesus Andino F, Otha Y, Robert J. Unusual evolutionary conservation and specific adaptations of a large family of nonclassical MHC class Ib genes across different degrees of genome ploidy in the amphibian subfamily Xenopodinae. Immunogenetics. 2014 doi: 10.1007/s00251-014-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goyos A, Guselnikov S, Chida AS, Sniderhan LF, Maggirwar SB, Nedelkovska H, Robert J. Involvement of nonclassical MHC class Ib molecules in heat shock protein-mediated anti-tumor responses. Eur J Immunol. 2007;37(6):1494–1501. doi: 10.1002/eji.200636570. [DOI] [PubMed] [Google Scholar]
- 84.Courtet M, Flajnik M, Du Pasquier L. Major histocompatibility complex and immunoglobulin loci visualized by in situ hybridization on Xenopus chromosomes. Dev Comp Immunol. 2001;25(2):149–157. doi: 10.1016/s0145-305x(00)00045-8. [DOI] [PubMed] [Google Scholar]
- 85.Nakanishi T, Toda H, Shibasaki Y, Somamoto T. Cytotoxic T cells in teleost fish. Dev Comp Immunol. 2011;35(12):1317–1323. doi: 10.1016/j.dci.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 86.Somamoto T, Koppang EO, Fischer U. Antiviral functions of CD8(+) cytotoxic T cells in teleost fish. Dev Comp Immunol. 2014;43(2):197–204. doi: 10.1016/j.dci.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 87.Toda H, Saito Y, Koike T, Takizawa F, Araki K, Yabu T, Somamoto T, Suetake H, Suzuki Y, Ototake M, Moritomo T, Nakanishi T. Conservation of characteristics and functions of CD4 positive lymphocytes in a teleost fish. Dev Comp Immunol. 2011;35(6):650–660. doi: 10.1016/j.dci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus . Dev Dyn. 2009;238(6):1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chida AS, Goyos A, Robert J. Phylogenetic and developmental study of CD4, CD8 alpha and beta T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis . Dev Comp Immunol. 2011;35(3):366–377. doi: 10.1016/j.dci.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laing KJ, Zou JJ, Purcell MK, Phillips R, Secombes CJ, Hansen JD. Evolution of the CD4 family: teleost fish possess two divergent forms of CD4 in addition to lymphocyte activation gene-3. J Immunol. 2006;177(6):3939–3951. doi: 10.4049/jimmunol.177.6.3939. [DOI] [PubMed] [Google Scholar]
- 91.Dijkstra JM, Somamoto T, Moore L, Hordvik I, Ototake M, Fischer U. Identification and characterization of a second CD4-like gene in teleost fish. Mol Immunol. 2006;43(5):410–419. doi: 10.1016/j.molimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Edholm ES, Stafford JL, Quiniou SM, Waldbieser G, Miller NW, Bengten E, Wilson M. Channel catfish, Ictalurus punctatus, CD4-like molecules. Dev Comp Immunol. 2007;31(2):172–187. doi: 10.1016/j.dci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 93.Venkatesh B, Lee AP, Ravi V, Maurya AK, Lian MM, Swann JB, Ohta Y, Flajnik MF, Sutoh Y, Kasahara M, Hoon S, Gangu V, Roy SW, Irimia M, Korzh V, Kondrychyn I, Lim ZW, Tay BH, Tohari S, Kong KW, Ho S, Lorente-Galdos B, Quilez J, Marques-Bonet T, Raney BJ, Ingham PW, Tay A, Hillier LW, Minx P, Boehm T, Wilson RK, Brenner S, Warren WC. Elephant shark genome provides unique insights into gnathostome evolution. Nature. 2014;505(7482):174–179. doi: 10.1038/nature12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohtani M, Hayashi N, Hashimoto K, Nakanishi T, Dijkstra JM. Comprehensive clarification of two paralogous interleukin 4/13 loci in teleost fish. Immunogenetics. 2008;60(7):383–397. doi: 10.1007/s00251-008-0299-x. [DOI] [PubMed] [Google Scholar]
- 95.Li JH, Shao JZ, Xiang LX, Wen Y. Cloning, characterization and expression analysis of pufferfish interleukin-4 cDNA: the first evidence of Th2-type cytokine in fish. Mol Immunol. 2007;44(8):2078–2086. doi: 10.1016/j.molimm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Castro R, Bernard D, Lefranc MP, Six A, Benmansour A, Boudinot P. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol. 2011;31(5):644–654. doi: 10.1016/j.fsi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 97.Horton TL, Minter R, Stewart R, Ritchie P, Watson MD, Horton JD. Xenopus NK cells identified by novel monoclonal antibodies. Eur J Immunol. 2000;30(2):604–613. doi: 10.1002/1521-4141(200002)30:2<604::AID-IMMU604>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 98.Robert J, Gantress J, Rau L, Bell A, Cohen N. Minor histocompatibility antigen-specific MHC-restricted CD8 T cell responses elicited by heat shock proteins. J Immunol. 2002;168(4):1697–1703. doi: 10.4049/jimmunol.168.4.1697. [DOI] [PubMed] [Google Scholar]
- 99.Goyos A, Cohen N, Gantress J, Robert J. Anti-tumor MHC class Ia-unrestricted CD8 T cell cytotoxicity elicited by the heat shock protein gp96. Eur J Immunol. 2004;34(9):2449–2458. doi: 10.1002/eji.200425105. [DOI] [PubMed] [Google Scholar]
- 100.Rau L, Cohen N, Robert J. MHC-restricted and -unrestricted CD8 T cells: an evolutionary perspective. Transplantation. 2001;72(11):1830–1835. doi: 10.1097/00007890-200112150-00020. [DOI] [PubMed] [Google Scholar]
- 101.Rau L, Gantress J, Bell A, Stewart R, Horton T, Cohen N, Horton J, Robert J. Identification and characterization of Xenopus CD8+ T cells expressing an NK cell-associated molecule. Eur J Immunol. 2002;32(6):1574–1583. doi: 10.1002/1521-4141(200206)32:6<1574::AID-IMMU1574>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 102.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162(1):161–167. [PubMed] [Google Scholar]
- 103.Zhao J, Weng X, Bagchi S, Wang CR. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci USA. 2014;111(7):2674–2679. doi: 10.1073/pnas.1323845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nieuwkoop PD, Faber J. Normal tables of Xenopuslaevis (Daudin) In: Nieuwkoop PD, editor. A systematic and chronological survey of the development from the fertilized egg till the end of metamorphosis. 2. Amsterdam: North-Holland; 1967. pp. 162–188. [Google Scholar]
- 105.Grayfer L, Andino FDJ, Chen G, Chinchar GV, Robert J. Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens. Viruses. 2012;4(7):1075–1092. doi: 10.3390/v4071075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Jesus Andino F, Chen G, Li Z, Grayfer L, Robert J. Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology. 2012;432(2):435–443. doi: 10.1016/j.virol.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haynes-Gilmore N, Banach M, Edholm ES, Lord E, Robert J. A critical role of nonclassical MHC in tumor immune evasion in the amphibian Xenopus model. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 109.Madden DR. The three-dimensional structure of peptide–MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 110.Hughes AL, Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335(6186):167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- 111.O’Callaghan CA, Tormo J, Willcox BE, Braud VM, Jakobsen BK, Stuart DI, McMichael AJ, Bell JI, Jones EY. Structural features impose tight peptide binding specificity in the nonclassical MHC molecule HLA-E. Mol Cell. 1998;1(4):531–541. doi: 10.1016/s1097-2765(00)80053-2. [DOI] [PubMed] [Google Scholar]
- 112.O’Callaghan CA, Tormo J, Willcox BE, Blundell CD, Jakobsen BK, Stuart DI, McMichael AJ, Bell JI, Jones EY. Production, crystallization, and preliminary X-ray analysis of the human MHC class Ib molecule HLA-E. Protein Sci. 1998;7(5):1264–1266. doi: 10.1002/pro.5560070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang CR, Lindahl KF, Deisenhofer J. Crystal structure of the MHC class Ib molecule H2-M3. Res Immunol. 1996;147(5):313–321. doi: 10.1016/0923-2494(96)89644-1. [DOI] [PubMed] [Google Scholar]
- 114.Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 115.Saper MA, Bjorkman PJ, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- 116.Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 117.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 118.Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3(8):772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kisielow J, Tortola L, Weber J, Karjalainen K, Kopf M. Evidence for the divergence of innate and adaptive T-cell precursors before commitment to the alphabeta and gammadelta lineages. Blood. 2011;118(25):6591–6600. doi: 10.1182/blood-2011-05-352732. [DOI] [PubMed] [Google Scholar]
- 120.Turpen JB, Smith PB. Precursor immigration and thymocyte succession during larval development and metamorphosis in Xenopus . J Immunol. 1989;142(1):41–47. [PubMed] [Google Scholar]
- 121.Gravenor I, Horton TL, Ritchie P, Flint E, Horton JD. Ontogeny and thymus-dependence of T cell surface antigens in Xenopus: flow cytometric studies on monoclonal antibody-stained thymus and spleen. Dev Comp Immunol. 1995;19(6):507–523. doi: 10.1016/0145-305x(95)00030-w. [DOI] [PubMed] [Google Scholar]
- 122.Du Pasquier L, Flajnik MF. Expression of MHC class II antigens during Xenopus development. Dev Immunol. 1990;1(2):85–95. doi: 10.1155/1990/67913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rollins-Smith LA, Barker KS, Davis AT. Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Dev Immunol. 1997;5(2):145–152. doi: 10.1155/1997/84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berzins SP, Uldrich AP, Pellicci DG, McNab F, Hayakawa Y, Smyth MJ, Godfrey DI. Parallels and distinctions between T and NKT cell development in the thymus. Immunol Cell Biol. 2004;82(3):269–275. doi: 10.1111/j.0818-9641.2004.01256.x. [DOI] [PubMed] [Google Scholar]
- 125.Gapin L. Where do MAIT cells fit in the family of unconventional T cells? PLoS Biol. 2009;7(3):e70. doi: 10.1371/journal.pbio.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant V alpha 14+ natural killer-T cell development. Eur J Immunol. 1999;29(10):3313–3318. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 127.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992;359(6393):330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 128.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2(10):971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 129.Robert J, Cohen N. Ontogeny of CTX expression in Xenopus . Dev Comp Immunol. 1998;22(5–6):605–612. doi: 10.1016/s0145-305x(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 130.Robert J, Cohen N. In vitro differentiation of a CD4/CD8 double-positive equivalent thymocyte subset in adult Xenopus . Int Immunol. 1999;11(4):499–508. doi: 10.1093/intimm/11.4.499. [DOI] [PubMed] [Google Scholar]
- 131.Chretien I, Robert J, Marcuz A, Garcia-Sanz JA, Courtet M, Du Pasquier L. CTX, a novel molecule specifically expressed on the surface of cortical thymocytes in Xenopus . Eur J Immunol. 1996;26(4):780–791. doi: 10.1002/eji.1830260409. [DOI] [PubMed] [Google Scholar]
- 132.Russ JH, Horton JD. Cytoarchitecture of the Xenopus thymus following gamma-irradiation. Development. 1987;100(1):95–105. doi: 10.1242/dev.100.1.95. [DOI] [PubMed] [Google Scholar]
- 133.Robert J, Chretien I, Guiet C, Du Pasquier L. Cross-linking CTX, a novel thymocyte-specific molecule, inhibits the growth of lymphoid tumor cells in Xenopus . Mol Immunol. 1997;34(2):133–143. doi: 10.1016/s0161-5890(97)00006-0. [DOI] [PubMed] [Google Scholar]
- 134.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174(6):3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 135.Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+ CD8+ cells. J Immunol. 2000;164(5):2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 137.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7(7):505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 138.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197(7):907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, Vera G, Latour S, Soudais C, Lantz O. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185(2):263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pellicci DG, Uldrich AP, Kyparissoudis K, Crowe NY, Brooks AG, Hammond KJ, Sidobre S, Kronenberg M, Smyth MJ, Godfrey DI. Intrathymic NKT cell development is blocked by the presence of alpha-galactosylceramide. Eur J Immunol. 2003;33(7):1816–1823. doi: 10.1002/eji.200323894. [DOI] [PubMed] [Google Scholar]