Abstract
The aim of this study was to investigate the effect of potentiation on stimulation-induced muscle function during and after an intense bout of self-paced dynamic exercise. Ten active subjects performed a time trial involving repetitive concentric extension-flexion of the right knee using a Biodex dynamometer. Electrical stimulation before and after a 5 s maximal isometric voluntary contraction was performed before the start of the time trial and immediately (< 5 s) after each 20% of the time trial as well as 1, 2, 4 and 8 min after time trial termination. Potentiation was observed before the time trial and as early as 1–2 min after the time trial, but no potentiation was detected during or immediately after the time trial for neither single or paired stimuli. At termination of the time trial, “potentiated” peak torque was significantly more reduced than “unpotentiated” peak torque for single stimulus (−65 ± 10% and −42 ± 18%, respectively) and paired stimuli at 100 Hz (−51 ± 10% and −33 ± 15%, respectively). Faster recovery for “potentiated” compared to “unpotentiated” peak torque indicate that potentiate peak torque measurements or delay the post-exercise measurements more than a few seconds, will underestimate peripheral fatigue. In conclusion, the potentiation after maximal contraction disappears during intense exercise. Whether the muscle is already potentiated during intense contraction or fatiguing mechanisms inhibits potentiation remains to be clarified.
Keywords: Electrical stimulation, twitch, peripheral fatigue, potentiation, time trial
Introduction
Post activation potentiation, subsequently termed potentiation, is defined as the increased response to electrical stimulation (ES) after an initial voluntary contraction (Vandervoort et al., 1983; McComas et al., 1983; Sale, 2002; Moore and Stull, 1984). Potentiation is explained by increased Ca2+ sensitivity mainly due to increased myosin regulatory light chain (RLC) phosphorylation (Macintosh et al., 2012), which increases muscle contractility. The magnitude of potentiation is striking because it can instantly increase evoked knee extensor force output by 40–60% (Green and Jones, 1989; Paasuke et al., 2007; Requena et al., 2008; Froyd et al., 2013a). This effect is important in studies quantifying peripheral fatigue (Place et al., 2010) in terms of changes in neuromuscular function (NMF) in response to ES during or after exercise. Accordingly, we define peripheral fatigue as a reduction in the peak evoked torque (PT) in response to ES on relaxed muscles.
When the muscles’ ability to produce force is re-measured in the post-exercise period, the change from the pre-exercise value will be the result of two opposing forces – potentiation which will increase -and repeated contractions which will induce fatigue and decrease PT in response to ES. It is not known to what extent either contributes to the change in PT measured after the exercise bout (Rassier and Macintosh, 2000). Several studies investigating the development of peripheral fatigue during prolonged (Millet et al., 2002; Gauche et al., 2006) or short duration (Skof and Strojnik, 2006; Gondin et al., 2006; Skurvydas et al., 2008) exercise however have performed the pre-exercise measurement in muscles that were not exposed to potentiation. Other studies have attempted to differentiate between potentiation and peripheral fatigue during exercise, but without measuring the maximal possible potentiation before exercise began (Fowles and Green, 2003; Morana and Perrey, 2009).
Previous studies have shown that potentiation disappears rapidly after a single maximal voluntary contraction (MVC) of the knee extensors (Green and Jones, 1989; Hamada et al., 2000; Froyd et al., 2013a). However, as far as we know, no study has yet reported the effects of potentiation kinetics during and after (i.e. during the recovery process) a bout of intense dynamic exercise. Also, the contractile response occurring as a result of different types of ES during exercise is unknown. Thus, this study was designed to compare different types of ES on NMF in muscles before and immediately after a 5 s isometric MVC before, during and after a high intensity time trial (TT) involving one-legged dynamic exercise in order to quantify the opposing effect of pre-exercise potentiation and the peripheral fatigue that develops during exercise. We hypothesized that the measurement of the extent of both potentiation and peripheral fatigue would differ with the type of ES used. In addition, we evaluated skeletal muscle contractile characteristics in order to explore the possible mechanical explanations for these phenomena.
Material and Methods
Participants
10 physically active (training > 4 times a week) subjects (2 women and 8 men) volunteered to participate in the study. Their average (± SD) age, body mass and height were 23.1 ± 6.0 years, 74.7 ± 9.0 kg, and 180.2 ± 9.0 cm, respectively. The subjects gave their written informed consent to participate in the study, after which they completed a health screening questionnaire. Subjects were given a full explanation of the details and rationale of the study and were informed that they were free to withdraw from the study at any time. The possibility that ES might cause discomfort was fully explained as was the nature of the risks involved. The study was approved by the Ethics Committee of the University of Cape Town, and the experiments were performed according to the latest (2008) revision of the Declaration of Helsinki.
Experimental design
The applied methods are described in detail in Froyd et al. (2013b). A short description of the methods is as follows: subjects made two preliminary visits to the laboratory during the 3 weeks immediately before the experiments commenced. During both visits, the subjects were familiarized by (I) performing the TT with knee extension-flexion of the right leg and (II) measuring NMF using a Biodex System 3 isokinetic dynamometer (Biodex Medical System, Shirley, NY). Pilot and familiarization testing found that the expected TT time was 4–8 min. NMF was assessed before, during and after the TT.
Protocol
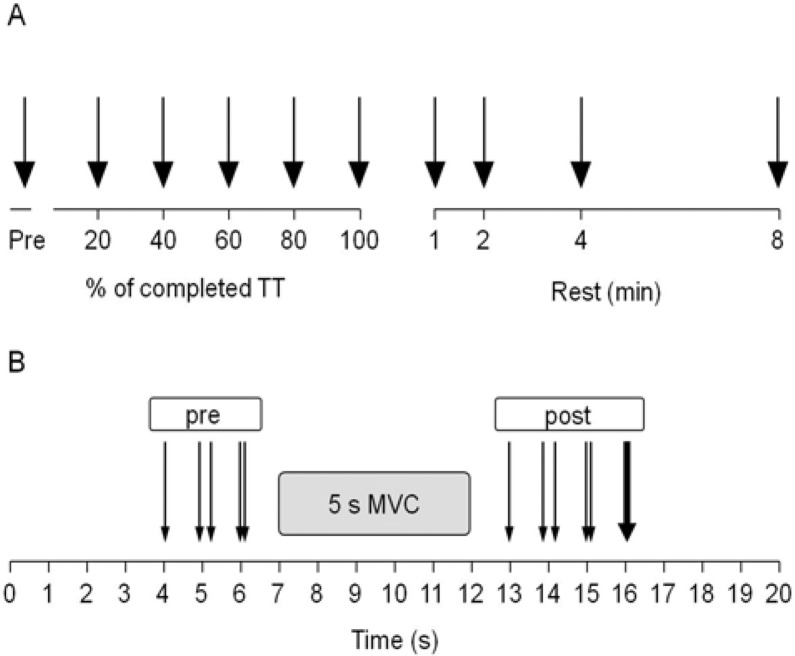
Subjects performed the TT on the isokinetic dynamometer by performing repetitive concentric extension-flexion movements of the right leg as previously described (Froyd et al., 2013b). Termination (100%) of the TT occurred when 30,000 J of work had been completed on the dynamometer (Figure 1A). NMF evaluation (Figure 1B) was performed before the start of the TT and immediately (mean for all subjects 2.8 ± 0.7 s) after each 20% (6000 J) of the TT had been completed, as well as 1, 2, 4 and 8 min after termination of the TT.
Figure 1.
Overview of the stimulation timing and methods during the time trial (TT) (A) and the neuromuscular function (NMF) evaluations (B).
A; during the TT, black arrows indicate electrical stimulation (ES) and maximal voluntary contraction (MVC) measurements.
B; one thin arrow indicates single stimulus (SS); paired thin arrows indicate paired stimuli at 10 Hz (PS10) and paired stimuli at 100 Hz (PS100).
A single solid arrow indicates tetanic stimulation.
ES was performed before the MVC and after the MVC. ES responses pre-MVC are referred as “pre” (e.g. PTpre for peak evoked torque) in the manuscript to distinguish from post-MVC referred as “post” (e.g. PTpost).
The figure is modified from Figure 1 in Froyd et al. (2013b).
During rest after the TT, subjects continued to sit in the dynamometer and were inactive except when performing the NMF evaluations. Pre-TT NMF was assessed twice separated by 1 min after an isometric warm up. Five maximal extension-flexion concentric isokinetic contractions were performed 3–4 min before the start of the TT.
Electrical stimulation
After detection of the femoral nerve with a ball probe cathode, ES was applied percutaneously via a 10 mm diameter self-adhesive cathode electrode (Kendall Meditrace, USA) pressed manually onto the skin over the femoral nerve. The anode, a 130×80 mm self-adhesive electrode (Cefar-Compex Scandinavia AB, Sweden) was applied to the gluteal fold.
A constant current stimulator (DS7AH, Digitimer, Hertfordshire, UK) delivered a square-wave stimulus of 200 μs duration at a maximum of 400 V. The optimal stimulation intensity for a single stimulus was determined by increasing the current gradually from 10 mA until a plateau in torque (50–115 mA) was reached. The current was then increased by a further 30% (70–150 mA) to ensure supramaximal stimulation. The intensity was kept constant for the same subject for all types of ES. The subjects were instructed to relax fully when the ES was applied.
Evaluation of neuromuscular function in response to electrical stimulation
As shown in Figure 1B, NMF evaluation consisted of the following sequence of stimuli before the MVC: single stimulus (SSpre); paired stimuli at 10 Hz (PS10pre); paired stimuli at 100 Hz (PS100pre); after a 5 s isometric MVC, SSpost, PS10post, PS100post and a tetanic stimulation at 100 Hz for 350–600 ms were evoked. During MVC, the subjects were instructed to reach maximum torque in 1 s and then to maintain this level for 4 s whilst they received strong verbal encouragement. ES responses pre-MVC are indicated with “pre” (i.e. PTpre) in the manuscript, to distinguish from post-MVC measurements indicated with “post” (i.e. PTpost). The interval between the stimulation techniques and between stimuli and MVCs was 1 s. PowerLab (ADInstruments Pty Ltd, Bella Vista NSW, Australia) was used to trigger the ES. The hip angle was positioned at 110 deg during all experiments, and the knee angle was positioned at 90 deg when isometric NMF was assessed.
Time trial
The goal of the TT was to complete 30,000 J of work in the fastest time possible. The subjects performed repetitive isokinetic concentric knee extension-flexions at 300 deg·s−1. In addition to knee extension, knee flexion was also used to mimic an activity such as cycling. The range of motion was from knee flexion at approximately 120 deg to full knee extension (anatomical zero) at 0 deg. As a result of familiarization testing, the subjects knew the approximate duration of the TT and were therefore able to pace themselves appropriately. After 18, 38, 58, 78 and 98% of the TT, the subjects were asked to report their rating of perceived exertion (RPEs; Borg, 1974). The TT was briefly stopped for NMF evaluation after 20, 40, 60 and 80% of the TT. These measurements were performed again immediately (< 5 s), and 1, 2, 4, and 8 min after completion of the TT (Figure 1A).
Torque measurements
Right leg torque was measured in the isokinetic dynamometer during both the TT and the NMF evaluation. During the TT, the dynamic maximum and minimum torque for every cycle of both extension and flexion was measured. Torque response from tetanic stimulation and TT concentric torque can be found in Froyd et al. (2013b).
Experimental variables and data analysis
In addition to PT, the SS torque response was analysed to determine contraction time (CT), which is the time from start of the contraction to PT, the rate of torque development (RTD) which is PT/CT, half relaxation time (½RT) which is the time from PT to 50% decline in PT, and the rate of relaxation (RR) calculated as PT/½RT. The PS10/PS100 torque ratio was calculated as an index of low/high-frequency fatigue (Verges et al., 2009). The comparative difference in PTpre and PTpost (Figure 3) was calculated by dividing the absolute PT value for post-MVC by the pre-MVC value.
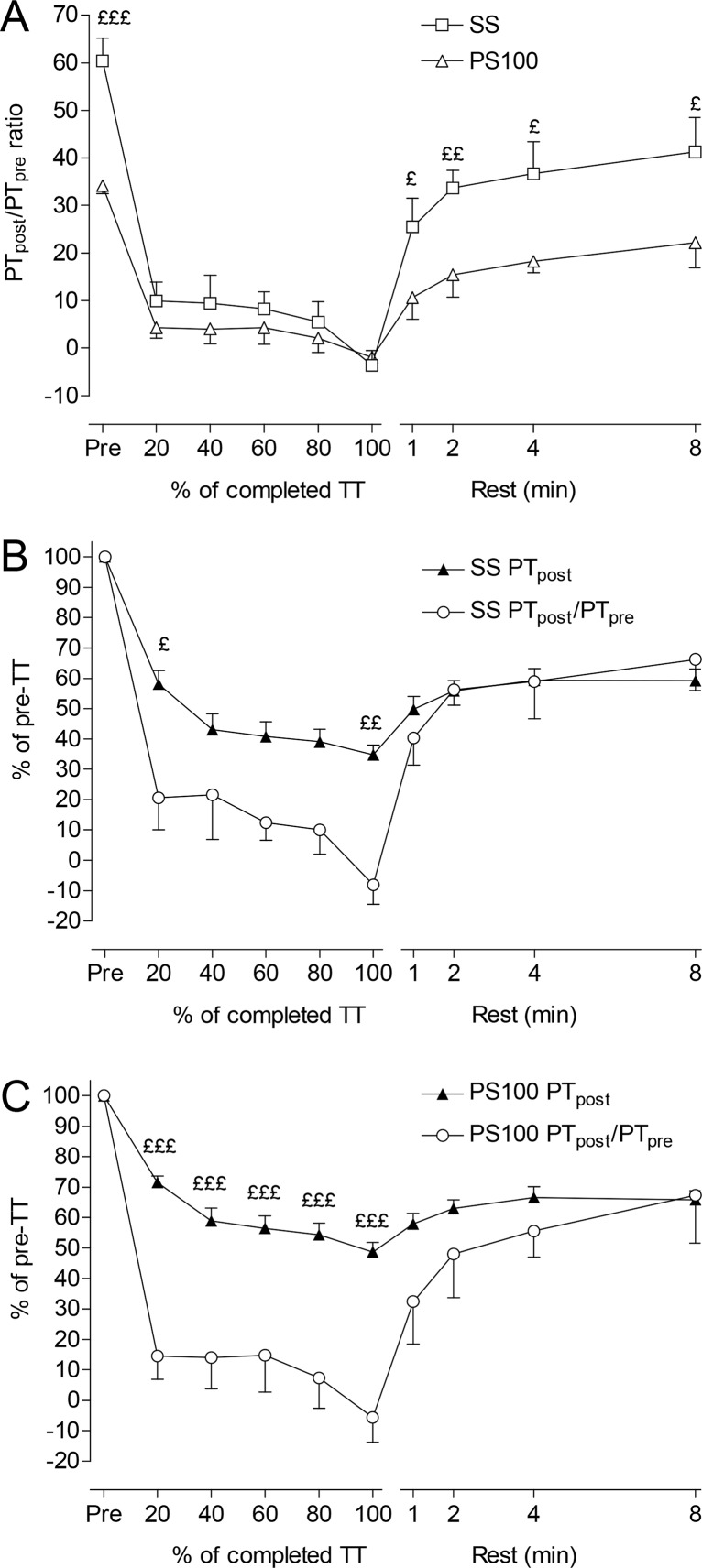
Figure 3.
Peak evoked torque post-MVC (PTpost) divided by pre-MVC (PTpre) for single stimulus (SS) and for paired stimuli at 100 Hz (PS100) shown as PTpost/PTpre ratio (A), PTpost/PTpre ratio and PTpost as percentage of pre-TT for SS (B), and PTpost/PTpre ratio and PTpost as percentage of pre-TT for PS100 (C), before, during and for 8 min after a knee extension-flexion TT in a dynamometer. Values are expressed as means ± SEM, n = 10, significant differences between SS and PS100 in panel A, and between PTpost/PTpre ratio and PTpost in panel B and C: £ p <0.05; ££ p <0.01; £££ p <0.001.
Statistical analyses
The data were analysed with Statistica 10.0 (Stat Soft. Inc., Tulsa, OK). Descriptive statistics are presented as means ± SD unless otherwise stated. Repeated-measures ANOVA was used to detect differences over time. A Tukey post hoc test was applied to determine the specific differences. Differences between pre-MVC and post-MVC responses to ES during different time segments (pre, during TT and resting condition) for the same variable were analysed using the General Linear Model. However, differences between pre-MVC and post-MVC values at one occasion were analysed using one way ANOVA. Correlation coefficients between PT and RTD, and between PT and RR were performed, and r2 values were presented. The statistical significance was defined at p <0.05.
Results
Total exercise duration was 347 ± 98 s for the 30,000 J. Average peak torque per extension–flexion cycle during the TT was 54 ± 13% of the maximal concentric torque measured pre-TT. Isometric MVC torque decreased significantly (p <0.001) by −48 ± 11% of the baseline value at the end of the TT and recovered significantly (p <0.05) after 2 min of rest. Those results and peak evoked torque responses without comparisons to “unpotentiated” measurements have been described in the study by Froyd et al. (2013b).
Changes in absolute and relative peak evoked torque
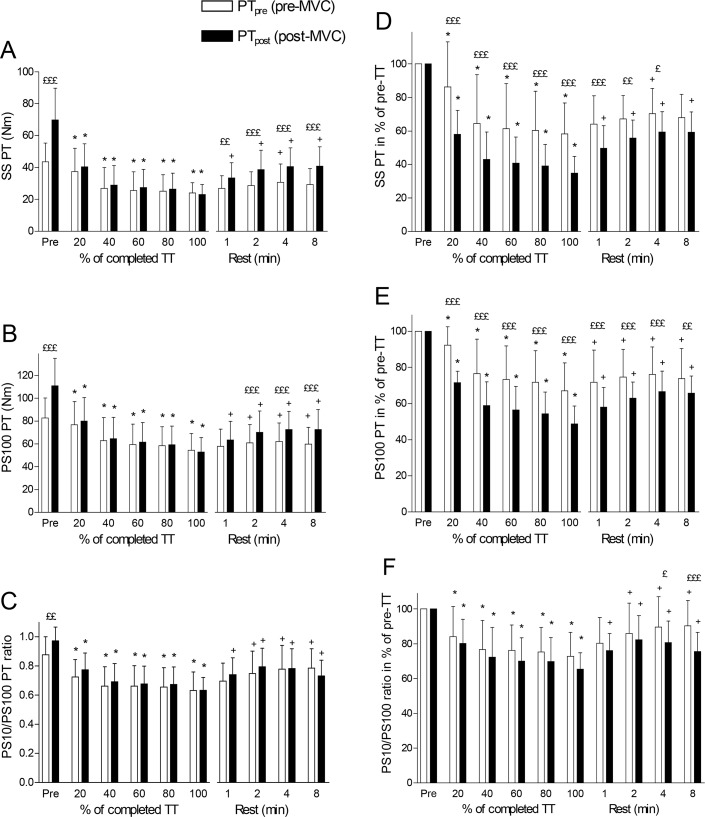
Prior to exercise (TT), PT increased significantly (p <0.001) by 60 ± 15%, 34 ± 5% and 51 ± 21%, respectively, when measured after MVC with SS (Figure 2A), PS100 (Figure 2B), or PS10 (data not shown), while the PS10/PS100 ratio increased significantly (p <0.01) by 12 ± 13% (Figure 2C). PS100 increased significantly less than SS (p <0.001) and PS10 (p <0.05). When measured with SS or PS100, both PTpre and PTpost decreased after 20% of the TT. There were no differences between PTpre and PTpost at any time during the TT for SS or PS100 (Figure 2A and 2B). After 1 min of recovery, the PTpost response to both SS and PS100 increased significantly. The recovery of PTpre was slower and increased significantly only 2–4 min after exercise termination in response to SS or PS100. Figure 2C shows that both PS10/PS100pre and PS10/PS100post ratios fell equally during exercise and increased similarly during the rest period, but did not reach pre-exercise levels 8 min after termination of exercise.
Figure 2.
Absolute and relative peak evoked torque (PT) response to electrical stimulation for pre-MVC (PTpre) and post-MVC (PTpost) single stimulus (SS) (A and D), paired stimuli at 100 Hz (PS100) (B and E), and PT ratio for paired stimuli at 10 Hz (PS10)/PS100 (C and F) before, during and for 8 min after a knee extension-flexion TT in a dynamometer. Values are expressed as means ± SD, n = 10. Significant differences between PTpre and PTpost: ££ p <0.01; £££ p <0.001; significant difference from pre-values for the same variable during the TT: * p <0.05; significant difference from 100% (end of TT) for the same variable during rest: + p <0.05.
The extent to which SS or PS100 identified changes in PTpre and PTpost as percentage of the corresponding pre-TT values is shown in Figure 2D–F. Correcting the pre-TT values to 100% adjusts for the pre-TT effect of potentiation. Figure 2D shows a significant difference in the extent to which PTpre and PTpost falls (reduced by −42 ± 18% and −65 ± 10%, respectively at termination of the TT) for SS; Figure 2E shows the same information in response to PS100 (reduced by −33 ± 15% and −51 ± 10%, respectively at termination of the TT). In both cases PTpost was significantly (p <0.001) more reduced than PTpre (SS: −40 ± 7%, PS100: −27 ± 7%, p <0.001). The PS10/PS100 ratio shows the same general pattern as for SS and PS100. However, the responses of PS10/PS100pre and PS10/PS100post ratios were not significantly different.
PTpost and PTpre between SS and PS100 during and after exercise
The PTpost/PTpre ratio was significantly (p <0.05) less for PS100 compared to SS prior to and after the TT (Figure 3A). However, during exercise none of the stimulation methods detected any effect of MVC on PT. From similar PTpost and PTpre values at TT termination for both SS and PS100, after 1 min recovery the PTpost/PTpre ratio was significantly greater (p <0.05) for SS (25 ± 19%) compared to PS100 (11 ± 14%) (Figure 3A). Figures 3B and 3C show the changes in PTpost as an indicator of fatigue, and PTpost/PTpre as an index of potentiation for SS and PS100, respectively. Changes in PT correlated with changes in PTpost/PTpre for SS (r2 = 0.81, p <0.001) and PS100 (r2 = 0.69, p <0.01).
Relationship between changes in PT, RTD and RR during and after exercise
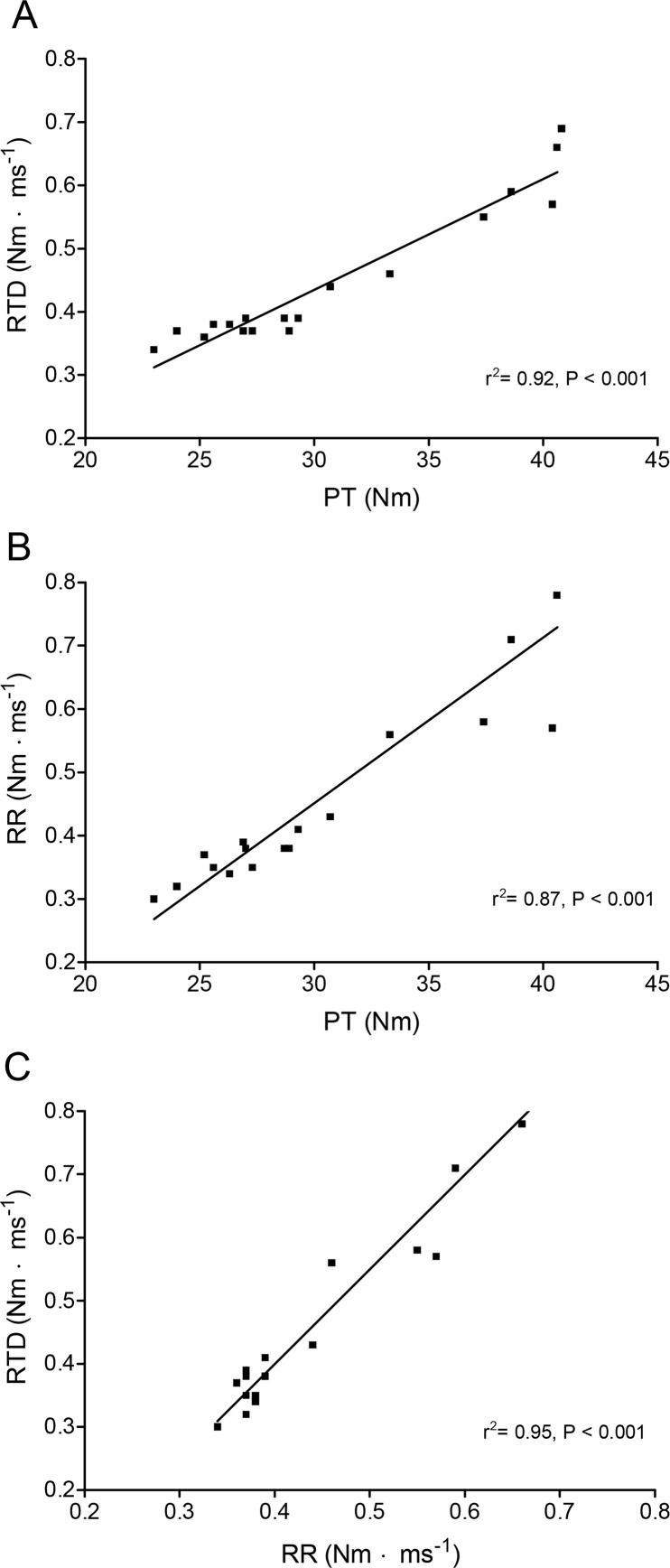
RTD and RR increased significantly (p <0.001) after a MVC before the TT (data not shown). Thereafter RTDpre and RTDpost in addition to RRpost and RRpre changed in a relatively similar way as PT (Figure 2A) during exercise and recovery. The relationship between PT and RTD, PT and RR, and RTD and RR during and after exercise for both pre-MVC and post-MVC values (r2 = 0.92, p <0.001, r2 = 0.87, p <0.001, and r2 = 0.95, p <0.001, respectively) are shown in Figures 4A, 4B and 4C. The strong correlation between these variable existed not only for the entire experiment, but also separately during or after the exercise or for pre-MVC or post MVC measurements separately (data not shown).
Figure 4.
Correlation between peak evoked torque (PT) and rate of torque development (RTD) (A), PT and rate of relaxation (RR) (B), and RTD and RR (C) for all pre-MVC and post-MVC measurements during and after the time trial. Values are expressed as means for all subjects, n = 10.
Discussion
The first finding of the present study was that a MVC potentiated the response to SS more than to PS100 prior to and as early as 1 min after the TT (Figure 3A). As far as we know, this is the first study to make this comparison during and after intense dynamic exercise. We concluded that SS could both be more potentiated and affected by fatigue than PS100 (Figures 2A–C and 3A). Since potentiation is an issue that must be controlled during fatigue measurements, PS100 may therefore be beneficial compared to SS for measurements of fatigue in exercise. The difference between SS and PS100 can be related to the shape of the force/frequency relationship (Edwards et al., 1977). Reduced PT during exercise may be related to decreased intracellular [Ca2+] and reduced myofibrillar Ca2+-sensitivity (Allen et al., 2008).
In Froyd et al. (2013b) it is shown that the extent to which PT falls during exercise is a function of the stimulation method, is greater with SS than with PS100 and is least with tetanic stimulation. Those results were collected immediately after a MVC to maximize potentiation of PT. In the present paper, we show that these differences between stimulation methods also exist when comparing PT before and after a MVC. The potentiation by a MVC disappeared as fatigue occurred and the time course of fatigue and potentiation was correlated. The correlation between potentiation and fatigue suggests that these two opposing phenomena are related. It is suggested that inorganic phosphate (Pi), which increases in the muscle during exercise, may both decrease intracellular [Ca2+] and myofibrillar Ca2+-sensitivity, which has been demonstrated in single animal muscle fibres (Millar and Homsher, 1990; Allen et al., 2008; Allen, 2013).
It is speculated that the same biological mechanisms that cause potentiation (changes in Ca2+ sensitivity) may also explain the development and recovery of peripheral fatigue since intracellular [Ca2+] is first decreased during exercise and then reversed during recovery. Several factors may influence Ca2+ sensitivity and intracellular [Ca2+], but RLC phosphorylation is thought to be the most important in affecting Ca2+ sensitivity (Macintosh, 2003). Although intracellular acidosis is often related to fatigue, it does not seem to reduce force production during exercise (Allen et al., 2008).
If correct, this interpretation supports the existence of a “peripheral governor” in skeletal muscle (Macintosh and Shahi, 2011; Westerblad et al., 2010) and is compatible with a key role for Ca2+ handling (Jones et al., 2009) and perhaps RLC phosphorylation (Sweeney et al., 1993; Macintosh et al., 2012) in these processes. This “peripheral governor” would act as a regulatory process to avoid ATP disturbance or metabolic catastrophe by decreasing Ca2+ release and thereby attenuating use of ATP by both Ca2+ ATPase and myosin ATPase (Macintosh and Shahi, 2011).
The next finding was that changes in PT were significantly correlated with RTD and RR during and after exercise (Figures 4A–B) as also found by others during exercise (Dolmage and Cafarelli, 1991). We (Froyd et al., 2013a) and others (Paasuke et al., 2007; Requena et al., 2008) have shown that the increase in PT caused by potentiation is associated with increases in both RTD and RR. In addition, a significant correlation between RTD and RR (Figure 4C) indicates that mechanisms related to torque development are also related to torque relaxation. This suggests that processes related to Ca2+-release from sarcoplasmic reticulum change similarly with Ca2+ pumping back to sarcoplasmic reticulum.
The last finding was that the calculation quantifying the extent of the fall in PT during exercise was influenced by the presence or absence of a MVC to potentiate PT before exercise. Figures 2D–E show that whereas PTpre fell to approximately 55% and 70% of pre-TT values at the termination of the TT, PTpost decreased much more since PTpost during exercise was probably already potentiated by the TT exercise. A MVC during a TT may cause both potentiation and fatigue, but we interpreted the results (Figures 2A–B) assuming that RLC phosphorylation was already high prior to the MVC during the TT. However, the potentiating effect of the MVC was re-established already within 1–2 min after the termination of exercise (Figures 2A–B). The difference seen between PTpost and PTpre before exercise confirms previous findings that a potentiated twitch is a better and more sensitive measure of peripheral fatigue than unpotentiated twitch (Alway et al., 1987; Kufel et al., 2002; Place et al., 2007). However, the originality of the present study compared to other studies is that PTpost and PTpre are measured not only prior to or after exercise, but also during and in the recovery phase after exercise. Always et al. (1987) found no reductions in PTpost or PTpre after exercise despite a 35% reduction in a MVC, and hence PTpost/PTpre was unchanged after exercise. This contrasts with the present study showing decreased PTpost/PTpre during exercise and recovery of PTpost/PTpre after exercise (Figure 3A).
The practical relevance of these findings is that studies that do not measure pre-exercise PT in potentiated muscles or that delay the post-exercise measurements of PT by more than a few seconds, will underestimate the extent to which peripheral fatigue develops. Such studies may conclude that central fatigue plays a larger role in impairing performance during exercise (Millet et al., 2002; Saldanha et al., 2008) than will studies that include these experimental methods (Matkowski et al., 2011). Although we did not measure central fatigue in this study, the levels of peripheral fatigue that we have documented are amongst the highest reported in the literature. This may suggest that the extent to which peripheral fatigue develops during exercise has been generally underestimated by previous studies.
The consequence for studies of NMF is that measures of PT that are not potentiated by a MVC will not detect the true nature of the recovery process that occurs in potentiated muscles after exercise. This confirms the conclusion from Kufel et al. (2002) and may explain the slow recovery for PT in other studies which used “unpotentiated” post exercise measurements (Behm and St-Pierre, 1997; Place et al., 2004; Lattier et al., 2004). However, in contrast to Kufel et al. (2002) who measured the effect of potentiation 15 minute after exercise, we compared the effect of potentiation immediately after the termination of exercise.
In conclusion, this study shows that potentiation influences the peak evoked torque response differently depending on the applied electrical stimulation method. Peak evoked torque was more potentiated by a MVC for SS than PS100 before and after, but not during exercise since no extra potentiation was observed during the intense dynamic exercise. More fatigue was measured during and after exercise when ES was applied after compared to before a 5 s MVC. In addition, the present findings suggest that potentiated measurements recovered faster than unpotentiated measurements after exercise and that SS can be more potentiated and fatigued than PS100. Studies that do not measure pre- or post-exercise peak evoked torque in muscles potentiated by a MVC or that delay the post-exercise measurements of peak evoked torque more than a few seconds, will underestimate the extent to which peripheral fatigue develops, and the underestimation will be more pronounced for SS compared to PS100. This may explain the slow recovery for peak evoked torque in other studies which used “unpotentiated” single stimulus after exercise.
Acknowledgments
This research was funded by the University of Cape Town Staff Research Fund, the Medical Research Council of South Africa, Discovery Health and the National Research Foundation.
References
- Allen DG. Dynamic changes in the contractile apparatus during exercise. Acta Physiol (Oxf) 2013;208:220–221. doi: 10.1111/apha.12109. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Alway SE, Hughson RL, Green HJ, Patla AE, Frank JS. Twitch potentiation after fatiguing exercise in man. Eur. J. Appl. Physiol Occup. Physiol. 1987;56:461–466. doi: 10.1007/BF00417776. [DOI] [PubMed] [Google Scholar]
- Behm DG, St-Pierre DM. Effects of fatigue duration and muscle type on voluntary and evoked contractile properties. J. Appl. Physiol. 1997;82:1654–1661. doi: 10.1152/jappl.1997.82.5.1654. [DOI] [PubMed] [Google Scholar]
- Dolmage T, Cafarelli E. Rate of fatigue during repeated submaximal contractions of human quadriceps muscle. Can. J. Physiol Pharmacol. 1991;69:1410–1415. doi: 10.1139/y91-211. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J. Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles JR, Green HJ. Coexistence of potentiation and low-frequency fatigue during voluntary exercise in human skeletal muscle. Can. J. Physiol Pharmacol. 2003;81:1092–1100. doi: 10.1139/y03-114. [DOI] [PubMed] [Google Scholar]
- Froyd C, Beltrami FG, Jensen J, Noakes TD. Potentiation increases peak twitch torque by enhancing rates of torque development and relaxation. J. Hum. Kinet. 2013a;38:83–94. doi: 10.2478/hukin-2013-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froyd C, Millet GY, Noakes TD. The development of peripheral fatigue and short-term recovery during self-paced high-intensity exercise. J. Physiol. 2013b;591:1339–1346. doi: 10.1113/jphysiol.2012.245316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauche E, Lepers R, Rabita G, Leveque JM, Bishop D, Brisswalter J, Hausswirth C. Vitamin and mineral supplementation and neuromuscular recovery after a running race. Med. Sci. Sports Exerc. 2006;38:2110–2117. doi: 10.1249/01.mss.0000235351.01438.5a. [DOI] [PubMed] [Google Scholar]
- Gondin J, Guette M, Jubeau M, Ballay Y, Martin A. Central and peripheral contributions to fatigue after electrostimulation training. Med. Sci. Sports Exerc. 2006;38:1147–1156. doi: 10.1249/01.mss.0000222843.04510.ca. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones SR. Does post-tetanic potentiation compensate for low frequency fatigue? Clin. Physiol. 1989;9:499–514. doi: 10.1111/j.1475-097x.1989.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Hamada T, Sale DG, MacDougall JD, Tarnopolsky MA. Postactivation potentiation, fiber type, and twitch contraction time in human knee extensor muscles. J. Appl. Physiol. 2000;88:2131–2137. doi: 10.1152/jappl.2000.88.6.2131. [DOI] [PubMed] [Google Scholar]
- Jones DA, Turner DL, McIntyre DB, Newham DJ. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. 2009;587:4329–4338. doi: 10.1113/jphysiol.2009.175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Lattier G, Millet GY, Martin A, Martin V. Fatigue and recovery after high-intensity exercise. Part II: Recovery interventions. Int. J. Sports Med. 2004;25:509–515. doi: 10.1055/s-2004-820946. [DOI] [PubMed] [Google Scholar]
- Macintosh BR. Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci. 2003;18:222–225. doi: 10.1152/nips.01456.2003. [DOI] [PubMed] [Google Scholar]
- Macintosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue - regulation of excitation-contraction coupling to avoid metabolic catastrophe. J. Cell Sci. 2012;125:2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- Macintosh BR, Shahi MR. A peripheral governor regulates muscle contraction. Appl. Physiol Nutr. Metab. 2011;36:1–11. doi: 10.1139/H10-073. [DOI] [PubMed] [Google Scholar]
- Matkowski B, Place N, Martin A, Lepers R. Neuromuscular fatigue differs following unilateral vs bilateral sustained submaximal contractions. Scand. J. Med. Sci. Sports. 2011;1:268–276. doi: 10.1111/j.1600-0838.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Quinlan J, Vandervoort AA. Twitch potentiation after voluntary contraction. The Journal of Physiology. 1983:33P–34P. doi: 10.1016/0014-4886(83)90163-2. [DOI] [PubMed] [Google Scholar]
- Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J. Biol. Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Millet GY, Lepers R, Maffiuletti NA, Babault N, Martin V, Lattier G. Alterations of neuromuscular function after an ultramarathon. J. Appl. Physiol. 2002;92:486–492. doi: 10.1152/japplphysiol.00122.2001. [DOI] [PubMed] [Google Scholar]
- Moore RL, Stull JT. Myosin light chain phosphorylation in fast and slow skeletal muscles in situ. Am. J. Physiol. 1984;247:C462–C471. doi: 10.1152/ajpcell.1984.247.5.C462. [DOI] [PubMed] [Google Scholar]
- Morana C, Perrey S. Time course of postactivation potentiation during intermittent submaximal fatiguing contractions in endurance- and power-trained athletes. J. Strength. Cond. Res. 2009;23:1456–1464. doi: 10.1519/JSC.0b013e3181a518f1. [DOI] [PubMed] [Google Scholar]
- Paasuke M, Saapar L, Ereline J, Gapeyeva H, Requena B, Oopik V. Postactivation potentiation of knee extensor muscles in power- and endurance-trained, and untrained women. Eur. J. Appl. Physiol. 2007;101:577–585. doi: 10.1007/s00421-007-0532-6. [DOI] [PubMed] [Google Scholar]
- Place N, Lepers R, Deley G, Millet GY. Time course of neuromuscular alterations during a prolonged running exercise. Med. Sci. Sports Exerc. 2004;36:1347–1356. doi: 10.1249/01.mss.0000135786.22996.77. [DOI] [PubMed] [Google Scholar]
- Place N, Maffiuletti NA, Martin A, Lepers R. Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve. 2007;35:486–495. doi: 10.1002/mus.20714. [DOI] [PubMed] [Google Scholar]
- Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: from observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur. J. Appl. Physiol. 2010;110:1–15. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- Rassier DE, Macintosh BR. Coexistence of potentiation and fatigue in skeletal muscle. Braz. J Med Biol Res. 2000;33:499–508. doi: 10.1590/s0100-879x2000000500003. [DOI] [PubMed] [Google Scholar]
- Requena B, Gapeyeva H, Garcia I, Ereline J, Paasuke M. Twitch potentiation after voluntary versus electrically induced isometric contractions in human knee extensor muscles. Eur. J. Appl. Physiol. 2008;104:463–472. doi: 10.1007/s00421-008-0793-8. [DOI] [PubMed] [Google Scholar]
- Saldanha A, Nordlund Ekblom MM, Thorstensson A. Central fatigue affects plantar flexor strength after prolonged running. Scand J Med Sci Sports. 2008;18:383–388. doi: 10.1111/j.1600-0838.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- Sale DG. Postactivation potentiation: role in human performance. Exerc. Sport Sci. Rev. 2002;30:138–143. doi: 10.1097/00003677-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Skof B, Strojnik V. Neuro-muscular fatigue and recovery dynamics following anaerobic interval workload. Int. J Sports Med. 2006;27:220–225. doi: 10.1055/s-2005-865632. [DOI] [PubMed] [Google Scholar]
- Skurvydas A, Masiulis N, Stanislovaitis A, Kamandulis S. Bi-modal recovery of quadriceps femoris muscle function after sustained maximum voluntary contraction at different muscle length. Medicina (Kaunas.) 2008;44:782–790. [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am. J. Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Quinlan J, McComas AJ. Twitch potentiation after voluntary contraction. Exp.Neurol. 1983;81:141–152. doi: 10.1016/0014-4886(83)90163-2. [DOI] [PubMed] [Google Scholar]
- Verges S, Maffiuletti NA, Kerherve H, Decorte N, Wuyam B, Millet GY. Comparison of electrical and magnetic stimulations to assess quadriceps muscle function. J. Appl. Physiol. 2009;106:701–710. doi: 10.1152/japplphysiol.01051.2007. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010;316:3093–3099. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]