Summary
Background
Circadian regulation of chemosensory processes is common in animals, but little is known about how circadian clocks control chemosensory systems or the consequences of rhythms in chemosensory system function. Taste is a major chemosensory gate used to decide whether or not an animal will eat, and the main taste organ in Drosophila, the proboscis, harbors autonomous circadian oscillators. Here we examine gustatory physiology, tastant-evoked appetitive behavior, and food ingestion to understand clock-dependent regulation of the Drosophila gustatory system.
Results
Here we report that single-unit responses from labellar gustatory receptor neurons (GRNs) to attractive and aversive tastants show diurnal and circadian rhythms in spike amplitude, frequency and duration across different classes of gustatory sensilla. Rhythms in electrophysiological responses parallel behavioral rhythms in proboscis extension reflex (PrER). Molecular oscillators in GRNs are necessary and sufficient for rhythms in gustatory responses, and drive rhythms in G protein coupled receptor kinase 2 (GPRK2) expression that mediate rhythms in taste-sensitivity. Eliminating clock function in certain GRNs increases feeding and locomotor activity, mimicking a starvation response.
Conclusions
Circadian clocks in GRNs control neuronal output and drive behavioral rhythms in taste responses that peak at a time of day when feeding is maximal in flies. Our results argue that oscillations in GPRK2 levels drive rhythms in gustatory physiology and behavior, and that GRN clocks repress feeding. The similarity in gustatory system organization and feeding behavior in flies and mammals, and diurnal changes in taste sensitivity in humans, suggest that our results are relevant to the situation in humans.
Introduction
In animals, plants, fungi and some prokaryotes endogenous circadian clocks drive daily rhythms in gene expression, physiology, metabolism and behavior, thus enabling organisms to anticipate daily environmental changes. At the molecular level, the circadian timekeeping mechanism in eukaryotes is comprised of core and interlocked transcriptional feedback loops [1]. In Drosophila, CLOCK-CYCLE (CLK-CYC) heterodimers bind E-boxes to activate transcription of period (per) and timeless (tim), then PER and TIM proteins nucleate the formation of protein complexes that feed back to repress transcription of per, tim and other CLK-CYC activated genes within these feedback loops [2]. Feedback repression is released when PER and TIM are degraded, thus initiating the next cycle of transcription [2].
Circadian clocks are present in both the central nervous system and peripheral tissues [1]. A circuit of ~ 150 brain neurons control locomotor activity rhythms in Drosophila [3, 4], while peripheral clocks in antenna, epidermis, oenocytes, and testis regulate local physiology [5–8]. In contrast to mammals, peripheral oscillators in Drosophila maintain synchrony in the absence of rhythmic input from the brain [9–12]. Although autonomous, light entrainable oscillators are known to be present in many Drosophila tissues including Malphighian tubules, proboscis, leg and wing [12, 13], relatively little is known about the rhythms that they control. Perhaps the best-understood peripheral oscillators in Drosophila reside in the antenna. These oscillators drive rhythms in spontaneous and odor-induced physiological responses in olfactory receptor neurons (ORNs), and are thought to control odor-driven chemotactic behavior in adult flies [11, 14, 15]. Circadian rhythms in odor-evoked physiological responses have also been described in humans, mice, cockroaches and moths, which implies a conserved and important function for circadian regulation of smell [16–19].
Drosophila senses taste via gustatory receptors (GRs) expressed in gustatory receptor neurons (GRNs) on the proboscis, leg, wing margins, and ovipositor [20]. At the tip of the main gustatory organ in Drosophila, the proboscis, is the labellum, which contains 31 pairs of taste-hairs, each housing 2 or 4 GRNs [21]. In insects, feeding is regulated by external signals such as gustatory stimuli and olfactory cues [22, 23], and internal signals such as feeding status and metabolic needs [23]. A set of conserved peptide hormones, Drosophila insulin-like peptides (DILPs) and the glucagon analog adipokinetic hormone (AKH), function reciprocally to control energy homeostasis in fruit flies and other animals [24]. Drosophila display daily rhythms in feeding that are regulated in part by circadian clocks in ORNs and the fat body [25]. Food intake is increased in Clock mutant mice [26, 27], demonstrating a conserved role for the circadian clock in the control of feeding.
Given the remarkable mechanistic and structural similarities between the Drosophila gustatory and olfactory systems and experiments demonstrating that the proboscis contains a self-sustaining oscillator [12], we reasoned that the proboscis clock controls rhythms in gustatory physiology and behavior. Here we show that GRN clocks control sugar and caffeine induced physiological and behavioral responses in the proboscis, and identify G-protein-coupled receptor kinase 2 (Gprk2) as a key signal transduction molecule that underlies these rhythms. Disrupting clock function in GRNs increases feeding, implying that GRN oscillators restrict food consumption.
Results
The amplitude, frequency and duration of GRN spikes are controlled by the circadian clock
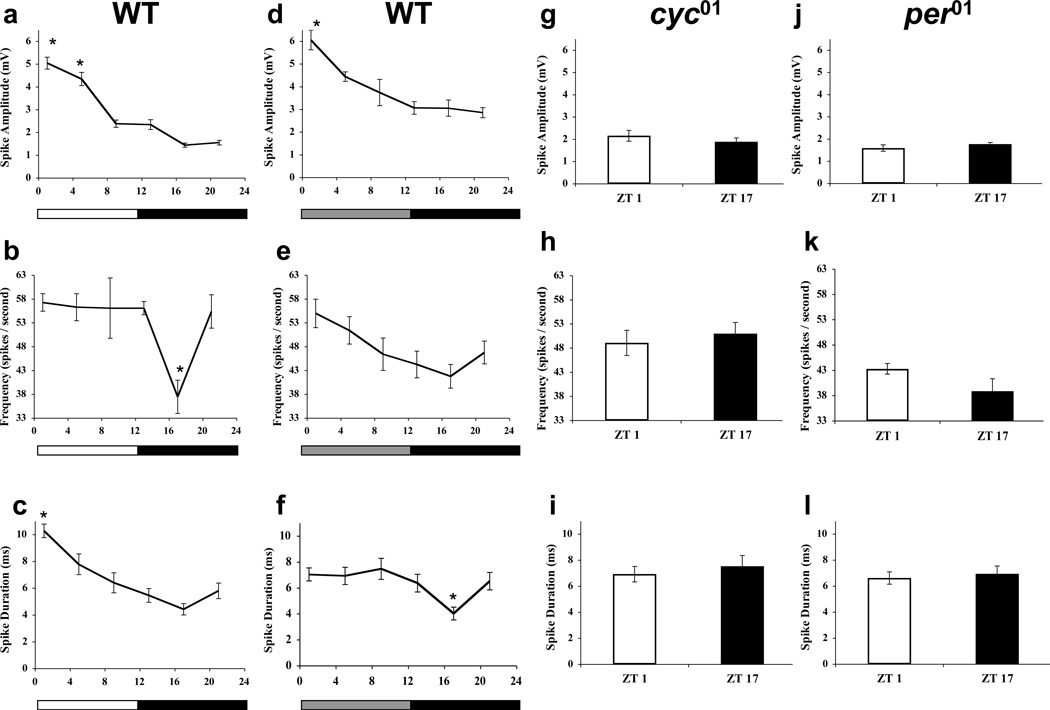
Based on their size and location, labellar taste hairs are divided into large (l-type), intermediate (i-type) and small (s-type) sensilla [20, 28]. GRNs housed in l-type and s-type sensilla are classified as S neurons (responsive to sugar), W neurons (responsive to water and low osmolarity), L1 neurons (responsive to low salt concentration), and L2 neurons (responsive to bitter compounds and high salt concentration) based on their electrophysiological response spectra [20, 29]. Recordings from single l-type sensillae were made in wild-type flies collected during 12h light:12h dark (LD) cycles. A different population of flies (n ≥ 6) was recorded at each timepoint. The sweet-sensitive S neuron was stimulated by application of 100 mM sucrose [30]. A ~3.5 fold rhythm in S spike amplitude was detected with a peak at Zeitgeber Time 1 (ZT1) and a trough at ZT17 (Fig. 1a; Fig. S1). The extent of diurnal influence on spiking activity of S neurons was determined by recording the rate of firing in response to 100 mM sucrose. A ~1.5 fold rhythm in spike frequency was detected, which showed a sharp trough at ZT17 (Fig. 1b). Since the waveforms of action potentials can encode biological information [31], we investigated changes in spike duration as a function of time of day. A ~2 fold rhythm in S spike duration was found, with a peak at ZT1 and a trough at ZT17 (Fig. 1c). These rhythms in spike amplitude, frequency, and duration persisted in constant darkness (DD) (Fig. 1d–f), thereby demonstrating that the rhythms are not a passive response to LD cycles but are driven by circadian clocks. These electrophysiological responses are constantly low in per01 and cyc01 null mutants even in LD cycles (Fig. 1g–l; Fig. S1), thus demonstrating that the clock is required for the daily increase in responses from S neurons.
Figure 1.
S-spikes are under circadian-clock control in the l-type sensilla. Spike amplitudes (a, d), frequencies (b, e), and durations (c, f) were measured in WT flies collected at the indicated time points during LD cycles (a–c) or on the second day of constant darkness (d–f). The overall effects of time of day are significant (p ≤ 0.02) by one-way ANOVA (a, c–f), and also significant (p = 0.002; heteroscedastic data set) by one-way Welch ANOVA (b). Asterisks indicate significant (p < 0.05) changes in spike parameters at a given time point compared to all other times of day. Spike amplitudes (g, j), frequencies (h, k), and durations (i, l) were measured in cyc01(g, h, i) and per01(j, k, l) flies collected at ZT1 and ZT17. The differences in mean amplitudes, frequencies, and durations of spikes at ZT1 and ZT17 are not significant (p > 0.18). Each time point represents amplitudes calculated from a minimum of 30 individual spikes in (a, d, g, j), frequencies calculated from a minimum of 10 individual GRNs in (b, e, h, k), and spike durations calculated from a minimum of 20 individual spikes in (c, f, i, l). All values are mean +/− S.E.M. Representative traces of single unit recordings are shown in Fig. S1.
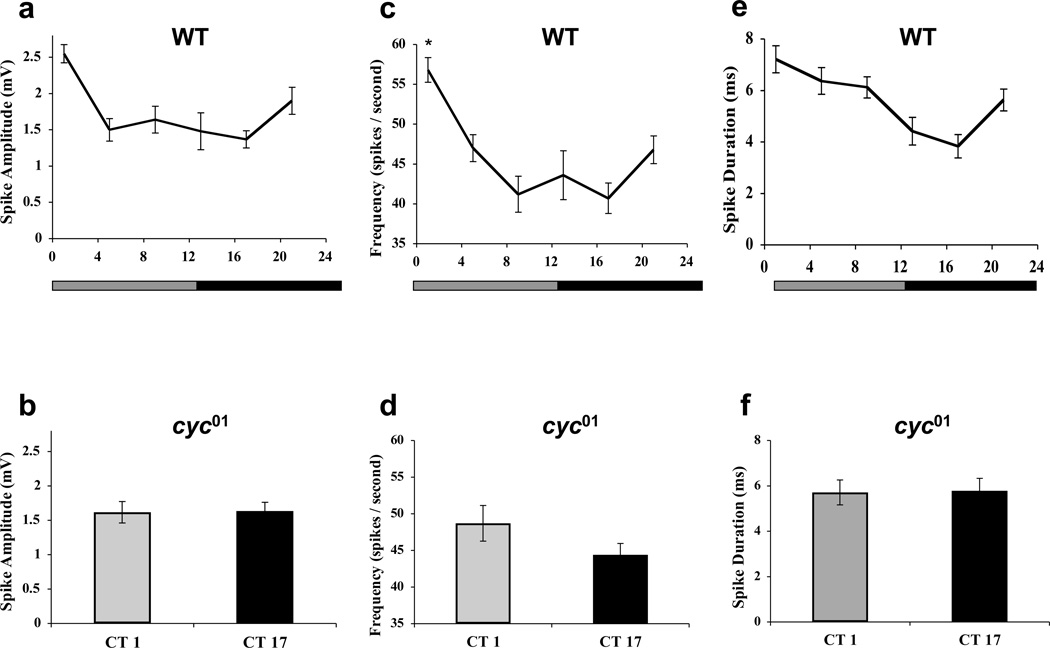
To determine whether other classes of GRNs and other types of sensillae exhibit circadian rhythms in spike activity, single-unit responses to the bitter compound caffeine (10 mM) were measured in L2 neurons from s-type sensilla during DD. Rhythms in spike amplitude, frequency and duration were detected that peaked at circadian time 1 (CT1), where CT0 is subjective lights-on and CT12 is subjective lights-off (Fig. 2a, c, e). These rhythms were abolished in cyc01 mutants in DD (Fig. 2b, d, f), where spike amplitude and frequency were near the wild-type trough and spike duration was between the wild-type peak and trough values. These results demonstrate that circadian control of spike activity is broad, encompassing bitter sensitive L2 neurons and sweet sensitive S neurons in s-type and l-type sensillae, respectively.
Figure 2.
L2-spikes are under circadian-clock control in s-type sensilla. Caffeine-induced L2 neuron spiking activity was measured from s-sensillae during the second day of constant darkness. (a) Spike amplitude was measured from ≥ 30 spikes in WT flies at each of the indicated time points. The overall effect of time of day is significant (p < 0.005) by one-way ANOVA. (b) Spike amplitude was measured from ≥ 30 spikes in cyc01 flies at CT1 and CT17. The difference in mean amplitudes of spikes at CT1 and CT17 is not significant (p > 0.92). (c) Spike frequency was measured from ≥10 individual GRNs in WT flies at each of the indicated time points. The overall effect of time of day is significant (p < 0.001) by one-way ANOVA. Asterisk indicates significant (p < 0.05) changes in firing frequency at CT1 compared to all other times of day. (d) Spike frequency was measured from ≥10 individual GRNs in cyc01 flies at CT1 and CT17. The difference in mean frequencies of spikes at CT1 and CT17 is not significant (p > 0.13). (e) Spike duration was measured from ≥ 20 individual spikes in WT flies at each of the indicated time points. The overall effect of time of day is significant (p < 0.001) by one-way ANOVA. (f) Spike duration was measured from ≥ 20 individual spikes in cyc01 flies at CT1 and CT17. The difference in the mean duration of spikes at CT1 and CT17 is not significant (p > 0.92). All values are mean +/− S.E.M.
Tastant-induced behavior is under clock control
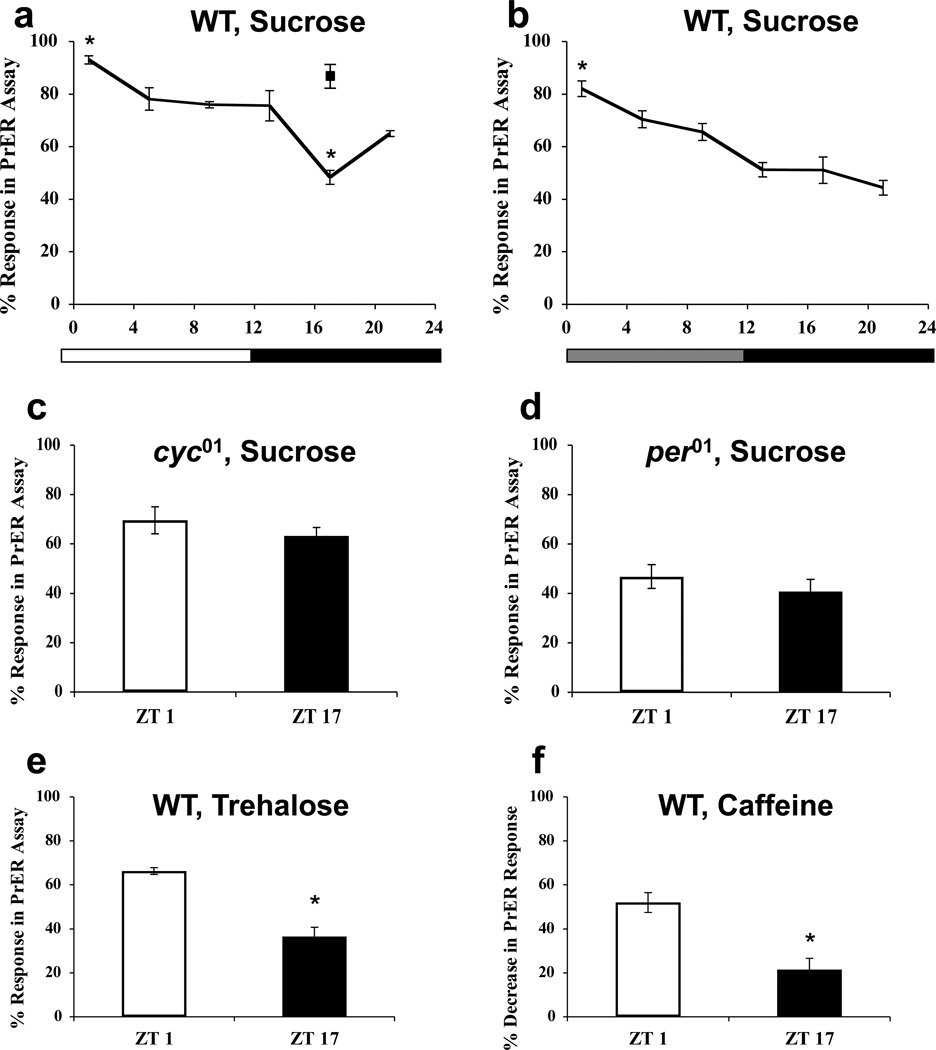
In response to contact-chemoreception with a phagostimulatory chemical, flies elicit a reflex-like appetitive behavior wherein they extend the proboscis to attempt feeding [32]. To determine whether the circadian clock controls tastant-driven behavior, we measured proboscis extension reflex (PrER) responses at different times of day in wild-type and clock mutant flies during LD and DD conditions. PrER responses to 100 mM sucrose in wild-type flies showed a diurnal fluctuation that peaks at dawn (ZT1) and falls to trough levels by mid-night (ZT17) in LD (Fig. 3a). These trough level PrER responses increase to near the peak level when stimulated with 500mM sucrose (Fig. 3a), indicating a clock-modulated change in sensitivity to sucrose. PrER rhythms persisted in wild-type flies during DD, demonstrating that these rhythms are under circadian control (Fig. 3b). Rhythms in PrER responses remained at constant low levels in per01 and cyc01 mutants in LD (Fig. 3c, d), showing that the clock is necessary for increased PrER responses and that light does not have a strong masking effect on PrER rhythms. PrER responses to the sugar trehalose (100mM), which also induces appetitive behavior, exhibited diurnal changes (Fig. 3e). Daily changes in responsiveness to a compound that deters appetitive behavior were measured by quantifying the reduction in PrER responses to a sucrose solution containing caffeine [28]. The presence of caffeine decreased the probability of PrER strongly at ZT1 and only weakly at ZT17 (Fig. 3f). These results demonstrate that gustatory behavior to attractive and repulsive stimuli is under clock control.
Figure 3.
Drosophila display circadian rhythms in gustatory behavioral responses. (a, b) PrER responses to 100 mM sucrose (black line) or 500mM sucrose (filled square) were measured in WT flies during LD cycles (a) or the first day of constant darkness (b). The overall effects of time of day in LD (a) and constant darkness (b) are significant (p < 0.001) by one-way ANOVA. Asterisks indicate significant (p < 0.05) changes in PrER behavior at ZT1 and ZT17 (a) or CT1 (b) compared to all other times of day. (c, d) PrER responses to 100 mM sucrose were measured in cyc01(c), and per01(d) flies at ZT1 and ZT17. The difference in mean PrER responses at ZT1 and ZT17 are not significant (p > 0.30) in cyc01 or per01 flies. (e) PrER responses to 100 mM trehalose were measured in WT flies at ZT1 and ZT17. Asterisks indicate a significant (p < 0.001) reduction in PrER responses at ZT17 compared to ZT1. (f) Decrease in PrER responses to a 100 mM sucrose solution containing 10 mM caffeine versus 100mM sucrose alone in WT flies at ZT1 and ZT17. Asterisk indicates significant (p = 0.025) decrease in PrER inhibition by caffeine at ZT17 compared to ZT1. All values are mean +/− S.E.M.
Clocks within GRNs are necessary and sufficient for PrER rhythms
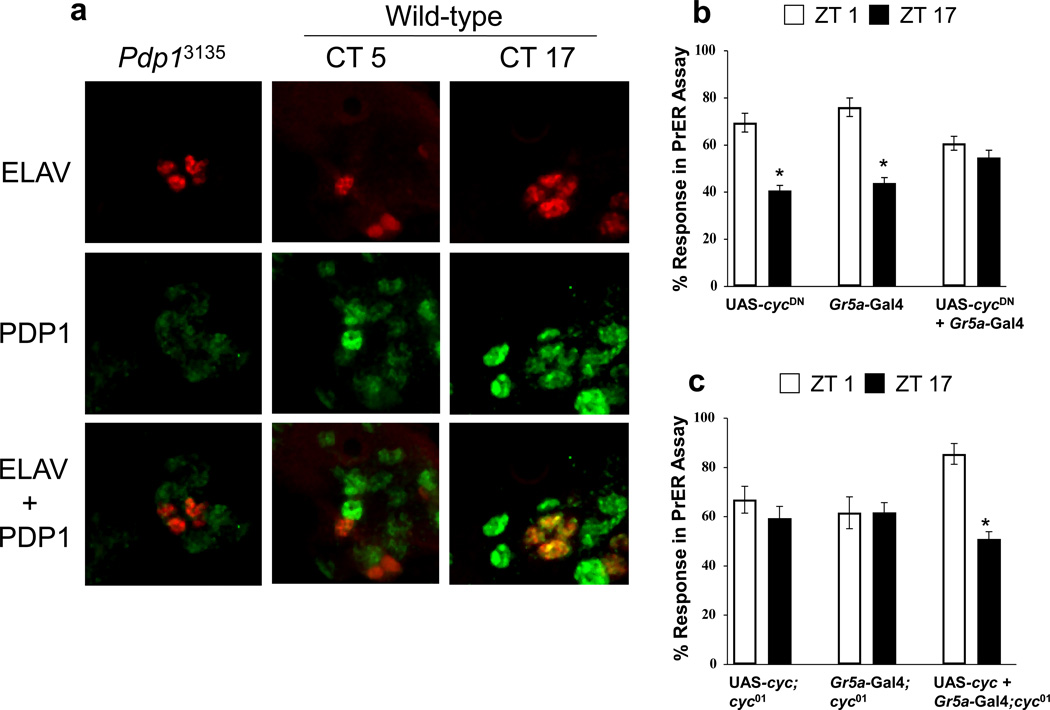
In the Drosophila olfactory system, peripheral clocks in OSNs drive rhythms in odor-induced physiological responses [8]. Given that the proboscis contains autonomous circadian oscillators and the PrER is initiated by GRNs [12, 33, 34], we hypothesized that peripheral oscillators in GRNs drive PrER rhythms. The presence of peripheral clocks in GRNs on the proboscis was first confirmed via immunocytochemistry. The constitutively expressed pan-neural nuclear antigen ELAV detects clusters of GRNs at the base of each sensillum (Fig. 4a). Coimmunostaining with anti-PDP1 revealed more intense PDP1 immunofluorescence in ELAV positive cells at ZT17 than at ZT5, but relatively constant immunofluorescence intensity in ELAV negative cells (Fig. 4a). All PDP1 immunostaining was eliminated in the PDP1ε-specific mutant Pdp13135 [35], indicating that only PDP1ε is expressed in these ELAV positive and negative cells. Rhythmic PDP1ε staining in ELAV positive cells is consistent with PDP1ε cycling in brain and peripheral oscillator cells [35–37], and demonstrates that the GRNs within gustatory sensilla contain circadian oscillators.
Figure 4.
Oscillators within GRNs are necessary and sufficient for PrER rhythms. (a) PDP1 and ELAV immunostaining in GRNs of Pdp13135 mutant flies collected at ZT17 and wild-type flies collected at CT5 and CT17. Anti-ELAV immunostaining (ELAV) is shown in red, anti-PDP1 immunostaining (PDP1) is shown in green, and co-localized PDP1 and ELAV immunostaining (ELAV + PDP1) is shown in yellow. (b) PrER responses were measured at ZT1 and ZT17 in wild-type flies bearing the Gr5a-Gal4, UAS-cycDN, or Gr5a-Gal4 + UAS--cycDN transgenes. The differences in mean PrER responses at ZT1 and ZT17 are significant (p < 0.001) in flies containing Gr5a-Gal4 and UAS-cycDN alone, but are not significant (p < 0.30) in flies carrying Gr5a-Gal4 + UAS-cycDN(c) PrER responses were measured at ZT1 and ZT17 in cyc01 flies carrying the Gr5a-Gal4, UAS-cyc, or UAS-cyc + Gr5a-Gal4 transgenes. There are no significant (p > 0.30) differences in PrER responses at ZT17 and ZT1 in cyc01 flies carrying either UAS-cyc or Gr5a-Gal4. The differences in mean PrER responses at ZT1 and ZT17 are significant (p < 0.001) in cyc01 flies carrying UAS-cyc + Gr5a-Gal4. Asterisks denote a significant (p < 0.05) change in PrER responses between ZT17 and ZT1. All values are mean +/− S.E.M.
To test the idea that local oscillators within GRNs are necessary for PrER rhythms, we expressed a dominant negative form of CYC (CYCDN) to abolish clock function in the sweet-sensitive S neurons that elicit PrER behavior in response to sucrose [33]. Under LD conditions, PrER responses were abolished in flies containing both the Gr5a-Gal4 driver, which is expressed in S neurons [34], and UAS-cycDN responder, but not in control flies containing the Gr5a-Gal4 driver or UAS-cycDN responder alone (Fig. 4b). This result demonstrates that circadian oscillators in GRNs are required for PrER rhythms.
We then sought to determine whether local clocks in GRNs are sufficient for PrER rhythms by generating flies with circadian oscillators only in S neurons. For this, oscillator function was rescued exclusively in S neurons by using Gr5a-Gal4 to drive UAS-cyc expression in cyc01 flies. PrER behavior in cyc01 flies containing both Gr5a-Gal4 and UAS-cyc was rhythmic, whereas cyc01 flies containing Gr5a-Gal4 or UAS-cyc alone were arrhythmic (Fig. 4c). These data demonstrate that clocks in GRNs are sufficient for PrER rhythms. Since clocks are not present elsewhere in cyc01 flies containing Gr5a-Gal4 and UAS-cyc, these data also show that central clocks in the brain are not necessary for PrER rhythms. Taken together, these results demonstrate that GRN clocks are necessary and sufficient to control rhythms in gustatory behavior.
Cycling GPRK2 levels drive PrER behavior rhythms
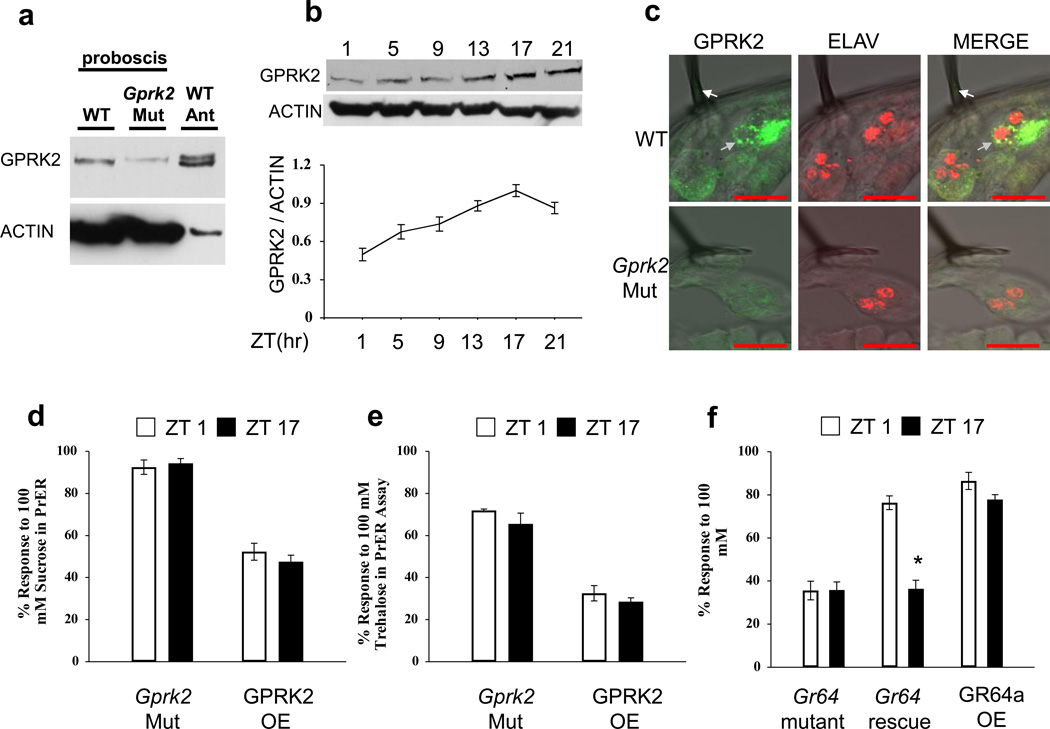
Since circadian oscillators in GRNs are sufficient for PrER rhythms, the clock output pathway that controls this rhythm must also reside in GRNs. To identify a clock-controlled molecule involved in gustatory signal transduction, we focused our attention on GPRK2, which is required for rhythms in olfactory responses in Drosophila [14, 38]. Western blot analysis shows that GPRK2 protein is expressed in the proboscis of wild-type flies, but that GPRK2 levels are reduced in the Gprk206936 mutant (Fig. 5a) [39]. In contrast to the two GPRK2 isoforms that are detected in antennae [38], only one GPRK2 band is seen in the proboscis of wild-type and Gprk206936 flies (Fig. 5a). The levels of GPRK2 cycled ~2-fold in wild-type proboscises with a peak at ZT17 and a trough at ZT1 (Fig. 5b). GPRK2 cycling was abolished in per01 and cyc01 flies (data not shown), indicating circadian clock control. GPRK2 immunostaining was detected in the cell body of GRNs at the base of taste-hairs that were co-immunostained with ELAV (Fig. 5c). GPRK2 was also detected in the shaft of the sensillar hair, which contains GRN dendritic projections, and possibly support cells closely associated with GRNs (Fig. 5c).
Figure 5.
GPRK2 and GR expression levels control rhythms in PrER behavior. (a) Western blot showing GPRK2 expression in proboscises from WT and Gprk206936 mutant (Gprk2 Mut) flies and antennae (Ant) from WT flies. GPRK2 runs as two isoforms in antennae and one isoform in proboscises. ACTIN was used as a loading control (b) Western blot showing GPRK2 levels in proboscises of WT flies collected at the indicated times during an LD cycle. The GPRK2:ACTIN values at ZT1, ZT5, ZT9, ZT13, and ZT21 are relative to the value at ZT17, which was set to 1.0. Each timepoint represents the mean of three independent experiments. The overall effect of time of day is significant (p < 0.005) by one-way ANOVA. (c) GPRK2 and ELAV immunostaining in labellar GRNs from WT and Gprk2 mutant flies. Anti-GPRK2 immunoreactivity is shown in green and anti-ELAV signal is shown in red. Scale bars represent 10 µm. Grey arrows, GPRK2 localization in the cytosol; white arrows, GPRK2 immunostaining in the shaft of a sensillar hair. (d, e) PrER responses to sucrose and trehalose were measured at ZT1 and ZT17 in WT flies carrying Gr5a-Gal4 and UAS-Gprk2, which overexpress GPRK2 in S neurons (GPRK2 OE), and in Gprk206936 mutants (Gprk2 Mut). Mean PrER responses to sucrose (d) and trehalose (e) at ZT1 and ZT17 were not significant (p > 0.16), and remained at constant low levels in Gprk2 Mut flies and constant high levels in GPRK2 OX flies. For each genotype, three or more groups of ≥ 10 flies were tested for PrER responses to sucrose and trehalose at each timepoint. Asterisks denote a significant (p < 0.05) change in PrER responses between ZT17 and ZT1. (f) PrER responses to 100 mM sucrose in Gr64 mutant (R1/+;R2/+;ΔGr64/ΔGr64), Gr64 rescue (R1/+;R2/+;ΔGr64/ΔGr64 carrying one copy of the UAS-Gr64abcd_GFP_f reporter), and Gr64a overexpressing flies at ZT1 and ZT17. The differences in mean responses at ZT1 and ZT17 are not significant in Gr64 mutants (p > 0.90) or Gr64a overexpressing flies (p > 0.05), but significant (p < 0.001) in Gr64 rescue flies. All values are mean +/− S.E.M. As with PrER responses, lower GPRK2 expression in Gprk2 Mut flies disrupts rhythms in GRN spike activity (Fig. S2).
The levels of GPRK2 in the proboscis are lowest when PrER responses peak, and peak when PrER responses are lowest. This antiphasic relationship suggests that GPRK2 levels may control rhythmic PrER behavior. Consistent with this possibility, PrER responses to sucrose and trehalose were constantly repressed when GPRK2 was overexpresssed, but were always high in the Gprk206936 mutant (Fig. 5d, e). Thus, these experiments argue that cycling GPRK2 levels drive rhythms in PrER behavior. Given that PrER responses are constantly high in Gprk206936 flies and that spike amplitude, frequency and duration in GRNs cycle in parallel to PrER responses, we reasoned that these spike activity parameters should be constant and relatively high in the GRNs of Gprk206936 flies. Rhythms in spike amplitude, frequency and duration were all abolished in Gprk206936 flies, where spike frequency was close to the wild-type peak, but spike amplitude was midway between the wild-type peak and trough and spike duration was only modestly higher than the wild-type trough (Fig. S2). These results suggest that certain aspects of GRN cell activity, particularly spike frequency and to a lesser extent spike amplitude, correlate with PrER behavior.
GPRK2 mediates circadian rhythms in the subcellular localization of Drosophila odorant receptors (ORs) [38]. Since Drosophila ORs and GRs belong to the same family of insect chemoreceptor proteins, we wished to determine whether GPRK2-dependent regulation of rhythmic PrER responses relies on GRs. A mutant that removes all six Drosophila Gr64 genes (ΔGr64) shows drastically reduced PrER responses to most sugars [33]. When ΔGr64 flies were stimulated with 100mM sucrose at ZT1 and ZT17 their PrER responses were not rhythmic, but ΔGr64 mutants rescued by a transgene containing the entire Gr64 gene cluster [33] recovered PrER rhythms (Fig. 5f). Overexpression of the sucrose receptor Gr64a resulted in arrhythmic PrER responses that were near the circadian peak value (Fig. 5f). Likewise, deletion of Gr5a, which is required for responses to trehalose [40, 41], resulted in constant low PrER responses to trehalose whereas Gr5a overexpression resulted in constant high responses to trehalose (data not shown). These results imply that GRs are not only required to detect tastants, but are necessary for sustaining rhythms in tastant-evoked appetitive behavior.
GRN clocks regulate feeding
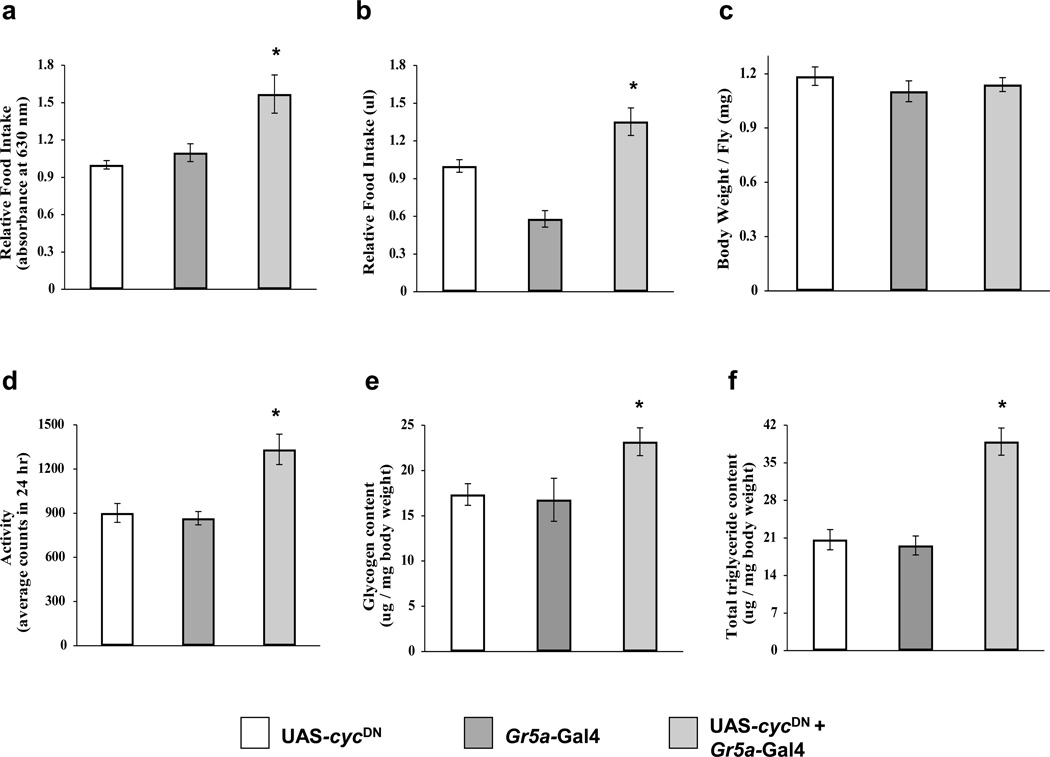
Both external sensory cues and internal metabolic state contribute to the regulation of feeding [23]. Recent work in Drosophila has shown that loss of clock function in fat body increases feeding by altering metabolic state [25]. We sought to determine whether GRN oscillators also regulate feeding since they modulate taste sensitivity. Food ingestion was measured using a blue food dye that can be quantified spectrophotometrically and the Capillary Feeder (CAFE) assay [25, 42]. Under LD conditions, flies that express CYCDN in sweet-sensitive Gr5a neurons consume significantly more food over 24 h than controls carrying the driver or responder transgenes (Fig. 6a, b). Moreover, food intake is higher in the morning (ZT0–4) than in the evening (ZT12–16), demonstrating that increased consumption is not uniform during a diurnal cycle (Table S1). This result shows that circadian clocks in a subset of GRNs act to limit the amount of food intake.
Figure 6.
Circadian clocks in Gr5a neurons regulate feeding, food storage and activity. (a, b) Relative food intake was measured after 24 h of feeding by quantifying the amount of blue food dye ingested (a) or by measuring the food consumed using the CAFE assay (b) (see Experimental Procedures). Flies carrying both the Gr5a-Gal4 and UAS-cycDN transgenes show significantly (p < 0.02) increased feeding compared to control flies containing either the UAS-cycDN or the Gr5a-Gal4 transgene. (c) The body weight of flies carrying both the Gr5a-Gal4 and UAS-cycDN transgenes was not different (p > 0.50) than control flies bearing the UAS-cycDN or Gr5a-Gal4 transgenes. (d) Overall activity was measured as the number of times flies crossed an infrared light beam during a 24 h period. Flies carrying both the Gr5a-Gal4 and UAS-cycDN transgenes show significantly (p < 0.001) increased activity compared to control flies containing either the UAS-cycDN or the Gr5a-Gal4 transgene. (e) Glycogen levels are significantly (p < 0.04) higher in flies carrying both the Gr5a-Gal4 and UAS-cycDN transgenes than control flies bearing the UAS-cycDN or Gr5a-Gal4 transgenes. (f) Triglyceride levels are significantly (p < 0.001) higher in flies carrying both the Gr5a-Gal4 and UAS-cycDN transgenes than control flies bearing the UAS-cycDN or Gr5a-Gal4 transgenes. Error bars represent +/− S.E.M. Increased feeding and activity in flies lacking clocks in Gr5a neurons is not uniform over the circadian cycle (Table S1).
Although flies that lack clocks in Gr5a neurons eat more, they do not gain weight compared to controls carrying the driver or responder transgenes alone (Fig. 6c). Nevertheless, loss of clock function in Gr5a neurons led to a considerable increase in triglyceride and glycogen content (Fig. 6d, e). Increased triglyceride and glycogen content in flies lacking clocks in Gr5a neurons is associated with higher levels of locomotor activity over a 24 h period (Fig. 6f), where increased activity levels coincide with increased feeding (Table S1). Thus, flies lacking clocks in Gr5a neurons eat more and store triglycerides and glycogen even though they expend more energy to fuel increased locomotor activity.
Discussion
Circadian rhythms in gustatory physiology and behavior
The ability to detect and discriminate tastants provides a survival advantage to animals ranging from flies to humans because chemosensation is universally employed to identify food sources and reject harmful substances [34]. Our results demonstrate for the first time that this fundamental sensory process is controlled by the circadian clock. The amplitude, frequency and duration of voltage spikes evoked by attractive and aversive tastants peak around dawn in multiple classes of GRNs and different types of taste-sensilla (Figs. 1 and 2). Ventrolateral clock neurons (LNvs) in the fly brain show rhythms in firing rate that also peak during the day [43–45], but whether a common mechanism controls rhythms in the electrical properties of GRNs, LNvs and OSNs is not known.
The PrER is a direct, robust, and all-or-none indicator of a fly's attraction and motivation to ingest a substance [32]. PrER response levels change as a function of time of day (Fig. 3), where the phase of this taste-behavior rhythm mirrors rhythms in the rate, amplitude, and duration of GRN impulses in wild-type flies under LD and DD conditions. These results suggest that spike amplitude and duration, in addition to spike frequency, are dynamic neuronal response properties capable of influencing sensitivity to chemical cues. Circadian rhythms in spike amplitude are also seen in the olfactory system of flies [14], where the phases of these electrophysiological rhythms coincide with rhythms in odor-dependent chemotactic behavior that peak during mid-night [15]. Our data suggest that rhythms in spike properties of GRNs tune the activity of downstream neurons in such a way that behavioral responses to the same stimulus show clock-regulated plasticity. Given that PrER behavior likely involves local circuitry with limited processing [20], it is surprising that this ‘hardwired’ behavior is subjected to daily functional remodeling by the clock, and that a straightforward predictive relationship emerges between rhythms in GRN responses and rhythms in tastant-driven appetitive behavior.
Control of PrER rhythms
We show that circadian oscillators in GRNs are necessary and sufficient for PrER rhythms (Fig. 4). To our knowledge, this is the first example where a single population of peripheral oscillator neurons is shown to generate behavioral rhythms. GRNs from the proboscis project primarily into the central portion of the suboesophageal ganglion (SOG) [46]. A number of SOG motor neurons are known to innervate muscles in proboscis and pharynx [47], which may be indirectly controlled by the GRN clock on a daily basis.
In Drosophila, GRNs express GPRK2, and rhythms in GPRK2 abundance are antiphase relative to PrER rhythms (Fig. 5a–f). Analyses of Gprk2 mutant and GPRK2 overexpression flies suggest that GPRK2 levels drive rhythms in PrER responses, and correspond to GRN spike frequency and to a lesser extent spike amplitude (Fig. 5d, e; Fig. S2). Rhythms in PrER responses are also abolished by altering GR levels; increasing or decreasing Gr64 or Gr5a levels results in constant high or low PrER responses, respectively (Fig. 5f; data not shown). PrER responses are constantly low in Gr64 and Gr5a deletion mutants and GPRK2 overexpression flies, but are constantly high in Gr64 and Gr5a overexpression flies and Gprk2 mutants, which argues that the balance between GR and GPRK2 abundance determine PrER response levels. Although the phase of GPRK2 cycling is the same in OSNs and GRNs, olfactory responses (e.g. spike amplitude) peak when gustatory responses are low, and gustatory responses peak when olfactory responses are low [14] (Fig. 1, 2). The difference in gustatory and olfactory response phases implies that GPRK2 has distinctly different activities in the olfactory and gustatory systems.
In OSNs, GPRK2 rhythmically promotes dendritic localization of ORs [38]. ORs and GRs are both seven transmembrane domain proteins that belong to the same superfamily of insect chemoreceptor proteins [20]. It is tempting to speculate that GPRK2 directly phosphorylates GRs, thereby controlling the abundance or activity of GR-dependent channels or ligand gated GR-channels in GRNs. This rhythmic regulation of neuronal excitability may be translated into rhythms in spike amplitude, frequency and duration. Thus, the PrER rhythm is likely a behavioral correlate of certain features of electrophysiological rhythms such as spike frequency and perhaps spike amplitude.
Functional significance of gustatory rhythms
Peripheral oscillators may play widespread roles in sensory processing, such that the perceived meaning of a sensory input is determined not just by the modality of the signal or its intensity, but also by the circadian time when the signal is registered. Our results indicate that the clock tunes the gustatory system to a higher gain level in the morning. This may allow the fly to temporally couple the morning bout of activity with food-detection machinery that works better at dawn, leading to increased feeding. This strategy can minimize energy expenditure by shutting down hardwired taste responses to weak stimuli (behavioral noise) at times when flies are resting, and selectively boost acuity at times when they are wakeful. Interestingly, the acrophase of feeding rhythms coincides with the early morning peak in gustatory response rhythms [25].
Social experience, which can influence behavior in Drosophila [48], is communicated by chemosensory cues such as pheromones. Moreover, circadian clocks in oenocytes regulate rhythms in the abundance of male pheromones including 7-tricosene [7] that are detected by GRNs [29, 49]. Thus, local clocks in oenocytes temporally gate the production of male pheromones and local clocks in GRNs may temporally gate pheromone reception and signaling. Such a system could function to define a time window for social interactions and mating, and the resulting social experience may in turn influence clocks that control pheromone production and/or gustatory sensitivity.
Feeding is modulated by the gustatory clock
Increased feeding in flies that lack circadian clock function in Gr5a neurons suggests that clocks in these cells act to restrict food consumption (Fig. 6). Given that clock function was compromised in GRNs that detect sugars [33, 50], increased food consumption may be due to a change in taste sensitivity. However, loss of clock function in Gr5a neurons decreases PrER responses at ZT1 and increases PrER responses at ZT17 (Fig. 4), indicating that the clock increases taste sensitivity in the morning and decreases it at night. When feeding was measured under the same conditions as PrER responses, food intake increased as PrER responses decreased in the morning and food intake decreased as PrER responses increased in the evening (Table S1). In control genotypes having clocks in Gr5a neurons, CAFE assays show that food intake is similar in the morning and the evening, consistent with estimates of food intake at different times of day in single flies using a novel assay that measures the proportion of time flies were observed extending their proboscis to feed [51]. Our results suggest that increased feeding is not due to altered taste sensitivity in flies lacking clocks in Gr5a neurons. It is possible that the increased food intake at ZT0–4 and the decreased food intake at ZT16–20 in flies lacking Gr5a neuron oscillators is due to metabolic feedback on tissues with functional clocks. Consistent with this possibility, food intake in ClkJrk and cyc01 mutant flies is the same as that in wild-type flies [25].
Flies lacking clocks in Gr5a neurons do not show a measurable gain in weight (Fig. 6c), presumably due to a high fixed level of cuticle, protein and water weight. However, their triglyceride and glycogen content increases substantially (Fig. 6d, e), indicating that the higher amounts of food consumed are being stored. Given this increase in food storage it was surprising that flies lacking clocks in Gr5a neurons were also more active (Figure 6f). Increased activity is typically observed when starved flies are searching for food [52], yet flies lacking clocks in Gr5a neurons consume more food than wild-type flies (Fig. 6a, b). The loss of clocks in Gr5a neurons may mimic starvation conditions, particularly during the day when feeding is increased and PrER responses are relatively low compared to wild-type flies (Table S1; Fig. 4). At night, even though PrER responses are higher in flies lacking clocks in Gr5a neurons than in wild-type flies, activity is already low and feeding is even lower than in wild-type flies. Thus, loss of clock function in Gr5a neurons may produce starvation signals during the day, thereby increasing activity and feeding, but not at night since feeding is decreased and flies are already inactive.
Experimental Procedures
Fly strains
0–7 day old flies reared on standard cornmeal media were entrained for 3 days in 12h light:12h dark (LD) cycles at 25°C. Lights were turned on at ZT0 and off at ZT12. Canton-S was used as our wild-type (WT) strain. The Gr5a-Gal4 driver [34], and the UAS-Gr64a [53], UAS-Gr5a [40], UAS-cycDN [8], UAS-cyc [8], and UAS-Gprk2 [38] responders were described previously. These experiments also employed the P-element insertion mutant Gprk206936 [39], the Pdp1ε-specific deletion mutant Pdp13135 [35], the Gr5a deletion mutant ΔEP(X)-5 [40], the ΔGr64 mutant (R1/+;R2/+;ΔGr64/ΔGr64) that lacks all six Gr64 genes [33], and the transgenically rescued ΔGr64 mutant (R1/+;R2/+;ΔGr64/ΔGr64 flies carrying one copy of the UAS-Gr64abcd_GFP_f reporter) [33].
Western blotting and Immunostaining
30–35 proboscises were dissected from flies entrained for at least 3 LD cycles. Western blots were processed as described [38]. Blots were probed with anti-GPRK2 antibody (1:1000 dilution) and anti-Actin antibody (Sigma-Aldrich, 1:10000 dilution) and visualized via ECL (Amersham). Immunostaining was carried out on cryosectioned proboscises as detailed in Supplemental Data.
Single-sensillum recording
Male flies (3–10 days old) entrained to LD cycles for ≥3 days were collected during LD or the second day of DD, mounted, and the proboscis was immobilized. Individual labellar sensillae were observed under 1200X magnification. Recordings in the dark were made using a <600nm filter. The indifferent electrode was inserted into the eye. The recording electrode contained tastant dissolved in 1 mM KCl, and was used to stimulate a sensillum by physical contact with the tip of that sensillum. All recordings using a given genotype and tastant were performed at least six times per time point from ≥ 6 flies. A new group of flies were recorded at each timepoint. 100 mM sucrose was used to stimulate S cells in accessible l-type sensilla, which respond to sugars in an identical manner [30]. 10 mM caffeine was used to stimulate s6 and s2 sensilla whose L2 neurons are responsive to bitter compounds [30]. The number of spikes initiated by the tastant was counted manually over 500 ms duration beginning 50 ms after the onset of stimulation. Spike traces were analyzed using Axoscope (Axon) software in offline mode, where the peak and trough values of individual spikes were used to compute amplitude. The time elapsed between the peak and trough values for an activity spike was used as a measure of spike duration [54].
Proboscis extension reflex (PrER) assay
3–7 day old male flies that had been entrained to LD cycles for ≥3 days were starved for 24 h, collected at different times during LD or the first day of DD, mounted on a slide, and allowed to recover for 30 minutes. Proboscis extension in response to 100 mM sucrose and 100 mM trehalose was recorded as described [33], with minor modifications detailed in Supplemental Data.
Feeding assays
3–10 day old male flies entrained for at least 3 LD cycles were given food containing 5% sucrose, 1% low melting point agarose and 0.5% brilliant blue FCF (Wako) for 24 h starting at ZT12. Flies were then collected and prepared for quantification of blue dye ingestion as described [25]. For a given genotype, at least 6 independent experiments, each set consisting of 10 flies, were carried out. CAFE (Capillary Feeding) assays were used to measure feeding behavior of grouped fruit flies [25, 42]. For each genotype, CAFE assays were conducted as described [25] except that flies were habituated to feeding from glass capillaries for 24 h and feeding was measured over 4 h. CAFE assays were repeated at least 5 times for each data point. Levels of glycogen and triglycerides were measured as previously described [25].
Activity measurement
For each line, 7–10 day old male flies were entrained for at least 3 days in LD cycles and placed in Drosophila Activity Monitors (Trikinetics Inc.). Activity was measured by counting the number of infrared beam breaks every 10 minutes and analyzed using Clocklab software.
Statistical analysis
Statistical analysis was done using Statistica (Statsoft). Analysis of the effects of time of day was examined by one-way analysis of variance (ANOVA). Welch’s ANOVA was used for heteroscedastic data set, provided Levene’s test indicated unequal variances. Posthoc comparisons were done using Scheffe’s test (α = 0.05). Unpaired Student’s t-test (two-tailed) was used to compare values at peak and trough time points.
Supplementary Material
Acknowledgements
We are thankful to Dr. Hubert Amrein, Dr. Craig Montell, and Dr. Anupama Dahanukar for generously providing fly strains. We would also like to thank Teiichi Tanimura and Kohei Ueno for their valuable technical suggestions. This work was supported by funds from Texas A&M University to PEH.
References
- 1.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 4.Sheeba V, Kaneko M, Sharma VK, Holmes TC. The Drosophila circadian pacemaker circuit: Pas De Deux or Tarantella? Crit Rev Biochem Mol Biol. 2008;43:37–61. doi: 10.1080/10409230701829128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:2134–2139. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito C, Goto SG, Shiga S, Tomioka K, Numata H. Peripheral circadian clock for the cuticle deposition rhythm in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:8446–8451. doi: 10.1073/pnas.0800145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupp JJ, Kent C, Billeter JC, Azanchi R, So AK, Schonfeld JA, Smith BP, Lucas C, Levine JD. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 8.Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Emery IF, Noveral JM, Jamison CF, Siwicki KK. Rhythms of Drosophila period gene expression in culture. Proc Natl Acad Sci U S A. 1997;94:4092–4096. doi: 10.1073/pnas.94.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giebultowicz JM, Stanewsky R, Hall JC, Hege DM. Transplanted Drosophila excretory tubules maintain circadian clock cycling out of phase with the host. Curr Biol. 2000;10:107–110. doi: 10.1016/s0960-9822(00)00299-2. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- 12.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila . Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 13.Giebultowicz JM, Hege DM. Circadian clock in Malpighian tubules. Nature. 1997;386:664. doi: 10.1038/386664a0. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan P, Chatterjee A, Tanoue S, Hardin PE. Spike amplitude of single-unit responses in antennal sensillae is controlled by the Drosophila circadian clock. Curr Biol. 2008;18:803–807. doi: 10.1016/j.cub.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Yuan C, Guo A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J Biol Rhythms. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- 16.Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlin C, Lucas P, Rochat D, Francois MC, Maibeche-Coisne M, Jacquin-Joly E. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis. J Biol Rhythms. 2007;22:502–514. doi: 10.1177/0748730407307737. [DOI] [PubMed] [Google Scholar]
- 18.Nordin S, Lotsch J, Murphy C, Hummel T, Kobal G. Circadian rhythm and desensitization in chemosensory event-related potentials in response to odorous and painful stimuli. Psychophysiology. 2003;40:612–619. doi: 10.1111/1469-8986.00062. [DOI] [PubMed] [Google Scholar]
- 19.Page TL, Koelling E. Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J Insect Physiol. 2003;49:697–707. doi: 10.1016/s0022-1910(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 20.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 21.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 22.Christiensen T. Methods in Insect Sensory Neuroscience. Boca Raton: CRC Press; 2004. [Google Scholar]
- 23.Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J Endocrinol. 2007;192:467–472. doi: 10.1677/JOE-06-0066. [DOI] [PubMed] [Google Scholar]
- 24.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8:169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 27.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amrein H, Thorne N. Gustatory perception and behavior in Drosophila melanogaster. Curr Biol. 2005;15:R673–R684. doi: 10.1016/j.cub.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Ebbs ML, Amrein H. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflugers Arch. 2007;454:735–747. doi: 10.1007/s00424-007-0246-y. [DOI] [PubMed] [Google Scholar]
- 30.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 31.Hochner B, Klein M, Schacher S, Kandel ER. Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A. 1986;83:8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dethier VG. The hungry fly. Cambridge: Harvard University Press; 1976. [Google Scholar]
- 33.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X, Koh K, Sowcik M, Smith CJ, Chen D, Wu MN, Sehgal A. An isoform-specific mutant reveals a role of PDP1 epsilon in the circadian oscillator. J Neurosci. 2009;29:10920–10927. doi: 10.1523/JNEUROSCI.2133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benito J, Zheng H, Hardin PE. PDP1epsilon functions downstream of the circadian oscillator to mediate behavioral rhythms. J Neurosci. 2007;27:2539–2547. doi: 10.1523/JNEUROSCI.4870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 38.Tanoue S, Krishnan P, Chatterjee A, Hardin PE. G protein-coupled receptor kinase 2 is required for rhythmic olfactory responses in Drosophila. Curr Biol. 2008;18:787–794. doi: 10.1016/j.cub.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider LE, Spradling AC. The Drosophila G-protein-coupled receptor kinase homologue Gprk2 is required for egg morphogenesis. Development. 1997;124:2591–2602. doi: 10.1242/dev.124.13.2591. [DOI] [PubMed] [Google Scholar]
- 40.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 41.Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 42.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park D, Griffith LC. Electrophysiological and anatomical characterization of PDF-positive clock neurons in the intact adult Drosophila brain. J Neurophysiol. 2006;95:3955–3960. doi: 10.1152/jn.00117.2006. [DOI] [PubMed] [Google Scholar]
- 45.Sheeba V, Gu H, Sharma VK, O’Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Rajashekhar KP, Singh RN. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J Comp Neurol. 1994;349:633–645. doi: 10.1002/cne.903490410. [DOI] [PubMed] [Google Scholar]
- 48.Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- 49.Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, Maniere G, Marion-Poll F, Ozaki M, Francke W, Cobb M, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS ONE. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 51.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 53.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gur M, Beylin A, Snodderly DM. Physiological properties of macaque V1 neurons are correlated with extracellular spike amplitude, duration, and polarity. J Neurophysiol. 1999;82:1451–1464. doi: 10.1152/jn.1999.82.3.1451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.