Abstract
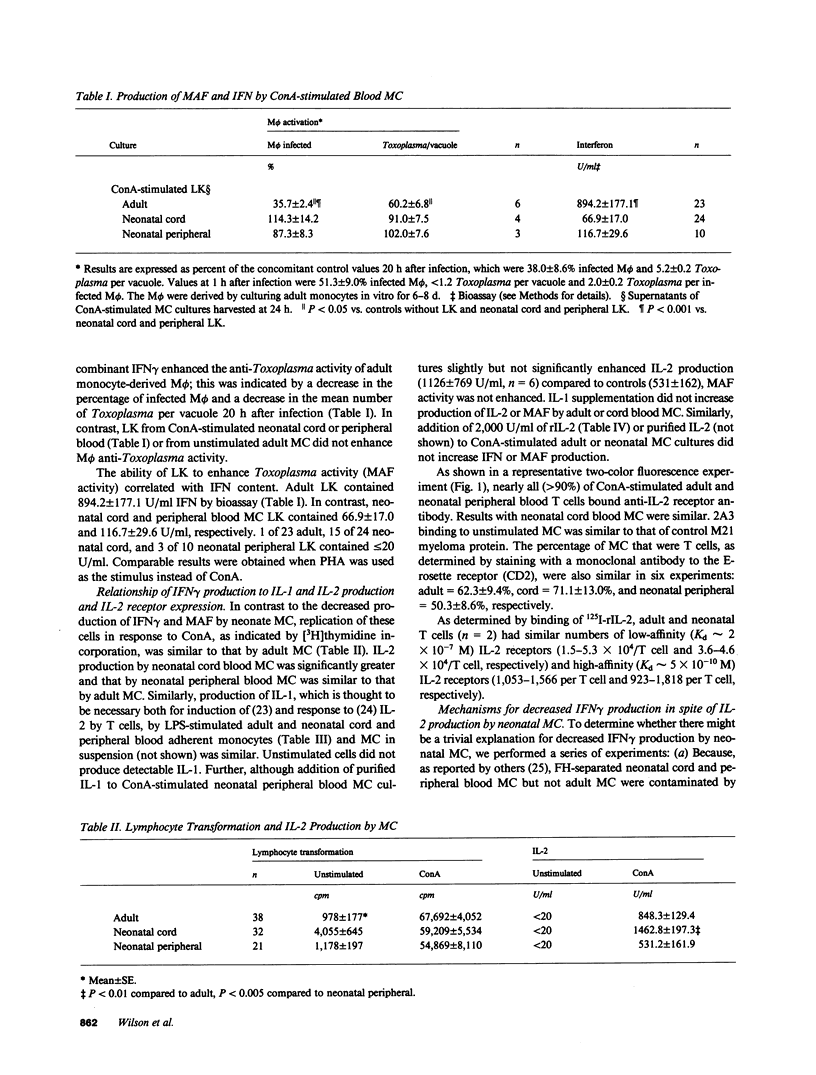
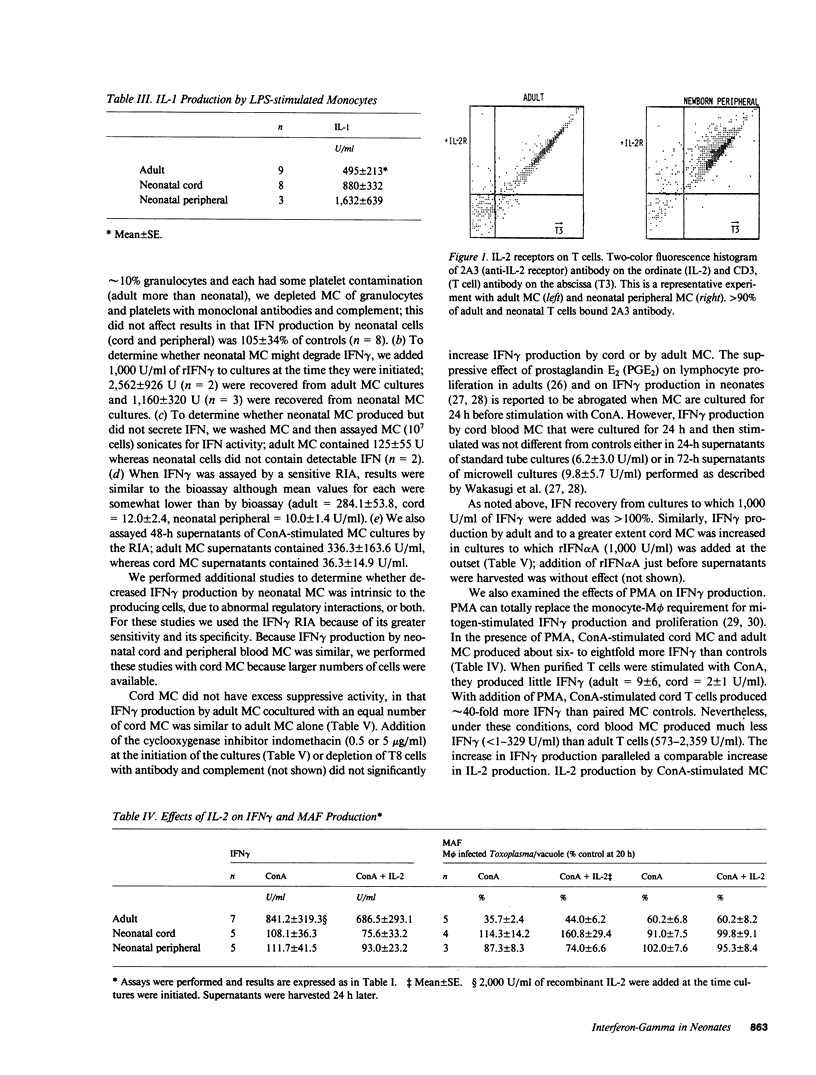
Human neonatal lymphocytes produced little macrophage activation factor in response to mitogens. This correlated with decreased production of interferon-gamma (IFN gamma): adult lymphokines contained 894.2 +/- 177.1 U/ml, whereas neonatal cord and peripheral lymphokines contained 66.9 +/- 17.0 and 116.7 +/- 29.6 U/ml by bioassay. Results by radioimmunoassay (RIA) for IFN gamma were similar. In contrast, the interleukin 2 content of cord lymphokines was greater (P less than 0.01) and that of neonatal peripheral blood lymphokines similar to that of adults. Interleukin 1 production and interleukin 2 receptor expression and affinity were similar for adult and neonatal cells. Interleukins 1 and 2 in amounts comparable to those in adult lymphokines did not increase production of macrophage activation factor or IFN gamma by neonatal cells. Neonatal cells did not contain intracellular IFN or degrade exogenous IFN. Excess suppressor activity was not found in neonatal cultures. Addition of IFN alpha, 10,000-50,000 U/ml of interleukin 2 or phorbol myristate acetate (PMA) to cord mononuclear cells or of adult monocytes or PMA to cord T cells increased IFN gamma production compared to cells stimulated with concanavalin A (ConA) alone. Nevertheless, under optimal conditions (T cells + PMA + Con A), adult cells produced much more IFN gamma (1,360 +/- 261 U/ml by RIA) than cord cells (122 +/- 37 U/ml). Staphylococcal enterotoxin A (SEA) stimulated cord cell IFN gamma production at low cell densities; nevertheless, adult cells produced more IFN in response to SEA 1,341 +/- 350 U/ml) than cord cells (350 +/- 33 U/ml). Decreased production of IFN gamma by neonatal cells appears to be due both to differences in their intrinsic capacity to produce IFN gamma and to differences in regulatory mechanisms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahronheim G. A. Toxoplasma gondii: human interferon studies by plaque assay. Proc Soc Exp Biol Med. 1979 Sep;161(4):522–526. doi: 10.3181/00379727-161-40588. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Leary P. L., Levin M. J. Gamma interferon production by combinations of human peripheral blood lymphocytes, monocytes, and cultured macrophages. Infect Immun. 1982 Feb;35(2):383–390. doi: 10.1128/iai.35.2.383-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenson E. B., Epstein M. B., Seeger R. C. Monocyte subsets in neonates and children. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):740–744. [PubMed] [Google Scholar]
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Chang T. W., McKinney S., Liu V., Kung P. C., Vilcek J., Le J. Use of monoclonal antibodies as sensitive and specific probes for biologically active human gamma-interferon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5219–5222. doi: 10.1073/pnas.81.16.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Shparber M., Kent E. F., Jr, Wolff S. M. Production of leukocytic pyrogen from phagocytes of neonates. J Infect Dis. 1981 Oct;144(4):337–343. doi: 10.1093/infdis/144.4.337. [DOI] [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Pilo S., Kaempfer R. Kinetics of induction and molecular size of mRNAs encoding human interleukin-2 and gamma-interferon. Nature. 1982 May 20;297(5863):236–239. doi: 10.1038/297236a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Handzel Z. T., Levin S., Dolphin Z., Schlesinger M., Hahn T., Altman Y., Schechter B., Shneyour A., Trainin N. Immune competence of newborn lymphocytes. Pediatrics. 1980 Mar;65(3):491–496. [PubMed] [Google Scholar]
- Hayward A. R., Kurnick J. Newborn T cell suppression: early appearance, maintenance in culture, and lack of growth factor suppression. J Immunol. 1981 Jan;126(1):50–53. [PubMed] [Google Scholar]
- Hirano T., Fujimoto K., Teranishi T., Nishino N., Onoue K., Maeda S., Shimada K. Phorbol ester increases the level of interleukin 2 mRNA in mitogen-stimulated human lymphocytes. J Immunol. 1984 May;132(5):2165–2167. [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Kronheim S. R., March C. J., Erb S. K., Conlon P. J., Mochizuki D. Y., Hopp T. P. Human interleukin 1. Purification to homogeneity. J Exp Med. 1985 Mar 1;161(3):490–502. doi: 10.1084/jem.161.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. E., Luft B. J., Remington J. S. Effect of murine interferon gamma on murine toxoplasmosis. J Infect Dis. 1984 Dec;150(6):961–962. doi: 10.1093/infdis/150.6.961. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., McGuffin R. W., Neiman P. E., Singer J. W., Thomas E. D. Toxicity and efficacy of human leukocyte interferon for treatment of cytomegalovirus pneumonia after marrow transplantation. J Infect Dis. 1980 May;141(5):555–562. doi: 10.1093/infdis/141.5.555. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Taga K., Sato H., Taniguchi N. Dissociated production of interleukin-2 and immune (gamma) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clin Exp Immunol. 1985 Feb;59(2):505–511. [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. G. The ontogeny of interferon production by human leukocytes. J Pediatr. 1970 Jan;76(1):94–98. doi: 10.1016/s0022-3476(70)80136-6. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. Signal requirements for T lymphocyte activation. I. Replacement of macrophage function with phorbol myristic acetate. J Immunol. 1979 Oct;123(4):1749–1754. [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Lindner S. G., Gootenberg J., Popovic M., Hemmi H., Sarin P. S., Gallo R. C. Lymphokine production by cultured human T cells transformed by human T-cell leukemia-lymphoma virus-I. Science. 1984 Feb 17;223(4637):703–707. doi: 10.1126/science.6320367. [DOI] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Production and properties of immune interferon from spleen cell cultures of Toxoplasma-infected mice. Microbiol Immunol. 1980;24(11):1109–1120. doi: 10.1111/j.1348-0421.1980.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S., Bryson Y. J. Impaired production of gamma-interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol. 1985 Mar;134(3):1493–1497. [PubMed] [Google Scholar]
- Urdal D. L., March C. J., Gillis S., Larsen A., Dower S. K. Purification and chemical characterization of the receptor for interleukin 2 from activated human T lymphocytes and from a human T-cell lymphoma cell line. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6481–6485. doi: 10.1073/pnas.81.20.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L., Arenzana-Seisdedos F., Rothhut B., Huerta J. M., Russo-Marie F., Fiers W. Defective IFN-gamma production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985 Jan;134(1):172–176. [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985 Jan;134(1):167–171. [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E. Cellular defenses against Toxoplasma gondii in newborns. J Clin Invest. 1984 Jun;73(6):1606–1616. doi: 10.1172/JCI111367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979 Dec;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Effects of monocytes from human neonates on lymphocyte transformation. Clin Exp Immunol. 1979 Jun;36(3):511–520. [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Westall J. Activation of neonatal and adult human macrophages by alpha, beta, and gamma interferons. Infect Immun. 1985 Aug;49(2):351–356. doi: 10.1128/iai.49.2.351-356.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Weiss A., Imboden J., Kamin-Lewis R., Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985 Mar;134(3):1599–1603. [PubMed] [Google Scholar]
- Yokoi T., Miyawaki T., Yachie A., Ohzeki S., Taniguchi N. Discrepancy in expression ability of Tac antigen and Ia determinants defined by monoclonal antibodies on activated or cultured cord blood T lymphocytes. J Immunol. 1982 Oct;129(4):1441–1445. [PubMed] [Google Scholar]



