Abstract
The prenatal brain develops under the influence of an ever-changing hormonal milieu that includes endogenous fetal gonadal and adrenal hormones, placental and maternal hormones, and exogenous substances with hormonal activity that can cross the placental barrier. This review discusses the influences of endogenous fetal and maternal hormones on normal brain development and potential consequences of pathophysiological hormonal perturbations to the developing brain, with particular reference to autism. We also consider the effects of hormonal pharmaceuticals used for assisted reproduction, the maintenance of pregnancy, the prevention of congenital adrenal hypertrophy, and hormonal contraceptives continued into an unanticipated pregnancy, among others. These treatments, although in some instances life-saving, may have unintended consequences on the developing fetuses. Additional concern is raised by fetal exposures to endocrine-disrupting chemicals encountered universally by pregnant women from food/water containers, contaminated food, household chemicals, and other sources. What are the potential outcomes of prenatal steroid perturbations on neurodevelopmental and behavioral disorders, including autism-spectrum disorders? Our purposes here are 1) to summarize some consequences of steroid exposures during pregnancy for the development of brain and behavior in the offspring; 2) to summarize what is known about the relationships between exposures and behavior, including autism spectrum disorders; 3) to discuss the molecular underpinnings of such effects, especially molecular epigenetic mechanisms of prenatal steroid manipulations, a field that may explain effects of direct exposures, and even transgenerational effects; and 4) for all of these, to add cautionary notes about their interpretation in the name of scientific rigor.
Introduction
-
Evidence That Prenatal Hormones Affect the Developing Brain
Hormones and the fetal brain: animal studies
Hormones and the fetal brain: human evidence
Exogenous hormones, environmental EDCs, and the developing brain
-
Evidence That Prenatal Hormones Affect Social Behaviors
Prenatal hormones, EDCs, and adult social behavior: animal studies
Prenatal hormones, EDCs, and adult social behavior: human evidence
-
Hormones, Sexually Dimorphic Behaviors, and Autism and Autism-Spectrum Disorders
Abnormal prenatal hormonal milieu
Correlations among physiological hormones, neurocognitive function, and autism
Cautions in interpretation of data
-
Molecular Mechanisms for Effects of Hormones on Brain and Behavior
Histone N-terminal modifications
DNA methylation
Noncoding RNAs
Epigenetics, social behavior, and autism
-
Evidence for Transgenerational Epigenetic Phenomena That Influence Brain and Behavior
Epigenetics and behavior
Transgenerational epigenetic actions of EDCs
Transgenerational EDCs and behavior
Caveats re the interpretation of data, to date
Outlook Regarding Autism and Neurodevelopment
I. Introduction
Millions of women from the last century were administered steroid hormones during their pregnancies for a variety of reasons, including the maintenance of pregnancy or the prevention of congenital adrenal hyperplasia (CAH). Exposures of fetuses to the pill in misuse or failure of birth control likely added hundreds of thousands of cases in which a developing human was exposed prenatally to progestins and/or estrogens (1, 2). Beyond these pharmaceuticals are thousands of environmental chemicals collectively termed endocrine-disrupting chemicals (EDCs) that can reach the fetus from the mother. Here, we consider the consequences for the development of brain and behavior in the offspring as well as possible multigenerational effects. We also discuss the evidence for underlying cellular, molecular, and epigenetic changes to the nervous system that may play a role in these processes. Our overaching question is whether prenatal steroid perturbations contribute to, or even cause, neurodevelopmental disorders, including autism and autism-spectrum disorders (ASDs).
II. Evidence That Prenatal Hormones Affect the Developing Brain
A. Hormones and the fetal brain: animal studies
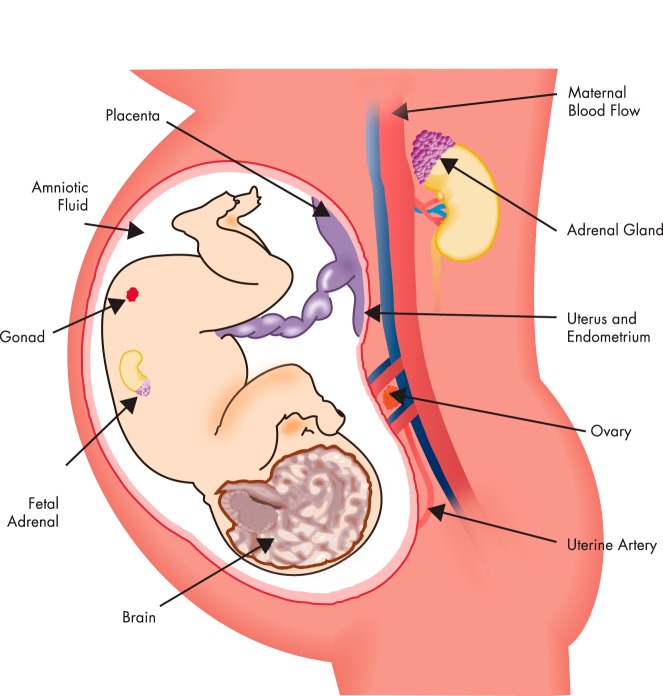
As the mammalian fetal brain develops, it encounters waves of steroid hormones arising from the mother, the placenta, and the fetus's own developing gonads and adrenals (Figure 1). The release of each of these endogenous hormones is highly regulated, with very precise timing and levels needed to accomplish normal development. Any mistiming or inappropriate levels, whether too high or too low, can result in a dysfunction or even death. In some cases, even extremely low levels of a hormone may have a dramatic effect in a fetus that may never have encountered that hormone before and whose hormone receptors are activated for the first time. This concept sets the stage for the exquisite sensitivity of the fetus, and the importance of critical periods, in neurobiological development. As this developmental programming by gonadal hormones continues, morphological sex differences begin to manifest, due to differences in the steroid milieu between males and females. In fact, biosynthetic capacities of the fetal testes and ovaries are quite distinct, with the testis more active than the ovary. This results in dramatic sex differences due to higher levels of testicular hormones, particularly testosterone. Moreover, sexually dimorphic effects of steroids in the nervous system are contributed to by enzymes such as p450 aromatase, encoded by the CYP19 gene, which converts testosterone to its major metabolite, 17β-estradiol in target tissues. The expression of these enzymes in the fetal brain differs by developmental stage and brain region, with sex differences persisting into adulthood in both animal models and humans (3, 4). Other steroidogenic or metabolizing enzymes with different actions or distributions between the sexes may further contribute to sex differences in actions and metabolism of steroids. Last, rodents such as mice and rats produce α-fetoprotein (5), which binds estradiol from the fetus's ovary, or maternal estrogens, effectively preventing any free estradiol from entering the brain.
Figure 1.
Depiction of the many modes of exposure of a developing fetus to natural hormones or exogenous hormonally active pharmaceuticals and EDCs.
Thus, as a whole, the male rodent brain is exposed to higher levels of testosterone and estradiol, both of which are needed for masculinization. Development proceeds in a sex-typical manner, a process that commences during early gestation, is apparent at birth, and is manifested for the rest of life due to effects of hormones on brain structure and function (Figure 1) (6). Although most research on hormones and brain sexual differentiation has focused on gonadal testosterone and estradiol, other steroid hormones such as progesterone and adrenal hormones play roles as well, although far less is known about these hormones' actions on brain sexual differentiation. These developmental changes in the brain are termed organizational and involve permanent structural and/or molecular changes that sculpt the ultimate neuron number and neurochemistry in a differential manner between the sexes (7–9). For example, prenatal gonadal hormones affect neuronal cell numbers, localization, migration, and targeting. The molecular mechanisms for these hormonal effects are still emerging and include effects of hormones on apoptosis (programmed cell death) of neurons, hormone actions on gene and protein expression in neurons, glial changes, and synaptic plasticity (Box 1).
Box 1. Molecular mechanisms by which hormones change the structure and function of the developing nervous system.
Apoptosis: In the developing nervous system, many more neurons are born than are used. Selective pruning occurs by apoptosis (programmed cell death).
Gene and protein expression in neurons: As neurons develop they undergo waves of gene and protein expression that ultimately determine the final individual neural phenotype.
Glial effects: Many glial cell types proliferate and differentiate in the developing nervous system in a brain-region-specific manner.
Synaptic plasticity: The establishment and maintenance of synapses, their properties, and other features are needed for neurotransmission. Hormones affect synaptic plasticity throughout life, including prenatal, perinatal, and pubertal periods.
Epigenetic and other molecular mechanisms: DNA methylation, histone modifications, expression of transcription factors, and noncoding RNAs (eg, microRNAs) undergo developmental change in the nervous system. Emerging evidence suggests that hormones play integral roles in all of these processes in the developing brain.
The first brain region discovered to be sexually dimorphic is the eponymous sexually dimorphic nucleus of the preoptic area (SDN-POA). This subregion of the hypothalamus is severalfold larger in male than female rats (10). Later studies showed that prenatal hormones organized this sex difference and that it could be reversed by fetal hormonal manipulations (11). Research has gone on to show that the mechanisms underlying this sex difference in SDN-POA size involved developmental effects of steroids on apoptosis and, furthermore, that neural phenotypes and neurochemistry are sexually dimorphic (12). Since the time of this original work, other brain nuclei are now accepted as being sexually dimorphic, both within the hypothalamus and in nonhypothalamic regions. Even humans have structural sex differences in brain regional sizes, numbers of cells, and the phenotype of cellular populations (13). Many sex differences in both normal neurobiological functions (eg, cognitive differences) and neuropathological, neurochemical, and/or neurobehavioral disorders originate in the embryo.
At least some of the organizing effects of fetal gonadal steroids on brain structure, phenotype, and behavior, best studied in rodents but also evident in monkeys, sheep, birds, and other species (14–17), are considered to be irreversible beyond a critical period of development, generally ending sometime during postnatal life. However, the most obvious functional consequences of sex differences in the brain are generally not manifested until after puberty, when increasing levels of gonadal steroid hormones activate those pathways that were organized earlier in life. This concept of organizational/activational effects of steroid hormones has been extended to recognize that puberty not only activates those neural circuits that were organized early in life but also that the pubertal brain undergoes further organizational changes (18).
For decades, it has been obvious from work with experimental animals that prenatal or neonatal testosterone or estradiol affects brain development and the manifestation of sexually differentiated behaviors. The earliest evidence for the hormonal basis of sexual dimorphisms in behavior was published more than 50 years ago (17) and centered on the effects of testosterone injected into experimental animals on the masculinization of behavior (19, 20). Twenty years after this initial discovery, McEwen and his colleagues (21) discovered that female rats treated neonatally with estradiol, and tested for sexual behaviors after they matured, showed a similar loss of female-typical behaviors as females treated neonatally with testosterone, meaning that estradiol and testosterone had similar actions. The chemical basis for this surprising similarity is due to aromatization, by which testosterone is metabolized into estradiol (22). As discussed above (Section II.A), the p450 aromatase enzyme encoded by the CYP19A gene is abundantly and dimorphically expressed in the nervous system and contributes to these activational effects. As we will discuss in Section III, nonreproductive behaviors, including many social behaviors, are also sexually dimorphic and influenced by the perinatal hormonal environment.
B. Hormones and the fetal brain: human evidence
All fetuses, including humans, are exposed to endogenous steroid hormones through the mother, the placenta, and from a fetus's own gonads and adrenals (Figure 1). Although still controversial, there is growing evidence for the existence of structural sex differences in the human brain, mainly based on histological measures conducted on postmortem adult human brains, and more recently from structural and functional magnetic resonance imaging (MRI). Even more controversial are whether and how early life hormonal exposures play into these structural/functional neurobiological differences, and we emphasize the caveat that little is known about the underlying cause and effect of prenatal hormones on a structural or functional outcome. Because of the complexity of this field and experimental differences, we refer readers to original and review articles on the subject (23–25) and provide a few representative examples. A cross-sectional study was performed using structural MRI on 121 healthy boys and girls aged 4 to 18 years (24). Results showed a slightly larger (9%) cerebral volume in boys than girls, even adjusted for height and weight. It should be noted that another group found a similar cerebral volume difference by sex in healthy adult men and women (26). The developmental study by Giedd et al (24) also found that boys have a greater globus pallidum volume and girls a greater caudate volume. When developmental age was factored in, specific changes in regional volumes were found that varied by sex, including in the lateral ventricles, globus pallidus, caudate, amygdala, and hippocampus. However, in that study, and probably not surprisingly, variability within a sex/age was often higher than developmental changes or sex differences (24). Other analyses using computed tomography scans and MRI found small but significant sex differences in relative gray and white matter volume and cerebrospinal fluid and demonstrated greater global cerebral blood flow in women than men (23). As reviewed by Swaab (27), postmortem histological analyses of the hypothalamus, a region that is highly sensitive to hormones and abundant in steroid hormone receptors in humans as well as all vertebrates, showed sex differences in size, shape, and/or volume of several subregions. Specifically, the suprachiasmatic nucleus and the intermediate nucleus of the anterior hypothalamus (speculated to be analogous to the rat's SDN-POA) differed between the sexes (27). More recent imaging studies using high-angular-resolution diffusion imaging tractography to study brain connectivity networks revealed that cerebral hemispheric connectivity is greater in women than men (28).
Information, however, on the causal influence of hormones on the development of these structural neurobiological sex differences is almost entirely lacking in humans. Inferences may be drawn from the natural differences in exposures to hormones in twins, in which hormones from one fetus affect another. Although there are very few studies in this realm, it has been proposed that prenatal testicular testosterone (29), and possibly its aromatization to estradiol, affects the development of functional cerebral lateralization, which is sexually dimorphic. Cohen-Bendahan et al (25) compared cerebral lateralization in opposite-sex and same-sex twin girls, based on the hypothesis that an opposite-sex twin girl would have higher prenatal testosterone exposure from her male twin than that found in same-sex twin girls. Using a dichotic listening task that reflects the dominance of the contralateral hemisphere, which is sexually dimorphic, this group showed that 1) boys had a higher laterality index score than girls and that 2) opposite-sex twin girls had greater laterality (more similar to that of boys) than did same-sex twin girls (25). However, whether the robustness of this task is adequate to enable wholesale conclusions to be drawn about hormones and sex differences in the developing brain is unclear. Thus, it is necessary to rely on animal studies, inferring from the high species conservation of the molecular processes by which hormones signal (eg, receptor binding and cellular signaling).
C. Exogenous hormones, environmental EDCs, and the developing brain
Both natural and xenobiotic hormones can reach the fetus through placental transfer (Figure 1). Although this concept is widely accepted in humans today, it was only first acknowledged in the early 1960s when severe developmental malformations of infants were linked to maternal use of thalidomide, usually for insomnia, anxiety, or nausea during the first trimester of gestation (30). In the case of hormonal pharmaceuticals, one of the most compelling and tragic examples of misuse is that of diethylstilbestrol (DES), a potent estrogen prescribed to an estimated 5 to 10 million women in the United States alone (31), with the intention of averting miscarriage. Ironically, DES was later found to be ineffective for this purpose, yet millions of infants were exposed. Although as infants, these exposed individuals appeared normal from external observation, with the exception of some cases of cryptorchidism in boys (reviewed in Ref. 32), a host of reproductive tract deformities, disorders, and rare cancers were diagnosed later in life, especially, although not exclusively, in females.
Although DES is no longer prescribed to pregnant women, a large number of hormonal pharmaceuticals are currently given to women undergoing assisted reproductive technologies or those with high-risk pregnancies. In addition, of the tens of millions of women who use oral contraceptives, close to half a million have unintended pregnancies (2). Some of these women, unaware that they are pregnant, continue oral contraceptive use well into the first trimester. These contraceptives typically include an estrogen, usually ethinyl estradiol, together with a progestin, or a progestin alone. Fortunately, doses of pharmaceutical estrogens and progestins have decreased significantly since the pill was first introduced half a century ago. Nevertheless, there is still no doubt that many fetuses continue to be unwittingly exposed to exogenous steroid hormones today, fortunately, mostly at lower concentrations than in the case of DES.
1. EDCs and their contemporary definition
The definition of EDCs has evolved over the past 2 decades. As reviewed in Zoeller et al (33), researchers in the field initially followed guidelines from the U.S. Environmental Protection Agency (EPA), stating that an endocrine disruptor is “an exogenous agent that interferes with the production, release, transport, metabolism, binding, action or elimination of natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes” (34). In 2012, the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) simplified this definition to “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations” (35), expanding upon the concept of mixtures as well as that of effects on subsequent generations (progeny), a concept we will expand upon greatly in Section V. An integral part of the EPA's report, and a theme echoed in the WHO-UNEP document, was the requirement of a proven adverse health effect. However, this issue is controversial, especially when considered in the context of development. Even in cases of low-dose prenatal exposures or perturbations, organizational effects of hormones may be affected, but these may not be manifest for months, years, or decades. For example, sexual development does not begin until the onset of puberty, a decade into life in humans, but prenatal hormone perturbations may have latent effects on this process such as advanced or delayed puberty or failure to attain reproductive competence. This type of adverse outcome cannot be measured at or even close to the time of exposure. Another example of how an adverse effect may not be noticed is the example of a reproductive biologist investigating whether an EDC has an effect on reproduction. She may conclude that the EDC has no effect if there is no discernible change in the system she is studying; however, the thyroid system might be impaired but overlooked.
Based on this background, our preferred definition of an endocrine disruptor was provided by a position statement of the Endocrine Society as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action” (33). This definition is deliberately intended to be broad and includes information from in vitro and in vivo laboratory work in combination with human evidence. The mechanisms by which EDCs act can range from gene expression to physiologic mechanisms, including steroid hormone receptor-mediated pathways. Other modes of steroid action may also be affected, such as steroidogenic enzymes, neurotransmitters, and other physiological processes (36). Of all the steroid hormone receptors, the estrogen receptor has the greatest capacity to bind compounds that are structurally quite different from endogenous estrogens (37). Thus, many EDCs are estrogenic or antiestrogenic. They may act through nuclear (estrogen receptor α or β) and nonnuclear (eg, G protein-coupled estrogen receptor or other membrane) estrogen receptors, which affect cellular signaling, gene expression, and protein synthesis or degradation (38). Other EDCs are antiandrogenic, with a few that may be androgenic, and some act on thyroid hormone systems. Far less is known about EDC effects on progesterone or glucocorticoid signaling processes, but emerging evidence suggests that these are targets of certain EDCs (39, 40).
2. Evidence for EDC exposures in humans
Environmental exposure to EDCs occurs in every human due to contact with plastics, pesticides, and industrial chemicals that have leached into the soil and water and gotten into the food chain; through personal care products, iv tubing, and food and water containers; and by many other routes. Exposure levels in most cases, with the exception of occupational exposure, contamination, or chemical explosions, tend to be low, but as a species, humans are exposed to a mixture of compounds virtually throughout our lives (41). Although the literature for EDC body burden is too extensive to be reviewed here, we summarize some representative findings for several EDC classes and, in some cases, provide statistical associations with adverse outcomes (Table 1). There are several points that are important to discuss in terms of human exposure. First, although there may seem to be controversy in the matter, there is absolutely no doubt that exposure in humans is ubiquitous and global (42–47). Measures of chemicals in blood, serum, and urine of thousands of individuals have consistently demonstrated dozens of chemicals detectable in these media in nearly all U.S. residents, including children (42). For example, bisphenol A (BPA) was found in 93% of urine samples of Americans over the age of 6 years (48) and in 97% of gestational and child urine samples collected in a study in Cincinnati, OH (49). Other studies performed before and since then have confirmed and extended that work around the world (50–64). The recent WHO-UNEP report has reviewed the literature for evidence of detection of these and other compounds in humans, including (but not limited to) persistent organic pollutants, plasticizers, polycyclic aromatic chemicals, pesticides, pharmaceuticals, and chemicals in personal care products (35). Second, most of the literature on human body burden analyses measures just a single or small number of chemicals or their metabolites, although those studies that measure a broader range of chemicals do indeed detect the presence of multiple compounds (58, 65). Thus, studies measuring single or few compounds are likely representing the tip of the iceberg with regard to the full spectrum of what is in each of our bodies. Third, each human likely has a unique composition, mixture, and levels of EDCs based on differences in where an individual resided/resides, diet, lifestyle, and many other factors. Fourth, body burdens of EDCs have been reported in parts of the world in which there is relatively little industrialization (reviewed in Ref. 57) due to combinations of air- and water-borne transport of EDCs, processed (canned and frozen) foods whose containers contain EDCs, and dietary dependence on migratory species that may feed in a contaminated part of the world but spend part of their lives elsewhere and serve as a food source. Fifth, we must consider the consequences of these environmental exposures during critical developmental life stages. However, in humans, when considering all of these points, the ability to directly link prenatal exposures to an endocrine outcome is rarely possible with notable exceptions in the case of DES or known acute contamination, discussed in the next paragraph. Thus, resolving what residual controversy remains relies on a combination of epidemiological studies, consideration of clinical data from humans with known exposures to environmental toxicants or pharmaceuticals with hormonal activity, and inference from experimental animal studies. Such an approach served as the basis for our selection of the literature to review, and conclusions to draw, in the current article.
Table 1.
Representative Examples of EDC Exposures, Body Burdens, and Neurobehavioral and Disease Associations in Humansa
| EDCs | Population Characteristics | Sample Size | Medium in Which EDCs Were Measured | Concentrations Detected | Neurobehavioral Outcome | Other Outcome | Observations | Reference |
|---|---|---|---|---|---|---|---|---|
| Atrazine | Prospective population-based cohort in Brittany (France): PELAGIE cohort from 2002–2006. | 579 urine samples were selected from women whose babies had major congenital or male genital anomalies, including FGR and small head circumference | Urine (single sample) collected before wk 19 of pregnancy | Median [atrazine] = 0.12 μg/L; other metabolites of atrazine and other herbicides were also measured | Adverse pregnancy outcomes (FGR, SHC) were associated with atrazine and its metabolites; for FGR, OR = 1.5 (95% CI = 1.0–2.2), and for SHC, OR = 1.7 (95% CI = 1.0–2.7); congenital abnormalities were not associated with exposure | Study was conducted after atrazine was banned so data interpretation should be conducted in this context | (51) | |
| BPA | Mothers during prenancy; children at 5 y of age | n = 292 mother-child pairs | Urine | Geometric mean [maternal BPA] (unadjusted or specific gravity adjusted) = 1.1, 1.3 μg/L; [child BPA] (unadjusted or specific gravity-adjusted) = 2.5, 3.7 μg/L | In boys, higher maternal BPA was associated with increased internalizing problems at age 7. Specifically, internalizing scores were increased by 1.8 points (95% CI = 0.3–3.3) by mothers' reports and 2.5 points (95% CI = 0.7–4.4) by teachers' reports; no association with inattention or hyperactivity was found in boys or girls or any behaviors in girls | (52) | ||
| BPA | Mothers and their 3-y-old children in the Cincinnati, OH, area | n = 244 mother-child pairs | Urine | Median maternal urine [BPA] = 2.0 μg/L), child urine [BPA] = 4.1 μg/L | Higher gestational [BPA] was associated with increased anxious and depressive behaviors on BASC-2 and poorer emotional control and inhibition on BRIEF-P; effects were more pronounced in girls | BPA was detected in 97% gestational and childhood urine samples | (49) | |
| BPA, phthalate metabolites, PCBs, organochlorine pesticides, brominated flame retardants, perfluoroalkyl substances | Mothers and their children (4–5 y old) in the HOME Study, Cincinnati, Ohio | n = 175 mother-child pairs | Urine, blood from pregnant women | Table 1 of this paper presents concentrations of urinary or serum EDCs in this study | Autistic behaviors were assessed by the mother completing the SRS; PBDE-28 was associated with more autistic behaviors [β = 4.1, 95% CI = 0.8–7.3]; fewer autistic behaviors were observed between women with detectable vs nondetectable PCB-178 [β = −3.0, 95% CI = −6.3, 0.2], β-hexachlorocyclohexane [β = −3.3; 95% CI = −6.1, −0.5], or PBDE-85 [β = −3.2, 95% CI = −5.9, −0.5] | Most EDCs were associated with negligible changes in SRS scores; only PBDE-28 had a positive association with autistic behaviors; other EDCs had a negative association; women in this study had from 21–52 (median 44) detectable EDCs in serum or urine during pregnancy | (134) | |
| BPA | Children from 2009–2010 NHANES | n = 710 children | Urine | Mean [BPA] = 1.889 (male) and 1.934 (female) ng/mL | The albumin-to-creatinine ratio was associated with urinary BPA (highest ratio in the highest quartile) consistent with modest albuminuria | (54) | ||
| BPA, tOP | Participants 6 y and older in the 2003–2004 NHANES | n = 2517 | Urine | Geometric mean [BPA] in the entire population was 2.6 μg/L, range 0.4–149 μg/L; tOP range = 0.2–20.6 μg/L | BPA was found in 93% and tOP in 57% of people; concentrations varied by sex, race/ethnicity, age, and household income | (48) | ||
| BPA | Participants from 6–19 y of age in the 2003–2008 NHANES | n = 2838 | Urine | Mean urinary [BPA] = 2.8 ng/mL | Children in the lowest quartile of BPA had lower prevalence of obesity (10%, 95% CI = 7.6%–12.5%); the second, third, and fourth quartiles had OR (95% CIs) of 2.22 (1.53–3.23), 2.09 (1.48–2.95), and 2.53 (1.72–3.74), respectively | (53) | ||
| BPA and metabolites | Second-trimester pregnancies (13–24 wk) in northern and central California | n = 85 participants from 2010–2012 | Cord blood | Geometric mean [BPA] = 0.16, [BPA-glucuronide] = 0.14, [BPA-sulfate] = 0.32 ng/mL | BPA and/or its metabolites BPA-glucuronide and BPA-sulfate was found in 100% of cord blood samples assayed by LC-MS/MS | (56) | ||
| PBDEs | Second-trimester pregnancies in California from 2008–2009 | n = 25 (pilot study) | Serum | 37 PBDE analytes were measured, along with TSH, free T4, and total T4 | Median [PBDEs] and [OH-PBDEs] are the highest reported in pregnant women; some association with transthyretin and TSH was found, but not with T4 | (47) | ||
| PCBs | Children recruited as newborns from 1980–1981, born to women known to have eaten Lake Michigan fish contaminated with PCBs | n = 212 children | Maternal serum, milk, and umbilical cord serum | Mean [PCBs] = 3 ng/mL (cord serum), 6 ng/mL (maternal serum), 841 ng/g fat (milk), and 1–2 ng/mL in the childrens' serum at 4 and 11 y | Prenatal PCB exposure was associated with lower verbal IQ, memory, and attention | DDT, lead, and mercury were also measured | (128) | |
| PCBs | Mother-infant pairs | n = 207 mother-infant pairs; 105 were breastfed, 102 bottle-fed | Maternal plasma in last month of pregnancy; levels in breast milk and duration of breastfeeding | Mean [PCBs] = 2.2 ng/g plasma; 419 ng/g milk fat | Higher prenatal PCBs (from maternal plasma) were associated with lower psychomotor scores on the Bayley test at 3 mo; postnatal PCB-dioxins were associated with poorer performance at 7 mo | Breastfed babies performed better than bottle-fed babies | (132) | |
| PCBs, dioxin | Dutch children at 42 mo of age, born from women recruited in 1990–1992 | n = 395 (cognitive abilities), a subgroup (n = 193) used for verbal comprehension | Plasma from 42-mo-old children and from maternal plasma | Median [PCB] in plasma (μg/L) was 2.04 (maternal), 0.38 (cord), and 0.35 (children at 42 mo); breast milk levels were about 200× higher | Maternal PCBs were associated with lower scores on aspects of the Kaufman Assessment Battery for Children, with the highest-exposed group having the poorest performance; lactational and the children's own PCB levels were not related to cognitive performance | Breast milk PCBs in the Netherlands at the time of the study were among the highest in the world | (129) | |
| PCBs, dioxin | Dutch Duisburg cohort recruited 2000–2002; children tested at 6–8 y of age. | n = 232 pregnant women, of which 121 fulfilled eligibility criteria | Maternal blood collected wk 28–43 or gestation; maternal milk during the first 3 wk after parturition | Mean concentrations of PCDD/F and PCBs in maternal blood were 14.5 and 6.9 pg/g blood lipid, respectively; mean levels in milk were 11.6 and 9.0 pg/g milk lipid, respectively | Sexually dimorphic behaviors in children were assessed by the PSAI scores for 3 categories (preferred toys, preferred activities, behavioral characteristics); significant interactions of sex and exposure were found, especially femininity scores, which showed positive correlations in boys with PCBs in milk and negative associations in girls | Exposure levels were relatively low compared with other studies | (279) | |
| PCBs | Milk samples from women aged 19–46 from 5 regions of Canada in 1992 | n = 497 milk samples | Breast milk | See Table 1 of this paper; medians ranged from 0–308 ng/g lipid; Most abundant PCBs were PCBs 138, 153, 118, 137, 170, 187, 49, 156, 180, 74 | 12 PCB congeners were detectable in 90% of samples, another 12 in 40%–90%; many of the major congeners were intercorrelated | (58) | ||
| PCBs | Infants at 6 and 12 mo from the Oswego Newborn and Infant Development Project, focusing on maternal consumption of Lake Ontario fish contaminated with chemicals | n = 230 (6 mo) and 219 (12 mo) | Cord blood, breast milk 1–3 mo after delivery | Cord blood median [total PCBs] = 0.52 ng/g wet; breast milk median [total PCBs] = 153 ng/g lipid | Small but significant associations between total PCBs and declining FTII performance at 6 and 12 mo of age; no association between breast milk total PCBs and FTII performance at either age | (130) | ||
| PCBs | Field study of children born in 1978–1985 whose mothers were poisoned by PCBs from 1978–1979 in the Yucheng incident in Taiwan (PCB-contaminated cooking oil); comparisons were made with nonpoisoned age-matched subjects | n = 118 | PCBs were not measured here, but participants' mothers had known exposure to PCB-contaminated cooking oil | Not applicable | Performance on the MDI and PDI (0.5–2 y of age) was slightly but significantly poorer in the Yucheng children; Stanford-Binet IQs were also significantly lower, especially at 4 and 5 y of age; in older children, performance continued to be poorer in the Yucheng group, although there appeared to be some catch-up as children developed | (131) | ||
| PCBs, pesticides, PBDE | Couples in Michigan or Texas in the Longitudinal Investigation of Fertility and the Environment (LIFE) study, discontinuing contraception to become pregnant | n = 501 couples | Serum from men and women collected at initial (prepregnancy) interview | See Table 2 of this paper for geometric means (GM, ng/g serum) of compounds associated with reduced fecundability OR; for those compounds with strongest association with reduced fecundability, PCB 167 in women: GM = 0.003 (achieved pregnancy) and 0.004 (no pregnancy); PCB 138 in men: 0.038 (achieved pregnancy) and 0.044 (no pregnancy) | A reduction in fecundability was associated with increases in PCBs 118, 167, and 209 and perfluorooctane sulfonamide in females (strongest for PCB 167 [OR = 0.79; 95% CI = 0.64–0.97)]; and p,p′-DDE and PCBs 138, 156, 157, 167, 170, 172, and 209 in males [strongest for PCB 138 [OR = 0.71; 95% CI = 0.52–0.98)] | (59) | ||
| Persistent organochlorines (PCBs, DDE, dieldrin, hexachlorobenzene) | CPP, which enrolled pregnant women in 1959–1965, and their children until 7 y of age | n = 1915 children (for BMI) and corresponding serum from mothers during pregnancy | Third-trimester blood from pregnant women | See Table 2 of this paper for median concentrations across selected percentiles (μg/L); for dieldrin, the 25th percentile was 0.60 μg/L and the 95th was 1.81 μg/L; levels in this CPP study were higher than those detected in NHANES | The only association found was for dieldrin; compared with the lowest quintile, the obesity OR was 3.6 (95% CI = 1.3–10.5) and 2.3 (95% CI = 0.8–7.1) for the fourth and highest quintile, respectively | 8.6% of children were considered overweight and 3.5% were obese; chemicals were found in nearly 100% of samples | (60) | |
| PFCs | Girls and mothers in the Avon Longitudinal Study of Parents and Children | n = 448 girls from singleton births | Serum | Median [PFOS] = 19.6 ng/mL; median [PFOA] = 3.7 ng/mL | Girls born to mothers whose PFCs were in the highest tertile weighed 140 g less than those in the lowest tertile (95% CI = −238-−42) at birth but weighed 580 g more at 20 mo (95% CI = 301–858) | 100% of samples had PFCs | (61) | |
| Phthalates | Mother-infant pairs studied from 2006–2009 in 3 cities (Seoul, Cheonan, Ulsan) in Korea | n = 460 mother-infant pairs | Urine (1 sample collected during third trimester of pregnancy) | Median [MEHHP] = 10.1 μg/L, [MEOHP] = 7.9 μg/L, [MBP] = 16.6 μg/L | There were inverse associations between prenatal MEHHP, MEOHP, and MBP in a third-trimester urine sample and MDI and PDI in male infants at 6 mo; no associations were found in females | (62) | ||
| Phthalates | Women from the Study for Future Families pregnancy cohort study at Los Angeles, CA; Minneapolis, MN; and Columbia, MS; from 1999–2002 | n = 85 boys 2–36 mo of age | Urine collected during pregnancy | Mean (median) concentrations of the 4 phthalates associated with shorter AGI are shown in Table 5, and range from: MBP 13.1 (11.5) to 38.7 (24.5); MBzP 10.6 (6.6) to 25.8 (16.1); MEP 124 (47.1) to 1076 (225); and MiBP 2.3 (1.5) to 7.7 (4.8) ng/mL; ranges are reported for quartiles with the longest AGI (lowest levels) to the shortest AGI (highest levels) | 4 phthalate metabolites (MEP, MBP, MBzP, MiBP) were inversely related to AGI (anogenital distance divided by weight); compared with lowest quartile, shorter AGI was found for MBP (OR = 10.2; 95% CI = 2.5–42.2) | (63) | ||
| Phthalates (DEHP) | Participants from 12–19 y of age in the 2003–2008 NHANES study | n = 766 | Urine | Low-molecular-weight phthalate metabolites were 0.508 and 0.729 μM in boys and girls, respectively; high-molecular-weight phthalates were 0.313 and 0.380 μM (boys and girls); DEHP was 0.224 and 0.273 μM in boys and girls | Prevalence of insulin resistance as measured by HOMA-IR was greater in groups with higher DEHP metabolites. | (55) | ||
| Pesticides | Cohort of urban minority women | n = 314 African American and Dominican women in northern Manhattan and the South Bronx | Ambient air monitoring | Ranges: diazinon, 2–6010 ng/m3; chlorpyrifos, 0.7–193 ng/m3; propoxur, 3.8–1380 ng/m3 | 85% of women reported that pest control was used in the house during pregnancy; 100% had detectable levels of diazinon, chlorpyrifos, and propoxur | (64) | ||
| Pesticides | CHARGE study; California residents living in proximity (within 1.5 km) to agricultural pesticide use during pregnancy; children were aged 2–5 y | Children (970) were recruited from those with ASD or DD; age-matched referents were recruited from the general population | Chemical body burdens were not measured but estimated based on geographical proximity to use of organophosphate, carbamate, pyrethroid, or organochlorine classes | Most common pesticides were organophosphates, especially chlorpyrifos, then acephate and diazinon; the next-frequent pesticide class was pyrethroids | Children with ASD were 60% more likely to have organophosphates applied near the home during pregnancy, and those with DD were 150% more likely to have carbamate pesticides applied near the home; For ASD, second- and third-trimester exposures had the strongest positive associations | (135) |
Abbreviations: AGI, anogenital index; BASC-2, Behavior Assessment System for Children-2; BMI, body mass index; BRIEF-P, Behavior Rating Inventory of Executive Function-Preschool; CHARGE, Childhood Autism Risks from Genetics and Environment; CCP, U.S. Collaborative Perinatal Project; DD, developmental delay; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DEHP, di-2-ethylhexylphthalate; FGR, fetal growth restriction; FTII (Fagan Test of Infant Intelligence); GluR1, glutamate receptor R1; HOMA-IR, homeostatic model assessment of insulin resistance; LC-MS/MS, liquid chromatography tandem mass spectrometry; MBP, mono-n-butyl phthalate; MBZP, monobenzyl phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthlate; MiBP, monoisobutyl phthalate; NHANES, National Health and Nutrition Examination Survey; NRI, N-methyl-d-aspartate receptor subunit 1; PBDE, polybrominated diphenyl ether; PCDD/F, polychlorinated dibenzo-p-dioxins and dibenzofurans; PFC, perfluorinated compound; PFOA, perfluorooctane; PFOS, perfluorooctane sulfonate; PSAI, preschool activities index; SHC, small head circumference; SRS, Social Responsiveness Scale; tOP, 4-tertiary-octylphenol.
Studies were selected based on their focus on prenatal/early postnatal/childhood exposure, and/or pregnancy outcomes.
Although experts concur that humans are exposed to EDCs and susceptible to their effects (36, 66), it is not possible to demonstrate a causal link because of the particular vulnerability of the fetus/infant and the often many years before a disease or dysfunction is manifested. Thus, causality has been limited to cases of industrial accidents or in the example of DES already described above. For the former, some of the best evidence for human exposure and subsequent disease comes from the Yusho (1968 Japan) and Yucheng (1979 Taiwan) incidents, in which cooking oil contaminated with polychlorinated biphenyls (PCBs) was consumed by thousands of individuals. Exposed adults demonstrated signs of organochlorine poisoning, whereas fetal and infant exposures were linked to poor cognitive development and motor skills (67, 68). A 1976 chemical plant explosion in Seveso, Italy, exposed thousands of people to dioxin, resulting in both acute toxicity as well as more latent effects such as increased risk of a number of cancers and other diseases, including endocrine disorders (69).
3. EDC actions: experimental animal studies and wildlife
Research in the last decade has revealed that developmental exposure to even low-level EDCs, especially in the fetus or infancy, can perturb normal brain maturation and subsequent functional outcomes in experimental laboratory animals, with effects on hypothalamic morphology and neuronal phenotypes. As reviewed previously (70, 71), EDCs including dioxins, BPA, PCBs, and pesticides (methoxychlor), to name a few, cause changes to the developing brain in a sexually dimorphic and region-specific manner. For example, prenatal/perinatal EDCs change the volume of sexually dimorphic hypothalamic regions and affect neural phenotype (eg, expression of proteins or genes for specific receptors, neurotransmitters, and neuropeptides). The SDN-POA is a good example, as is the anteroventral periventricular nucleus, important for the control of ovulation and steroid feedback in females. These regions are altered in their size and volume, including neuron numbers, neurochemistry, and cellular phenotype, after exposures to prenatal EDCs such as PCBs (72–77). A common outcome of such exposures was masculinization or defeminization of the female hypothalamus, and demasculinization or feminization of this region in males (72, 75, 76, 78–85). Beyond the hypothalamus, developmental EDC exposures also altered neural development. In hippocampus, cortex, amygdala, and brainstem, EDCs altered synaptic plasticity, neural development, and expression of genes and proteins in specific cellular populations (86, 87). This developmental programming undoubtedly has implications for the subsequent manifestation of behaviors. This is best studied for reproductive behaviors, because the field of endocrine disruption has its roots in initial observations of reproductive failure as early as the 1962 publication of Rachel Carson's Silent Spring, and much research into reproductive toxicological effects of EDCs has been published. Moreover, the roles of natural hormones in many nonreproductive behaviors implicate EDCs in perturbations of social behaviors, including complex neurodevelopmental disorders in humans, such as ASDs.
As alluded to above, the first realization of the hazards of EDC exposures came from field observations of toxic pesticides and effects on birds and fish. Although the purpose of this article is ultimately to link exposures to pharmaceuticals and EDCs with human neurobehavioral and ASDs, for the sake of completeness we provide a brief update on the state of the science from wild species. As discussed in the WHO-UNEP document (35), there is abundant evidence for the presence of many classes of EDCs in all animals examined, from marine mollusks to insects to all of the vertebrate classes. Among the most abundant are the persistent organic pollutants, not surprising considering their resistance to breakdown in the environment and their very long half-lives. Persistent organic pollutants are usually found in greatest concentrations in species at the top of the food chain, including polar bears, carnivorous reptiles, predatory fish and birds, and humans, due to bioaccumulation. Plastics and plasticizers, polycyclic aromatic chemicals, and pesticides and herbicides are also found in wildlife around the world. Hormonal pharmaceuticals from humans (eg, ethinyl estradiol, progestins, and others) and livestock (eg, trenbolone acetate used in beef cattle) that get into sewage treatment can be detected in water systems and in bodies of marine organisms (88–90). Even BPA, although generally considered a shorter-lived chemical, is detectable in wastewater and sewage effluent (91).
Of relevance to this article, observational studies suggest that there are behavioral effects of environmental chemicals in wildlife. An excellent review on the subject by Zala and Penn (92) summarizes some of the field literature, together with studies on nontraditional animals brought into captivity. Common behavioral outcomes associated with exposures to dichlorodiphenyltrichloroethane (DDT), PCBs, and pesticides in particular included aberrant courtship, mating and nesting behaviors; disturbances in parental behaviors; aggressive and dominance displays; and social behaviors. A growing literature shows associations between EDC exposures and behavioral outcomes in fish, a vertebrate class that is likely to be among the most vulnerable to EDCs due to their literally swimming in contaminated environments (93–95).
4. Bringing together the literature from experimental animals, wildlife, and humans
The exquisite sensitivity of the fetal brain to gonadal hormones underscores the importance of understanding the consequences of exogenous exposure through pharmaceuticals and EDCs. The case of EDCs raises the additional spectre that all humans are exposed throughout their lives to varying amounts and mixtures of these compounds. However, there is still ambiguity as to whether EDCs are actually causing neurobiological changes through developmental exposure in humans. As already mentioned, this can be addressed only by inference from a combination of in vitro work and animal models showing effects of EDCs on hormonal pathways, the evolutionary conservation of hormones and their receptors, knowledge about endogenous hormone actions from both normal physiological and pathophysiological development (a concept we will discuss in Section IV.A), and epidemiological work. Although most humans are probably not exposed to overtly toxic levels of pharmaceuticals and EDCs, there is growing concern about the particular vulnerability of the fetus and that exposures of pregnant women to even very low-dose EDCs may have lifelong consequences on the offspring's health and disease propensity (96). This field is rooted strongly in a discipline called the developmental origins of adult disease (97), posited first for metabolic disorders but extended to many other biological processes, including EDC exposures and adult neurobehavioral deficits that will be discussed below.
III. Evidence That Prenatal Hormones Affect Social Behaviors
Animal models consistently show that both the natural hormone environment as well as xenobiotics encountered during early life can permanently change adult social interactions in a sexually dimorphic manner. These behaviors are well-conserved in that all mammals must engage in sexual and social behaviors to reproduce and survive. However, the specific nature of these behaviors, and extrapolating complex behaviors from animals to neurodevelopmental disorders in humans, is difficult. Without meaning to sound anthropocentric, we believe it is fair to say that the human brain and social system is unique. This is even captured in the language of sex; humans and animals have a sex, but only humans have a gender. Nevertheless, because we wish to build a case that the etiology of complex neurobehavioral phenotypes such as autism may include hormonal influences, we will provide a few examples, again with the cautionary note that we do not believe that animals are autistic in the same way as humans. The fact that animal species have complex social systems and many are highly gregarious and live in colonies or herds, whereas others, although largely solitary, must engage in social interactions to defend territory, locate mates and engage in sexual behaviors, and may need to share resources underscores the point that in much more general terms, behavioral neurobiological research in animals is relevant to humans. Both in the lab and the wild, animals are highly sensitive to hormonal, physiological, and behavioral differences, and tests of sociability, social memory, and sociosexual behaviors underscore the importance of one individual's behavior in influencing the behavioral response of a conspecific, be it aggression, sexual interest, or another. The question then is how the prenatal hormonal environment, which affects development of brain morphology, structure, synaptic plasticity, and neurogenesis, leads to changes in social behaviors.
A. Prenatal hormones, EDCs, and adult social behavior: animal studies
The organizational/activational effects of hormones are most obvious for the neurobiological control of reproductive processes such as reproductive cycles and ovulation in females and reproduction-related behaviors such as mate preference, sex-typical mating behaviors, and proceptive/receptive behaviors. Beyond the reproductive system, however, it is now well accepted that many other types of behaviors are strongly influenced by organizational/activational effects of gonadal and adrenal steroid hormones. Regions of the brain that control learning and memory, anxiety, and affective and social behaviors (eg, hippocampus, amygdala, and hypothalamus, among others) are sexually differentiated and undergo phenotypic changes in response to natural or exogenous hormone manipulations.
We will begin with the developmental programming of cognition, learning, and memory as an example because the role of gonadal steroids in this function has a relatively long history and is well established. Most of this literature is based on models of castration/ovariectomy of adult male/female animals followed by hormone treatment, but relatively little has examined hormonal changes during the neonatal period. Prenatal treatment of rats with estradiol, DHT, or testosterone resulted in sexually dimorphic changes in performance in a water maze test of memory, concomitant with alterations in the sizes/volumes of CA1 and CA3 hippocampal pyramidal cells (98). Neonatal castration of rats, with or without estradiol treatment, altered spatial memory, with estradiol enhancing spatial acquisition behaviors (99). Manipulations of prenatal progestin levels in pregnant rats resulted in significant changes in performance on hippocampal-dependent tasks in the offspring when tested in late adolescence (100). The aforementioned results differed between the sexes, with females much more affected than males.
What about hormones and the neurobiology of social behaviors? Many social behaviors are sexually differentiated, and each species has its own set of social structures, hierarchies, and interactions, making it difficult to draw inferences from one species to another, although there may be some parallels (101). The neural control of social behaviors involves the interactions of multiple brain regions, with numerous neural pathways and neurotransmitters. There is particular interest in the roles of oxytocin and vasopressin (102, 103) and their regulation by steroid hormones (104). For example, the vasopressin neural system is sexually dimorphic in its distribution, and neonatal hormones or castration can affect cell numbers (105, 106). In birds, vasotocin (the avian homolog of mammalian vasopressin) is sexually differentiated early in life and is regulated by estrogens (107). Physiological oxytocin responses are also dimorphic and altered by neonatal androgens (108). In rats, juvenile social play behavior differs between the sexes and is altered by perinatal manipulations of androgens and estrogens (reviewed in Ref. 109). These examples indicate that first, there are natural sex differences in the structures of many brain regions, and second, that there are behavioral sex differences in a wide variety of behaviors; and third, that early life hormones have organizational effects on neural circuits and phenotypes in hippocampus, amygdala, cortex, and beyond, that become activated later in life.
With this as brief background, we now turn to what is known about EDCs and nonreproductive behaviors in animals, summarized in Table 2. The literature for cognitive effects is quite consistent, with EDC exposures consistently linked to diminutions in function (71, 110–112). In rodents, developmental exposure of mice to BPA, a known estrogenic endocrine disruptor, caused significant impairments in performance on tests of anxiety and learning in adulthood, together with changes in N-methyl-d-aspartate receptors and dopaminergic systems in the hippocampus and prefrontal cortex (113). Social, anxiety, and aggressive behaviors are also vulnerable to EDCs, with much of the literature focusing on BPA. A study on effects of prenatal BPA exposure demonstrated increases in social contacts in female, but not male, mice along with changes in glutamate neurotransmission (114). Similarly, deer mice showed dimorphic effects of BPA on sociosexual behaviors (115). Other EDCs, including polybrominated flame-retardants (polybrominated diphenyl ether) (116), pesticides (86), and PCBs (87, 112, 117) resulted in considerable changes in markers of neural plasticity and synaptic function, together with behavioral differences. In pine voles, a monogamous species, perinatal exposure to the pesticide methoxychlor caused increased aggression of the females toward a stranger and an increase in the amount of time spent alone (118). In this latter study, autoradiography of the oxytocin receptor was performed, revealing less oxytocin receptor binding in the cingulate cortex of female voles exposed to methoxychlor, compared with vehicle-exposed females. Binding in other regions was not affected by treatment. Developmental exposures to EDCs such as BPA were also associated with increases in anxiety behavior in adolescence and adulthood (119, 120). As a whole, this literature and other reports (121–123) support effects of early-life EDC exposures on the development of sexually dimorphic social and anxiety behaviors across a variety of animal models. It should be noted that all of this work (Table 2) was conducted in rodents, so it is very important for future research to include nonhuman primates for greater translatability to humans.
Table 2.
Examples of Developmental EDC Effects on the Neurobiology of Cognitive and Social Behaviors in Animals
| Species | Sex | EDC Treatment (Route, Dosage) | Age at Treatment | Endpoints Tested | Brain Effect | Behavior Effect | Other Effects and Comments | Reference |
|---|---|---|---|---|---|---|---|---|
| Mouse | Male, female | Oral BPA (100 or 500 μg/kg/d | Gestational d 7 through P36 | Behavior on open-field, elevated plus, Y-maze, novel object tests; receptor autoradiography for dopamine-1 and -2 receptors, dopamine transporter, and NMDA receptors in caudate-putamen, frontal cortex, and hippocampus | Dopamine-1 receptor binding was unaffected in caudate-putamen. D2 receptor binding was increased in the low-dose BPA group, and dopamine transporter binding was decreased in caudate-putamen; NMDA receptor binding was decreased in frontal cortex and in CA1, CA3, and dentate gyrus of the hippocampus | In open-field test, mice traveled more distance in the center; in elevated plus, mice had increased open arm entries (eg, decreased anxiety); on Y-maze, there was reduced alternation behavior; object recognition was impaired in the low-BPA-dosage group | (113) | |
| Mouse | Male, female | Oral BPA (0.4 or 4 mg/kg/d) in oil | Gestational d 7–20 or P1–14 | Behavior in open-field, light-dark transition, mirrored maze, elevated plus maze, forced swim; protein expression (Western blots) in hippocampus and amygdala | Western blots of GluR1 (AMPA receptor subunit) showed decreased protein in hippocampus and amygdala of BPA females and males; there was no effect on NR1 (NMDA) receptor subunit expression | Open field: gestational BPA increased grooming frequency in females; light-dark transition: in general, BPA decreased time in the light chamber in both sexes; mirrored maze: small effects in a sex-dependent manner; elevated plus: BPA decreased time spent in the open arms, and number of open arm entries; forced swim: BPA animals had increased immobile time; overall, effects of BPA were consistent with being anxiogenic | Table 1 of this paper summarizes effects of BPA on anxiety and locomotor activity in rodents; BW was affected by BPA: females given gestational BPA had lower BW, and lactational BPA males and females had higher BW | (279) |
| Mouse | Male, female | Dietary BPA (1.25 mg/kg chow) given to dams beginning 1 wk before mating; intake is estimated at 5 μg daily; all pups were cross-fostered to dams on a control diet | Gestation only | Juvenile social interactions with a age-, sex-, treatment-matched mouse (elevated plus maze; social preference); gene expression in whole embryo brain | Gene expression in whole embryo brains: Slc1a1 and Dnmt3a were increased in BPA compared with control females; Dnmt1 was decreased in BPA compared with control females; Oxtr was decreased in BPA compared with control males | BPA females had increased side-by-side behaviors, decreased self-grooming, more nose-nose sniffing, more following another animal, and more approaches; BPA males had more nose-nose sniffing and approaches; social preference and anxiety behaviors were unaffected | Plasma BPA was in a human-relevant dose range | (114) |
| Deer mouse | Male, female | Dietary BPA (50 mg/kg feed) given in chow to the mother beginning 2 wk before mating through weaning | Gestation and lactation | Sensory and motor function, olfaction, spatial learning and memory in the Barnes maze, exploratory and anxiety behavior in the elevated plus maze, mate choice (female chooses BPA- or control-exposed males), serum testosterone and corticosterone in males | Behavior in the Barnes maze was impaired in BPA males but not females; in the elevated plus maze, BPA decreased exploratory behaviors in males but not females; females showed a reduced sexual preference for males exposed to BPA | No effect on litter size or sex ratio in BPA compared with control litters; sensory systems were intact; in males, serum testosterone and corticosterone were not affected | (115) | |
| Pine vole | Female | Oral DES (0.2 μg/kg/d) or methoxychlor (2 mg/kg/d) in corn oil, fed to the voles | Gestation and lactation | Mate preference, maternal behaviors, other social behaviors, oxytocin receptor binding | Oxytocin receptor binding was lower in the cingulate cortex of methoxychlor females; no effects were seen in the lateral septum, and DES had no effect on receptor binding | DES females were significantly more aggressive to a strange male; methoxychlor females tended to be less aggressive; time spent alone or with a conspecific male was differentially affected by the 2 treatments; no differences in time spent side by side with a male or in maternal behaviors | No effect on litter size, sex ratio, birth weights of pups; no effect anogenital distance; uterine weight was unaffected by DES but was reduced in methoxychlor females | (118) |
| Rat | Male, female | BPA (1 mg/L) in drinking water, beginning 1 wk before mating through weaning; animals received a soy-free or soy diet | Gestation and lactation | Gene expression in amygdala of juveniles; anxiety-like and exploratory behavior in juveniles and adults | In amygdala (P34), 48 genes were measured by quantitative PCR; gene expression was affected (mainly decreased) by BPA for Esr2, Mc4r, Mc3r, Tac2, Kiss1, Bdnf, Gal, and Gad2 | In juveniles, BPA was associated with increased anxiogenic responses in the light-dark box and elevated plus maze in both sexes; some anxiogenic effects persisted into adulthood | No effect on litter size or sex ratio; some behavioral and gene expression effects of BPA were mitigated by the soy diet | (119) |
| Mouse | Male, female | Oral BPA (2, 20, and 200 μg/kg/d) in corn oil | Gestation | Maternal behavior of dams (dosed with BPA) to pups; gene expression in prefrontal cortex, hippocampus, hypothalamus on P28; DNA methylation of Esr1; social and anxiety behaviors | BPA had dose-, region-, and sex-specific effects on mRNAs of Esr1, Esr2, and Esrrg; BPA affected expression of Dnmt1 and Dnmt3a in hypothalamus and cortex, but little effect in hippocampus; Esr1 methylation was affected, especially in the prefrontal cortex of males and hypothalamus of females | BPA affected social behaviors in the home cage and disrupted play behaviors; it increased anxiety-like behavior in females and decrased it in males with overall diminishing sex differences; few effects on maternal behavior of the dosed dams | (120) | |
| Rat | Male, female | BPA (0.1, 1 mg/L), resveratrol (5 mg/L) in drinking water | Gestation through weaning at P21 | Sexual development, anogenital distance, open-field test, sexual behavior, estrous cycles, sperm counts, serum hormones, reproductive organ weights, SDN-POA, and locus coeruleus volume | The sex difference in the SDN-POA (male > female) was not affected by treatment; the sex differences in the locus coeruleus size and the number of cells (female > male) were reversed by both BPA doses and by resveratrol | Sex differences in the open-field test were abolished in the higher-BPA-dose group, due to males behaving more similarly to females | BPA had no effect on sexual development of males and females; resveratrol delayed day of vaginal opening | (121) |
| Rat | Male | PCBs 47 and 77 mixture, 12.5 or 25 mg/kg diet in food | Gestation through weaning | Social recognition in juveniles, conspecific-directed investigation in adults, size of PVN | PVN area was significantly smaller in young adult PCB males of both doses compared with control males | In the social recognition test (juveniles), the higher-dose PCB groups spent more time in the social box whereas the low-dose PCB and control males did not; in the social investigation test (adults), the high-dose PCB group differed modestly from other groups in one aspect of the test | No effect on food consumption, weight gain, or BW in dams; the 25 mg/kg diet males as adults were significantly heavier than other groups | (123) |
| Rat | Male, female | Reconstituted mix of PCBs 138, 153, and 180 (equimolar) plus PCB 126, 10 mg/kg/d given sc | Gestational d 15–19 | Enzyme expression in the hypothalamus of offspring at gestational d 20 and P12, 21, and 60; timing of puberty; learning and memory (Morris water maze) and passive avoidance | Aromatase mRNA in hypothalamus was increased in PCB-treated rats only at P21 in males; mRNA of 5α-reductase-1 was lower in PCB females at P21 and in PCB males and females at P60; 5α-reducatase-2 mRNA was elevated in PCB females at P60 | Spatial memory (Morris water maze) was unaffected by treatment; passive avoidance was impaired only in PCB males | BW at puberty was lower in PCB-exposed males and females; age at vaginal opening was 1 d earlier; age at testicular descent was 1 d later | (117) |
| Rat | Male, female | Phthalates (DEHP), 30 mg/kg BW/d in drinking water | P1 to weaning | Behavior in elevated plus maze at P30, P45, and P60 | DEHP males were affected at P45 and P60 with decreased frequencies of entries into the open arm and time spent in open arm and increased time spent in the closed arm; no effect in females | (122) |
Abbreviations: BW, body weight; DEHP, di-2-ethylhexyl phthalate; NMDA, N-methyl-d-aspartate; P36, postnatal day 36; PVN, paraventricular nucleus.
B. Prenatal hormones, EDCs, and adult behavior: human evidence
Men and women are not identical in how their brains work. For example, there are sex differences in human play, cognitive processes, learning and memory, and many other functions (124). In terms of emotion, men and women are also not equally susceptible to certain neurological/psychiatric maladies. Although some behavioral sex differences may seem purely qualitative, there are morphological, phenotypic, and quantifiable behavioral sex differences (13). Furthermore, the sexes differ in their risk and severity of psychiatric and neurological disorders such as depression (female-biased) and attention deficit hyperactivity disorder (male-biased) (125–127). Autism and ASDs, which typically manifest early in childhood, and schizophrenia, a disorder that usually presents in young adulthood, are more prevalent in males. In fact, neurodevelopmental and neuropathological disorders are nearly always more prevalent in one sex than the other, leading us and others to speculate that some of these dimorphisms may be due to organizational and/or activational effects of steroid hormones.
Epidemiological data for associations between higher body burden of EDCs with neurobehavioral and neurocognitive effects is growing (Table 1). Despite limitations in being able to draw causal relationships, some of the earliest EDC research linked cognitive impairments to early-life EDC exposures in humans (111, 128). In fact, Jacobson and Jacobson (128) studied a population of humans living near Lake Michigan whose mothers, although pregnant, consumed fish contaminated with relatively high levels of PCBs. Children with the highest PCB concentrations had the lowest IQ scores and deficits in memory and attention. Subsequent studies relating concentrations of PCBs or dioxins in maternal plasma or breast milk showed lower overall cognitive scores on a behavioral battery, an effect that was particularly pronounced in the mostly highly exposed group (129). Many other human studies with PCBs have shown, and continue to show, associations with poorer performance on neurobehavioral and cognitive tests (130–132), underscoring the point that despite the ban on PCBs in the late 1970s in the United States, their persistence in environment and tissues have lingering consequences (67). A Korean study of 460 mother-infant pairs measured phthalate exposures in third-trimester urine samples and related it to performance at 6 months on mental developmental index (MDI) and psychomotor developmental index (PDI) function (62). Results showed that depending upon which phthalate was measured, inverse relationships with MDI and PDI were found in boys, but not girls. Organophosphorus pesticides are well established as being negatively associated with cognitive development (reviewed in Ref. 133). Associations between BPA concentrations in urine of pregnant women, or in the urine in 5-year old children, revealed behavioral changes related to anxiety and hyperactivity, with effects differing between the sexes (52). In fact, a burgeoning literature on human exposures to BPA and associated endocrine and neurodevelopmental effects, including potential links to autism (134), quite consistently supports these studies and are summarized in Table 1. Finally, a recent study of children whose mothers resided near agricultural pesticide application revealed positive associations between second- and third-trimester organophosphate pesticides and ASD, and some associations between pyrethroids and developmental delay (135). As a whole, the literature supports an association between exposure to environmental contaminants and neurodevelopmental and cognitive effects. Causal associations and underlying mechanisms are lacking and should be a priority for research in humans if and when this is possible.
IV. Hormones, Sexually Dimorphic Behaviors, and Autism and Autism-Spectrum Disorders
In general, the diagnosis of autism and ASDs in humans is based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) cardinal signs of autistic behavior: impaired social and communication skills, and restricted and/or repetitive behaviors (272). Other aspects of attention and cognitive profile associated with the autistic phenotype may also be considered in the diagnosis. For a more complete description of the autistic behavioral phenotype based on DSM-IV, see Supplemental Material (note that although DSM-V was recently released, research to date has been based on DSM-IV or earlier criteria). The fact that autism and ASD are approximately 4 times more prevalent in males than females and that Asperger's syndrome (high-functioning ASD individuals with intact language) is 9 times more prevalent in males than females (136) has led some to propose a role of androgens and/or their estrogenic metabolites in the development of autism (137). This extreme male brain theory of autism has been invoked to explain sex differences in prevalence due to higher prenatal androgens (138, 139). However, this is highly controversial. For example, other hormones, including thyroid hormones, have been considered for their possible links to autism and ASDs (140, 141). Genetic disorders, pathophysiological conditions, and pharmaceutical treatments resulting in changes in the hormonal milieu of the developing fetus have provided some insight into the roles of prenatal hormones, but again, there are some inconsistencies in the relationships between hormones, neurodevelopment, and the autistic phenotype. To follow, we will discuss 5 examples from humans with possible links between the prenatal hormone environment, sexually dimorphic behaviors, and autism/ASDs (Table 3).
Table 3.
Prenatal Hormonal Environment and Links to Autism in Humans
| Disorder or Treatment | Etiology/Exposure | Hormonal Phenotype or Exposure in the Fetus | Link to Autism/ASD |
|---|---|---|---|
| CAH | Deficiency in 21-hydroxylase | Elevated progestins, androgens in fetus | Mixed evidence for links to autism, although high prenatal androgens can masculinize behavior in females in some (but not all) studies |
| PCOS in pregnancy | Multifactorial | Elevated androgens in fetus, more so in females than males | Sons, no link; daughters, increased autistic-like behaviors; both, amniotic testosterone was positively correlated with autistic-like behaviors |
| Pharmaceutical progestins in pregnancy | Progestins administered during high-risk pregnancies | Elevated prenatal progestin (sometimes in combination with estrogens and/or androgens, depending upon nature/combination of the pharmaceutical) | Links to autism were not studied; some sexually dimorphic behaviors were affected |
| ART to facilitate pregnancy | Various, including ovulation-inducing drugs (clomiphene and gonadotropins/GnRHa) | Exposure of preconceptual ovum to various pharmaceuticals and/or altered maternal hormonal milieu | Slight but nonsignificant link between gonadotropins used in ART and ASD risk |
| SLOS | Mutation in DHCR7 gene | Deficiencies in steroid hormones including sex steroids, glucocorticoids, and mineralocorticoids | Children are microencephalic, and 50%–75% meet criteria for ASD |
A. Abnormal prenatal hormonal milieu and autism/ASDs
1. Congenital adrenal hyperplasia
CAH is a naturally occurring disorder in which the activity of a key adrenal enzyme required for normal steroid synthesis, usually 21-hydroxylase, is deficient, resulting in deficiencies in cortisol, a lack of negative feedback to the hypothalamus and pituitary, and hence increased prenatal production of adrenal progestin and androgens, including testosterone. In untreated female fetuses, excessive gestational androgen exposure masculinizes the genitalia and induces ovarian changes typical of those seen in polycystic ovary syndrome (PCOS) (see Section IV.A.2). Cortisol replacement therapy, in the form of dexamethasone, improves outcomes (142). By contrast, in males with CAH, the normally high prenatal testosterone levels minimize the impact of elevated adrenal androgens (143, 144). Some studies have suggested that high levels of androgens during prenatal development in CAH females resulted in masculinized behavior and cognitive skills (124, 145–147), although the risk of developing autism/ASDs in males or females was not significantly increased. Nevertheless, higher prenatal testosterone levels in girls were associated with more autistic behavioral and cognitive traits (147). Compared with unaffected females, CAH females preferred male-stereotypical play behavior (148) and male peer interactions (149), and they also showed increased male-stereotypic visual-spatial abilities (150). However, other studies found no differences in measures of masculinity, femininity, or sex-typical cognitive ability (151). Baron-Cohen and colleagues (152) looked for differences in autistic traits in CAH females and males using their questionnaire, the autism spectrum quotient (AQ), which measures autistic traits related to social skill, communication, and cognitive features seen in ASDs (153, 154). When comparing CAH and non-CAH male siblings, Knickmeyer et al (147) found no difference in autistic traits in any domain. However, females with CAH showed higher scores on the AQ than their unaffected sisters and had reduced vocabulary scores. Unexpectedly, attention to detail was less ASD-like in the female CAH cases vs controls. Thus, the sex-specific behavioral outcome data in CAH are consistent with the hypothesized impact of sex-specific alterations in prenatal testosterone levels. We note that most work on CAH has focused on prenatal testosterone; the consequences of elevated progestin levels in CAH boys and girls have received little consideration in the literature, something that merits further study. Estrogenic metabolites of testosterone similarly have not been adequately considered in the CAH literature.
2. Polycystic ovary syndrome
PCOS is a condition associated with high androgen levels and characteristic ovarian changes, often associated with infertility. Palomba et al (155) measured androgen levels at the time of amniocentesis in pregnant women with PCOS (27% using assisted reproductive technology [ART]) and non-PCOS controls (16% using ART) and examined autistic-like behaviors at follow-up in the offspring at 4 to 11 years of age using the AQ children's version and questionnaires assessing empathy and systemizing, traits that are associated with the broader autistic phenotype. Testosterone levels in amniotic fluid of PCOS compared with non-PCOS women were significantly higher in female but not male fetuses. Sons born to PCOS mothers showed no significant increase in autistic-like behavior on any measure, but daughters were rated as showing increased autistic-like behaviors compared with female controls on all tests, although none met DSM-IV criteria for autism. Interestingly, amniotic testosterone levels were positively correlated with autistic-like behaviors on all 3 test instruments, irrespective of maternal diagnosis or offspring gender, such that the higher the testosterone level, the greater the number of autistic traits. These data support an effect of prenatal testosterone on the development of brain systems related to the broader autistic phenotype in offspring of mothers with PCOS, with a larger impact in females compared with males, presumably due to the increased fetal testosterone levels in the females.
3. Pharmaceutical progestins in pregnancy
Limited studies on behavioral outcomes after prenatal exogenous hormone exposure were reported in the 1970s and 1980s for treatment of pregnancies deemed to be at risk for early fetal loss, toxemia, and premature birth. Results showed an impact of these hormones on sexually dimorphic behaviors (eg, tomboyishness in girls) in the offspring, but little or no effects on cognition and no evidence of autistic features, although the studies at the time were not designed to seek autistic traits (156). Reinisch (157) examined IQ and personality traits in late childhood after first-trimester gestational exposure to synthetic progestins and estrogens (most commonly Colprosterone, Norlutin, Delalutin, Deluteval, Provera, Provest, and DES), some with androgenizing effects, using unexposed siblings as controls. There was a high degree of variability in which hormones were used and their timing, dosing, and duration. No differences in IQ were found, but differences in personality were significant, with the high-progestin group more independent, individualistic, self-assured, self-sufficient, and sensitive. The high-estrogen group was more group-oriented and group-dependent (157). None of these children met existing criteria for any disorder, and none were thought to show autistic traits, but again, these were not specifically sought.
4. ARTs/in vitro fertilization (IVF)
The advent of IVF and other ARTs over the last few decades have provided another patient population in which periconceptual and prenatal hormone treatment can be studied relative to long-term developmental outcomes. These studies have not indicated a strong link to ASDs (158–160), although a slight (albeit nonsignificant) association between use of ovulation-inducing drugs and ASDs was described. In a study published just last year from the Nurses' Health study in North America, ART and history of infertility again were not associated with an increased risk of having a child with ASD: adjusted odds ratio (OR) = 0.70 (95% confidence interval [CI] = 0.33–1.47) and 0.93 (0.73–1.19), respectively (161, 162). A significant crude OR for having an autistic child after ovulation-inducing agents plus another fertility therapy (usually artificial insemination [AI]) lost significance once adjusted for parental age, birth order, etc. but the use of AI alone survived adjusting for confounders with a doubling of ASD risk (OR 2.17 [CI 0.90–5.21]). When looking at individuals with advanced maternal age, the incidence of infertility was high (35%), yet there was no difference in prevalence of IVF or ART overall across women with and without ASD affected children. However, among mothers 35 years of age or older, using a combination of non-ART fertility therapies had nearly a doubling in risk of having offspring with ASD compared with those using no fertility therapies (OR 1.92 [95% CI 1.03–3.60]). Conversely, mothers over 35 with ASD affected children showed a significantly higher chance of having used fertility therapy, mostly ovulation-inducing agents or AI, when compared to over 35 mothers of unaffected children, 14.6% vs 8.6% (P = .008); but again the only fertility treatment that had a significant effect after removal of confounders was AI with an adjusted OR of 3.73 [CI 1.38–10.1]). Interestingly, in women over 35, the risk of having a child with milder forms of autism, Aspergers and PDD-NOS (pervasive developmental disorder not otherwise specified), was significantly increased with use of either ovulation-inducing agents or AI, adjusted OR 2.12 [CI 1.06–4.23] and 4.52 [CI 1.48–13.9]). Individuals using a combination of non-ART fertility therapies had nearly a doubling in risk of ASD compared with those using no fertility therapies (OR = 1.92 [95% CI = 1.03–3.60]). These findings add credence to the most recent Danish studies showing a trend relating FSH and human chorionic gonadotropin treatment to ASD risk (162). Although none of these papers directly showed sex steroids themselves as contributors to ASD, further study is warranted because gonadotropin treatment clearly leads to sex steroid release from the ovaries of the mother and potentially affects the fetus.
5. Smith Lemli Opitz syndrome
Smith Lemli Opitz syndrome (SLOS) is a single-gene disorder with mutation in the DHCR7 gene and reduced activity of its protein, 7-dehydroxycholesterol reductase. This enzyme converts 7-dehydrocholesterol to cholesterol, the lack of which results in low serum levels of cholesterol, increased levels of precursor molecules, and failure to produce normal levels of sex steroids, glucocorticoids, and mineralocorticoids (163). These individuals may also have altered neurosteroid production (164). Children with SLOS show microcephaly and characteristic dysmorphisms, in association with mental retardation, language impairments, and sleep and behavioral problems. Between 50% and 75% of children with SLOS meet diagnostic criteria for an ASD (165). Sikora et al (165), using the Autism Diagnostic Observation Schedule on a sample of 14 subjects with this rare genetic disorder, found 12 of the 14 met autism/ASD criteria. The strong incidence of autism in SLOS brings to light the possibility that elevated brain neurosteroids may play some role in triggering autism and that elevated sex steroids may act as neurosteroids and thereby trigger ASDs.
B. Correlations among physiological hormones, neurocognitive function, and autism
1. Hormones in cord blood
Cord blood has been used to look for the influence of prenatal steroids on later cognitive and behavioral profiles and, recently, brain morphogenesis. Language pragmatics, such as the ability to integrate the perspective of others into communication, and which have long been associated with the autistic language phenotype, were assessed in school-age children relative to their cord free testosterone levels. Increased cord free testosterone was highly correlated with weaker performance in pragmatic language ability in girls, but not in boys (166), although direct associations with ASD have not been made. Jacklin and colleagues (167, 168) and Marcus et al (169) examined cord blood sex steroids relative to temperament, mood, and cognition in infants, toddlers, and young children. They reported sexually dimorphic relationships between steroids with behaviors, with cord testosterone and androgens affecting temperament, mood, and cognition and cord estrogens and progesterone influencing temperament and mood. Marcus et al (169) found sexually dimorphic effects of sex hormones on mood, such that happy/excited behavior increased with cord blood sex steroids in boys and tended to decrease with cord blood sex steroids in girls. It is important to note that cord blood is always sampled at the end of gestation and may not reflect steroid hormone influences in critical periods of brain development in gestation.
2. Hormones in maternal blood
In a very large clinical sample, Hines et al (170) related maternal testosterone and sex hormone-binding globulin (SHBG) levels between 5 and 36 weeks of gestation to sex-stereotypical play behaviors in male and female offspring at 3.5 years of age. Increased levels of testosterone, but not SHBG, were positively correlated with male-type play in girls. Play behavior in the sons was not correlated with maternal testosterone levels.
3. Hormones in amniotic fluid
Routine amniocentesis is done between 14 and 20 weeks gestation; therefore, studies on amniotic fluid hormones are best related to the first half of the second trimester of pregnancy. Amniotic testosterone is measured most commonly and is a reliable proxy of fetal exposure to testosterone. Finegan et al (171) first demonstrated higher fetal amniotic fluid testosterone (afT) levels in male compared with female fetuses. This group (172–174) examined afT as a predictor of later cognitive abilities and found a positive effect on girls' spatial processing speed, but no effect in boys. A study on afT relative to the development of brain lateralization yielded sexually dimorphic results, with high afT in girls associated with increased left lateralization for language and high afT in boys associated with increased right lateralization for affect. A strong tendency to right lateralization has been documented in autism (175).
The vast majority of the studies since then on amniotic steroid hormones with behavioral outcomes have looked only at fetal testosterone levels, in an effort to test the extreme male brain theory of autism propounded by Baron-Cohen and colleagues (138, 139) at Cambridge University in the United Kingdom. In a series of correlational analyses carried out over the last decade, the Cambridge Fetal Testosterone Project found that increased afT predicted decreased eye contact in infant boys at 12 months (176), poorer quality of social relationships and skills in 4-year-old boys and girls, and restricted interests in 4-year-old boys (177, 178). In 2009, Auyeung and colleagues (179) at Cambridge found that afT levels predicted the development of sex-typical play in children from the general population, with higher afT levels predicting more male stereotypic behaviors in both sexes. They subsequently found that high aFT, but not postnatal plasma testosterone, was correlated with increased autistic behavioral traits based on questionnaires completed with the mother (180, 181). Most recently, Auyeung et al (182) studied the effects of afT on a specific visuospatial ability in school-age boys and girls using the embedded figures task, which requires exquisite attention to detail. They found a positive correlation between afT and this visual spatial task in both boys and girls. Success on the embedded figures task has been associated with an autistic phenotype in some studies but not in others (183).
A very recent study was conducted in which amniotic fluids were assayed for 4 hormones in the Δ4 pathway, progesterone, 17-OH progesterone, androstenedione, and testosterone, along with cortisol (184). Using the Danish Historic Birth Cohort and the Danish Psychiatric Central Register, comparisons were made between fluids of women whose male children subsequently developed autism (n = 128) compared with nonautistic male controls (n = 217). A significant elevation in the Δ4 hormones was found in the males with autism compared with controls. To our knowledge, this is the first study linking steroidogenic activity to autism (184).
C. Cautions in interpretation of data
There are several reasons for uncertainty. First, the physiological range of hormones has considerable variability even across normal pregnancies. Second, the effective hormonal compounds proximal to the behavior may not be the steroids administered, but one or more of its metabolites. Furthermore, both the parent steroid and its metabolites may be active (reviewed in Ref. 185). Third, behavioral effects may vary according to sex and genetic background. In the Section V, molecular mechanisms elucidated in animal models will be discussed. Although it has been argued that many neuroendocrine mechanisms and hormone actions on the nervous system have been conserved from the animal to the human brain (185), that point of view does not necessarily apply universally to the phenomena considered below.
V. Molecular Mechanisms for Effects of Hormones on Brain and Behavior
Several molecular mechanisms plausibly explain how long-lasting effects of prenatally administered natural or synthetic steroids could affect brain and behavior. These mechanisms usually go under the heading epigenetic: modifications to the DNA that occur independently of changes in DNA sequence but that regulate gene expression. Most DNA is unavailable for gene transcription because of its condensation and tight association with histones. Only regions of DNA that are in a relaxed state and accessible to the transcriptional machinery are transcribed within a cell. It is now widely accepted that several molecular epigenetic mechanisms, principally DNA methylation and histone modifications, profoundly affect a gene's propensity to be expressed. In addition, posttranscriptional modifications including noncoding RNAs change the stability of RNA, adding to the complexity of whether (or not) a gene is transcribed and translated to the protein product.
The developing nervous system undergoes substantial epigenetic modifications beginning in the fetus and continuing through life. There is evidence for all of the known molecular mechanisms as key determinants of which genes are expressed, in what brain regions, and when, throughout the life cycle. Recent evidence shows that hormonal exposures, especially to the developing (fetal and infant) brain, are involved in normal brain sexual differentiation and, ultimately, play out as an adult phenotype in terms of brain function and the control of behavior. Because these epigenetic processes provide an interface between the organism and its environment, even a brief exposure to environmental insults can have long-lasting effects on nervous cellular functions (186). Due to the explosion of research and review papers on molecular epigenetic mechanisms, we only briefly introduce the mechanisms before turning to examples of how they are modified by hormones and EDCs.
A. Histone N-terminal modifications
Unstructured amino-terminal tails of histone proteins protrude from the nucleosome core and are subject to large numbers of possible posttranslational modifications. These modifications include addition of small chemical groups through acetylation (187, 188), methylation (189, 190), phosphorylation (191, 192), or additions of larger peptides (ubiquitination and sumoylation) to the side chains of specific and remarkably conserved amino acids of each of the core histone proteins (193). It has been proposed that specific combinations of histone posttranslational modifications and their temporal sequences create the so-called histone code that largely determines the functional outcome, activation, or repression of transcription (194), a hypothesis that has received considerable support (195).
Histone modifications in the brain are affected by natural prenatal hormones; they are sexually dimorphic, and they are altered by prenatal perturbations to the hormonal milieu. For example, levels of acetylated and methylated (K9me3) histone H3 were sexually dimorphic in the ventromedial hypothalamic nucleus and POA of newborn mice (Figure 2) (196). Notably, histone protein status is labile at this critical developmental stage, because higher levels of H3 acetyl and H3K9me3 observed in the brains of male mice on postnatal day 0 were either absent (in ventromedial hypothalamic nucleus) or reversed (POA) on postnatal day 1. Moreover, H3 acetylation but not H3K9 methylation, was affected by estradiol treatment of neonatal mice. Another group showed sex differences in acetylated H3K9/14 in the cortex and hippocampus of late embryonic mice, a pattern that was reversed by prenatal exposure to testosterone (197). By contrast, although HeK9Me3 is sexually dimorphic in these same regions, prenatal testosterone did not affect this pattern. Also, the natural sex difference in size and number of cells in the bed nucleus of the stria terminalis of rats was reversed by neonatal testosterone treatment in females and prevented by treatment with a histone deacetylase inhibitor, valproic acid (198). A follow-up study from this latter group showed that neonatal valproic acid treatment to mice reversed the sex difference in vasopressin immunoreactivity in the bed nucleus of the stria terminalis, together with inducing subtle behavioral alterations in an olfactory preference test (199). As a whole, these data suggest that chemical make-up of histone proteins is sensitive to hormonal influences and, as a consequence, may contribute to the development of the sexually dimorphic brain and behaviors (186, 196). This work is also consistent with evidence that prenatal EDC exposures are associated with histone modifications in the brain and correlate with changes in behavioral outcomes in adulthood (reviewed in Ref. 200).
Figure 2.
In perinatal animals, histone acetylation and methylation are sexually dimorphic; higher levels of histone H3 acetylation and histone H3 lysine 9 methylation have been detected in neuroendocrine tissues (medial basal hypothalamus and POA) as well as in limbic areas (cortex/hippocampus) of the male brain. In both neuronal groups, whereas histone 3 acetylation was affected by neonatal hormone treatment (left), H3K9me3 (right) was not. [Based on K. Gagnidze and D. W. Pfaff: Hormone-dependent chromatin modifications regulating sexually differentiated animal behavior. Multiple Origins of Sex Differences in Brain: Neuroendocrine Functions and their Pathologies (edited by D. W. Pfaff and Y. Christen), Springer, 2013, p 1 (196); and H. W. Tsai et al: Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4:47, 2009 (197)].
B. DNA methylation
DNA methylation involves the addition of a methyl group (-CH3) at the 5′ position on the cytosine base within CpG dinucleotides. It is an important mode of regulation of constitutively repressed chromatin found within imprinted genes, inactive X-chromosomes, retrotransposons, and other genomic elements. In addition, active DNA demethylation may take place in postmitotic neural cells (201).
DNA methylation is implicated in developmental neuroendocrine phenomena. There are substantial sex differences in methylation of genes whose protein products mediate sexually dimorphic behaviors, including estrogen receptors, progesterone receptor, and glucocorticoid receptor (202–204). These methylation patterns are strongly affected by manipulations of prenatal/perinatal hormones such as estrogens and androgens.
Results from studies on developmental exposures to EDCs in animals are consistent with work on prenatal hormones. For example, in utero and early postnatal exposure to BPA results in postnatal changes in DNA methylation status and altered expression of specific genes in offspring (120, 205). That these expression patterns can last a lifetime was shown in a study in which rats were exposed to estrogenic endocrine disruptors in late gestational/early postnatal development. Expression of numerous hypothalamic genes was altered in these animals even when they were very aged (18 months), and DNA methylation analysis of the estrogen receptor-α gene (Esr1) showed altered methylation patterns (206). However, it is important to note that contrary to original beliefs about the permanence of DNA methylation marks, DNA methylation patterns may undergo postnatal developmental changes, as shown for the Esr1 gene in the hypothalamus of rats (207).
C. Noncoding RNAs
Recently, RNA species called noncoding RNAs have become the focus of much attention as important regulators of chromatin structure and gene expression at the transcriptional as well as posttranscriptional levels (208). Long noncoding RNAs (lncRNAs) are transcribed from intergenic and gene regulatory regions (ie, promoters and enhancers) in antisense, overlapping, intronic, and bidirectional orientations relative to protein-coding genes and can be between 200 bases and tens of kilobases long (209, 210). Unlike mRNAs, many lncRNAs are retained in various subnuclear compartments after transcription and participate in chromatin remodeling and targeted gene silencing via the recruitment of transcription factors and chromatin-modifying complexes to specific nuclear and genomic sites (210–212). The lncRNAs inhibit expression of multiple genes spread over a large genomic region or even a whole chromosome, as is the case of X-chromosome inactivation in the female by Xist lncRNA (210, 212–214). In addition, lncRNAs such as retrotransposons have been detected in human neural progenitor cells (215) and in the human brain (216). They themselves can be modulated in neurons by DNA methylation (217) and, indeed, are active during neuronal differentiation (218).
Among noncoding RNAs, microRNAs are 20- to 23-nucleotide-long single-stranded RNA species mainly involved in posttranscriptional regulation of gene expression (209) (Box 2). These and other noncoding RNAs are beginning to be investigated in their roles in neuroendocrine function, especially stress responsiveness and the neuroendocrine control of reproduction. In the case of the stress system, Mueller and Bale (219, 220) showed that stressing pregnant rats during the first trimester had sexually dimorphic effects in offspring, with larger effects on the behaviors of males than females. That same group went on to assay the epigenomic microRNA environment in prenatally stressed compared with control animals across 2 generations. The second-generation stressed males had significant reductions compared with nonstressed counterparts of 3 microRNAs, miR-322, miR-574, and miR-873, as measured in the whole brain on postnatal day 1 (221). These results offer the possibility that stress-altered microRNA expression lies on the mechanistic path by which behaviorally important epigenetic programming is transmitted.
Box 2. MicroRNAs.
MicroRNAs are small single-stranded RNAs, 20 to 23 nucleotides in length, that are involved in posttranscriptional/posttranslational regulation of gene expression. The majority of microRNAs are encoded within the intergenic space or gene introns, and only a small portion are transcribed from exons (275, 276). Biogenesis of microRNAs involves processing of longer primary transcripts (primary microRNA) by ribonuclease-3 containing complex DROSHA, transport into the cytoplasm, and cleaving of an intermadiate by the DICER1 ribonuclease. Mature microRNAs are then loaded onto Argonaute proteins and integrated into multicomponent RNA-induced silencing complexes. By binding to complementary sequences predominantly found in 3′-untranslated regions, microRNAs direct the RNA-induced silencing complex to target mRNAs leading to repression or degradation of these transcripts (277, 278).
Further evidence for the involvement of microRNA chemistry in the regulation of neuroendocrine signaling comes from work on the regulation of puberty. Sangiao-Alvarellos et al (222) studied the expression of 2 RNA binding proteins (Lin28 and Lin28b) that regulate the maturation of microRNAs of the let-7 family. In both male and female rats, Lin28 showed high expression in the hypothalamus during the neonatal period and then declined to a nadir at the time of puberty. Conversely, the let-7 microRNAs showed the opposite developmental profile of expression. Neonatal injections of an estrogen or androgen, which altered the timing of puberty, likewise altered Lin28 and let-7 expression at the time of puberty. Thus, noncoding RNAs appear to participate in the mediation of hormone effects on the brain.
D. Epigenetics, social behavior, and autism
These are early days for the application of measurements of DNA methylation, histone modifications, and noncoding RNAs to specific brain regions for the analysis of prenatal influence on social behaviors and their abnormalities. With respect to possible epigenetic links to autism, these studies fall into 2 main categories: genome-wide epigenetic analyses and targeted searches of obvious candidate genes such as those in oxytocin systems. As an example of the former type of study, Ladd-Acosta et al (223) examined patterns of DNA methylation in prefrontal cortex, temporal cortex, and cerebellum in postmortem brain tissue of 19 autism cases compared with 21 controls. In doing so, they identified 4 genomic regions that were differentially methylated between autistic and control subjects. In a similar type of study, Wong et al (224) examined monozygotic twins who were either concordant or discordant for autism. Numerous significantly different methylated chromosomal regions were identified, suggesting a role for altered DNA methylation in the genesis of autism. Levitt and his colleagues (225) have followed up on discoveries on the TAC1 gene (encoding tachykinins such as substance P), and reported TAC1 promoter hypermethylation in a small study of females with autism (n = 17) compared with controls (n = 19).
Regarding oxytocinergic systems, based on previous results connecting oxytocin signaling to the promotion of sociosexual behaviors (226–228), we would expect for the social disabilities of autism to be associated with epigenetic changes that lead to decreased expression from the oxytocin gene or the oxytocin receptor gene (reviewed in Ref. 229). Indeed, methylation analysis of the CpG island known to regulate oxytocin receptor expression in blood cells and in postmortem brain revealed increases in the DNA methylation status in individuals with autism compared with controls (230). These authors also reported decreased oxytocin receptor mRNA in temporal cortex of autistic patients. Mamrut et al (231) confirmed the reciprocal relationship between higher methylation status of the oxytocin receptor promoter and reduced transcription. The importance of these findings for the perception of social stimuli has been pointed out by Jack et al (232), who found significant correlations between the degree of oxytocin receptor gene methylation and the brain's response to ambiguous abstracted-social signals in 2 regions of the brain: the temporal cortex near the temporalparietal junction and the dorsal anterior cingulate cortex. Both of these areas of the human brain have been linked to social perception and emotional function.
Because of a rapid increase in the precision and availability of assays for DNA methylation, histone N-terminal modifications, and noncoding RNAs, it is likely that the literature connecting these epigenetic phenomena to autism will grow quickly. However, the ability to measure gene expression and epigenetic modifications in the brain is clearly limited to postmortem tissues. Using peripheral tissues (eg, serum) may not be a good proxy for epigenetic events in the nervous system. Thus, we reiterate that this is a relatively new field that merits much additional research.
VI. Evidence for Transgenerational Epigenetic Phenomena That Influence Brain and Behavior
Although most of the data referred to in this section derive from experiments with laboratory animals, we are ultimately interested in human behavior. The field of environmental epigenetics in human health is rooted in the long-term understanding of interactions between environmental factors (toxicants, stressors, etc) and an individual's susceptibility to develop a disease or dysfunction. Nearly all discussion of this concept includes the gene-environment interaction and builds into it the molecular epigenetic processes described above as providing the interface between gene and environment. However, it is important to recognize that monogenic disease accounts for a small percentage of most complex diseases, estimated at about 2% to 5% of a single gene's contribution to a disease (233). In the case of mental illness and affective disorders, there is widespread agreement that there is no single gene for schizophrenia, autism, or major depressive disorder. This begs the question of how important the gene is in the equation of gene × environment. Recently, in collaboration with David Crews (234), one of us (A.C.G.) hypothesized that a more accurate equation is Eg × Ec, with Eg representing germline-dependent epigenetic processes and Ec representing context-dependent (126, 235), sometimes referred to as experience-dependent (236), epigenetic modifications. This concept is fundamental to understanding the link between the environment and complex disease. The germline part of the equation explains the molecular epigenetic changes caused by environmental insult, whereas the context (experience) part of the equation underlies how an individual's epigenome will be subject to further modification via that individual's own life experiences and environment. Of course, the definition of environment in this case can be exogenous compounds (eg, EDCs), but it can also include maternal hormones, stress, diet, or other factors now known to induce epigenetic modification, including changes to germ cells that are propagated across generations. Evidence for this concept will be discussed below.
A. Epigenetics and behavior
Links between epigenetic modifications and behavior are elegantly exemplified by the work of Michael Meaney, Frances Champagne, Moshe Szyf, and colleagues (120, 205, 236, 239, 258), who imposed experimental variations of maternal care in rodents. Over more than a decade, these groups have demonstrated that maternal behavior of rats toward their young, particularly licking and grooming of pups, had substantial effects on behaviors in the offspring, including their stress responsiveness and, in the case of the females, their own maternal behavior toward their young (237). Furthermore, these offspring differed in hippocampal expression of glucocorticoid receptors, an effect that involves epigenetic modifications such as histone modifications and DNA methylation (238). Importantly, although the behavioral phenotype was passed from mother to daughter to granddaughter, implying some heritability, it was abolished by cross-fostering pups to mothers with a different mothering style (239). This finding is consistent with the importance of the social context, Ec, in the epigenetic transmission of a behavioral phenotype. Studies on nonhuman primates also suggest that mothering style has an impact on social behavior in offspring (240, 241).
This body of work likely has high relevance to humans. A comparison of offspring of Holocaust survivors with or without posttraumatic stress disorder revealed differences in their urinary cortisol levels (242, 243). Although there is much speculation on the propagation of undesirable social behaviors (eg, abuse) or in response to adversity (244), little is known about underlying epigenetic mechanisms in humans (245). The literature on transgenerational effects on brain function as studied in animals has recently been reviewed (246). To date, we consider that the most convincing data are those from studies of the imposition of stressful experiences early in life, causing depressive-like symptoms and changes in social behavior in the F2 generation. Thus, even as we proceed with caution, there are transgenerational behavioral phenomena to be accounted for.
B. Transgenerational epigenetic actions of EDCs
A landmark publication in the field of environmental endocrine disruption came from Michael Skinner's laboratory (247), demonstrating that not only does prenatal exposure to an EDC (vinclozolin, a fungicide) cause adult late-onset disease, but it was also heritable to future generations via the male germline. The mechanism of transmission is due at least in part to DNA methylation changes in the germ cells during a critical period of embryonic development, when DNA methylation marks are erased and then reset in the fetal germ cells. This mode of transmission had been shown previously in other experimental contexts (eg, nuclear transplantation) (248). Although work in this area is in its infancy and is not without controversy (see Ref. 235 for a review), there is increasing support for germline-dependent transgenerational effects of EDCs. A study of vinclozolin transgenerational effects in mice (249) also showed methylation changes in the germline. Other EDCs, including PCBs, DES, and BPA, are now associated with transgenerational effects (114, 235). Although the focus of this section is exogenous hormonally active substances, for which there is the greatest evidence, daughters of experimentally induced PCOS monkeys have impaired oocyte developmental competence (250).
C. Transgenerational EDCs and behavior
A transgenerational behavioral phenotype of developmental EDC exposure has been shown for 2 EDCs. In the case of vinclozolin, third-generation (F3) offspring of pregnant rats treated with vinclozolin, thereby exposing their F1 fetuses, were tested in a mate-preference paradigm (251). In a 2-male partner preference test, in which a female was allowed to discriminate and choose between an F3-vinclozolin lineage and an F3-vehicle lineage male, female rats strongly preferred the nonexposed (F3-vehicle lineage) offspring (251). A second study using this same model showed alterations in anxiety-like behaviors, together with substantial changes to the transcriptome of the amygdala and hippocampus, of male and female F3-vinclozolin lineage compared with F3-vehicle lineage rats (252). More recently, a transgenerational model of BPA exposure demonstrated differential behavioral outcomes in the F1 offspring (fewer social interactions in BPA- vs vehicle-treated mice) and in the F2 and F4 generations (more social interactions in BPA- than vehicle-treated mice) (114). Transgenerational BPA effects were also seen in expression of mRNA for vasopressin and oxytocin in whole embryonic brains (253). These latter studies are important for several reasons, among which are that they show a transgenerational behavioral phenotype, but also because in the case of Wolstenholme et al (253), this phenotype differed across the generations. This result may at first glance seem counterintuitive, but it should be considered that the F1 generation is directly exposed to the EDC through placental transfer; the F2 generation's exposure occurs to these individuals as germ cells; and the F4 exposure is nonexistent. Thus, the concept of Eg × Ec becomes very important. In the F2 and F4 generations but not F1, most effects are liable to be through germline transmission, whereas the context of exposure differs across the F1, F2, and F4 generations. We also note that although animal models of social behavior may not model autism with true fidelity, such behavioral differences across generations are important first steps in developing preclinical models.
There is a caveat to this work, because to our knowledge, rigorous testing of the Eg × Ec hypothesis has been published in only 2 papers (254, 255). In the first study, which used the vinclozolin transgenerational model, F3 male offspring of the vinclozolin or vehicle lineage were subjected to a significant contextual challenge, that of adolescent stress (254). The behavioral, physiological, metabolic, and transcriptome phenotype of these F3 animals differed by both germline-dependent (Eg) and context-dependent (Ec) epigenetic mechanisms. In a follow-up study (255), substantial sex differences in stress reactivity, transgenerational vinclozolin inheritance, and their interactions, the latter concept referred to as synchronicity due to the complexity of how ancestral inheritance affects proximate stress responses, were observed in male and female rats.
The heritability of Eg can be altered by factors that change DNA methylation or histone modifications. An example of this process is provided by the Agouti-viable yellow mouse (Avy), whose coat color ranges from brown to yellow due to levels of DNA methylation of an inserted retrotransposon in the Agouti gene (256). BPA treatment of pregnant Avy mice caused hypomethylation of the gene, an effect that was mitigated by supplementation of the maternal diet with a methyl donor (folic acid or genistein, a phytoestrogen) (257). Similarly, from the maternal behavior perspective, the effects of different mothering styles of licking/grooming on pup phenotype, an effect caused by histone modifications, was reversed by infusion of a histone deacetylase inhibitor into the brain of the adult offspring (258).
It is worth mentioning that histone modifications and posttranscriptional mechanisms involving microRNAs (259, 260) may have heritability. As for the environment, many factors other than EDCs cause transgenerational epigenetic change. For example, a recent study of maternal and paternal diet and epigenetic change has shown effects on body size and insulin sensitivity in a sex-specific manner, together with alterations in DNA methylation (261, 262).
D. Caveats re the interpretation of data, to date
In the most general terms, the field relating to transgenerational effects of hormones and EDCs on behavior is relatively new, with respect to humans and even with respect to laboratory animals. Studies in this field defy the possibility of full control over all relevant experimental variables as we expect in short-term behavioral and neuroendocrine studies. It is impossible to control for everything in such studies. As a result, particular cautions in ferreting out false-negative as well as false-positive results are strongly warranted.
VII. Outlook Regarding Autism
Our purpose here was to evaluate the possibility that prenatal exposures to hormones, or hormone-disrupting compounds, influence the chance of a child developing autism or other neurocognitive developmental disorders. In addition, epigenetic modifications caused by prenatal hormonal exposures or disruptions, especially if they affect germ cells, can be transmitted to future generations. These latter events have the potential to differentially affect F1, F2, and F3 generations, and beyond, such that behavioral changes can even skip a generation.
At the moment, there is no direct proof that ASDs result from prenatal steroid exposure or are transgenerationally inherited. However, we can ask, in light of the studies reviewed here, what are the chances that certain prenatal exposures influence the development of brain and behavior in such a manner as to affect cognitive and emotional functions? Baron-Cohen and his colleagues (263) have hypothesized that early exposures to testosterone account for the sex difference in autism, and that research group's recent measurements of fetal testosterone support that theory (264, 265). As already discussed, amniotic fluid progesterone, 17-OH progesterone, androstenedione, and testosterone, but not cortisol, are elevated in fetuses who later went on to develop ASDs compared with those that later developed typically (184). One of us (D.P.) has proposed how that testosterone effect may take place: hormonal effects on central nervous system arousal systems ascending from the brainstem to potentiate amygdaloid mechanisms that elevate social anxiety (137), although this model is highly speculative. Clearly, the neuroscience related to early effects of androgenic hormones, along with those of estrogens, progestins, and glucocorticoids, on social behavior, both normal and abnormal, requires further attention.
A review of the current literature on prenatal stress and hypothalamic-pituitary-adrenal (HPA) hormones in the fetus is beyond the scope of this paper, but we mention it briefly here as an important area of research. The epidemiological studies on maternal emotional and physical stress during various periods of gestation are mixed regarding autism outcome (266). Further research is warranted especially relative to the role of HPA activation in early vs late gestation and for acute vs chronic stress in gestation. There is a considerable literature on steroidogenic HPA pathways and autism related to maternal immune activation (267), particularly for the cytokine IL-6, which activates the HPA axis. It is important to note, however, that Baron-Cohen et al (184) did not find increased cortisol in the amniotic fluid samples of fetuses that later developed ASDs. Also, treatment of CAH with cortisol during gestation has tended to decrease rather than increase ASD features at outcome. Therefore, only limited conclusions can be drawn at this time.
We have also reviewed the evidence that all humans are exposed to chronic, low-level mixtures of EDCs. The question then is whether this contributes to physiological and neurobehavioral effects, similar to animal studies. These authors believe that the answer to this question is yes, because the hormonal and neurobiological processes targeted by EDCs are highly conserved across all vertebrates, including humans. It is also notable that the increased incidence of neurobehavioral and cognitive disorders, ASDs, metabolic syndrome, and other disease states exhibit a similar upward trajectory as the increase in chemicals detectable in the environment since the beginning of the chemical revolution after World War II (Figure 3) (268). However, prenatal EDCs are not the only explanation but a contributing factor.
Figure 3.
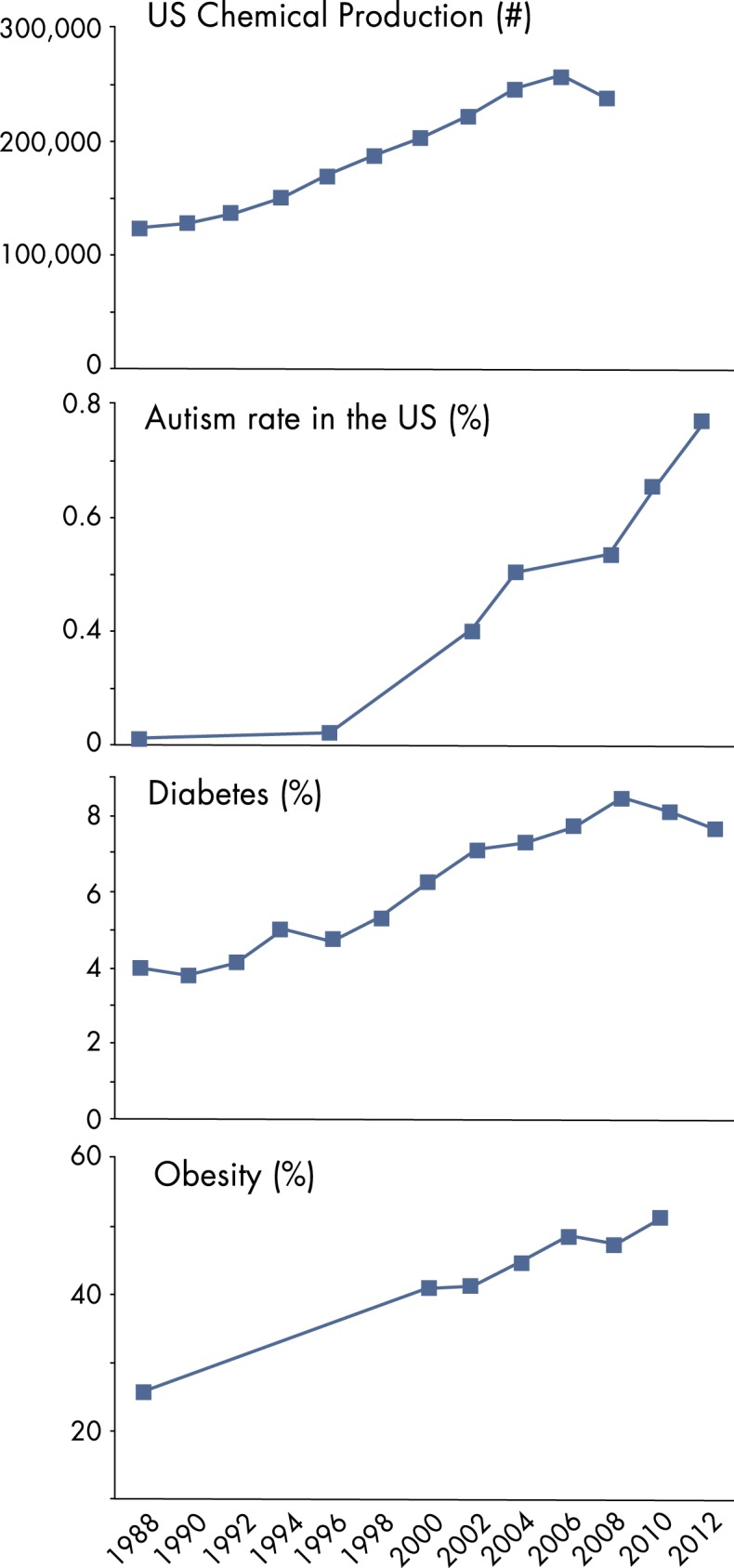
Chemical production and increased disease prevalence since the mid-1980s are shown here. Data were modified from Refs. 273 and 274; http://www.cdc.gov/nchs/hus/contents2012.htm#063; http://www.cdc.gov/diabetes/statistics/incidence/Figure2.htm; and Autism Speaks!/CDC. Data on chemical production were collated by Rachel Delaney (U.S. EPA AWBERC Library) and Robert Sargis, MD, PhD, University of Chicago. To generate data for the chemical production graphic, Dr Sargis used a combination of U.S. Tariff Association data and C&E News production data. [Modified from B. A. Neel and R. M. Sargis: The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes 60:1838, 2011 (273); and C. A. Boyle et al: Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 127:1034, 2011 (274)].
Environmental health scientists suspect that the chemical environment is one such contributing factor in the etiology of autism (269). However, there is little basic or clinical research on the subject. A study conducted on 48 children with ASD measured phthalates and their metabolites in comparison with a reference population of 45 children (270). Significantly higher levels of several metabolites were found in the ASD population, suggesting an association between this class of EDCs. A commentary on this subject proposed that efforts to find the autism gene have revealed that each candidate gene is not likely to account for more than 3% of cases and that even taking all potential genes into consideration only explains about 30% to 40% of this disorder (269). Instead, the authors suggest that it is the environment, and particularly developmental exposures to toxic chemicals including EDCs, that is more likely to explain the upsurge in prevalence. A recent review proposed that autism prevalence is increasing at least in part due to exposures to pesticides (271). These authors also postulated that the male bias in autism prevalence is consistent with animal models of organochlorine pesticide exposures, in which males are more cognitively impaired than females. In light of the correlation over decades between increasing industrial chemical production and increasing rates of ASD diagnoses (Figure 3), further examination of the effects of EDCs clearly is warranted. Sample sizes in the studies reviewed are in some cases quite small, evidence in some cases is weak or indirect, and autism is quite heterogeneous with multiple sources contributing to its manifestation and prevalence. Therefore, this review is not intended to be prescriptive but, instead, is a guide for further study.
Acknowledgments
We thank Professors Bruce McEwen, Isabelle Rapin, and Frances Champagne, who graciously agreed to review and criticize an early draft of this paper. Dr Robert Sargis and Rachel Delaney were extremely helpful in obtaining and providing the chemical production data used in Figure 3.
This work was supported by National Institutes of Health Grants HD05751 (to D.P.), ES020662 (to A.C.G.), and ES023254 (to A.C.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- afT
- amniotic fluid testosterone
- AI
- artificial insemination
- AQ
- autism spectrum quotient
- ART
- assisted reproductive technology
- ASD
- autism-spectrum disorder
- BPA
- bisphenol A
- CAH
- congenital adrenal hyperplasia
- DES
- diethylstilbestrol
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- EDC
- endocrine-disrupting chemical
- HPA
- hypothalamic-pituitary-adrenal
- lncRNA
- long noncoding RNA
- IVF
- in vitro fertilization
- MDI
- mental developmental index
- MRI
- magnetic resonance imaging
- PCB
- polychlorinated biphenyl
- PCOS
- polycystic ovary syndrome
- PDI
- psychomotor developmental index
- SDN-POA
- sexually dimorphic nucleus of the preoptic area
- SLOS
- Smith Lemli Opitz syndrome.
References
- 1. Ansbacher R. Interchangeability of low-dose oral contraceptives. Are current bioequivalent testing measures adequate to ensure therapeutic equivalency? Contraception. 1991;43:139–147. [DOI] [PubMed] [Google Scholar]
- 2. Ansbacher R. Low-dose oral contraceptives: health consequences of discontinuation. Contraception. 2000;62:285–288. [DOI] [PubMed] [Google Scholar]
- 3. Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biegon A, Kim SW, Alexoff DL, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bakker J, De Mees C, Douhard Q, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy MM. Sex and the Developing Brain. San Rafael, CA: Morgan & Claypool Life Sciences; 2011. [Google Scholar]
- 7. Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 8. Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. [DOI] [PubMed] [Google Scholar]
- 9. de Vries GJ, Södersten P. Sex differences in the brain: the relation between structure and function. Horm Behav. 2009;55:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. [DOI] [PubMed] [Google Scholar]
- 11. Gorski RA. Hypothalamic imprinting by gonadal steroid hormones. Adv Exp Med Biol. 2002;511:57–70; discussion 70–73. [DOI] [PubMed] [Google Scholar]
- 12. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann Rev Neurosci. 2002;25:507–536. [DOI] [PubMed] [Google Scholar]
- 13. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. [DOI] [PubMed] [Google Scholar]
- 14. Roselli CE, Resko JA, Stormshak F. Expression of steroid hormone receptors in the fetal sheep brain during the critical period for sexual differentiation. Brain Res. 2006;1110:76–80. [DOI] [PubMed] [Google Scholar]
- 15. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci. 1984;7:413–442. [DOI] [PubMed] [Google Scholar]
- 16. Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. [DOI] [PubMed] [Google Scholar]
- 17. Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. [DOI] [PubMed] [Google Scholar]
- 18. Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. [DOI] [PubMed] [Google Scholar]
- 19. Arnold A. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the 20th century, with special reference to the brain. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, eds. Hormones, Brain and Behavior. Vol 4 San Diego, CA: Academic Press; 2002:105–136. [Google Scholar]
- 20. De Vries GJ, Simerly R. Anatomy, development and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, eds. Hormones, Brain and Behavior. Vol 4 San Diego, CA: Academic Press; 2002:137–191. [Google Scholar]
- 21. McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. [DOI] [PubMed] [Google Scholar]
- 22. McCarthy MM, Wright CL, Konkle ATM. Aromatase and sexual differentiation of the rodent brain. The old, the new and the unexpected In: Balthazart J, Ball GF, eds. Brain Aromatase, Estrogens and Behavior. New York, NY: Oxford University Press; 2012:315–336. [Google Scholar]
- 23. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. [DOI] [PubMed] [Google Scholar]
- 25. Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Cohen-Kettenis PT. Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology. 2004;29:911–916. [DOI] [PubMed] [Google Scholar]
- 26. Nopoulos P, Flaum M, O'Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. [DOI] [PubMed] [Google Scholar]
- 27. Swaab DF. Development of the human hypothalamus. Neurochem Res. 1995;20:509–519. [DOI] [PubMed] [Google Scholar]
- 28. Duarte-Carvajalino JM, Jahanshad N, Lenglet C, et al. Hierarchical topological network analysis of anatomical human brain connectivity and differences related to sex and kinship. NeuroImage. 2012;59:3784–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23:519–547. [DOI] [PubMed] [Google Scholar]
- 30. Myren M, Mose T, Mathiesen L, Knudsen LE. The human placenta–an alternative for studying foetal exposure. Toxicology In Vitro. 2007;21:1332–1340. [DOI] [PubMed] [Google Scholar]
- 31. Goodman A, Schorge J, Greene MF. The long-term effects of in utero exposures–the DES story. N Engl J Med. 2011;364:2083–2084. [DOI] [PubMed] [Google Scholar]
- 32. Strohsnitter WC, Noller KL, Hoover RN, et al. Cancer risk in men exposed in utero to diethylstilbestrol. J Natl Cancer Inst. 2001;93:545–551. [DOI] [PubMed] [Google Scholar]
- 33. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kavlock RJ, Daston GP, DeRosa C, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996;104(Suppl 4):715–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds. State of the Science of Endocrine Disrupting Chemicals. Geneva, Switzerland: United Nations Environment Programme and the World Health Organization; 2013. [Google Scholar]
- 36. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Rev. 2009;30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agras K, Shiroyanagi Y, Baskin LS. Progesterone receptors in the developing genital tubercle: implications for the endocrine disruptor hypothesis as the etiology of hypospadias. J Urol. 2007;178:722–727. [DOI] [PubMed] [Google Scholar]
- 41. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Environmental Health; 2009. [Google Scholar]
- 42. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Environmental Health; 2005. [Google Scholar]
- 43. Skakkebaek NE, Toppari J, Söder O, Gordon CM, Divall S, Draznin M. The exposure of fetuses and children to endocrine disrupting chemicals: a European Society for Paediatric Endocrinology (ESPE) and Pediatric Endocrine Society (PES) call to action statement. J Clin Endocrinol Metab. 2011;96:3056–3058. [DOI] [PubMed] [Google Scholar]
- 44. Meeker JD. Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med. 2012;166:952–958. [PubMed] [Google Scholar]
- 45. Li W, Chen B, Ding X. Environment and reproductive health in China: challenges and opportunities. Environ Health Perspect. 2012;120:A184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- 47. Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lang IA, Galloway TS, Scarlett A, et al. Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults. JAMA. 2008;300:1303–1310. [DOI] [PubMed] [Google Scholar]
- 51. Chevrier C, Limon G, Monfort C, et al. Urinary biomarkers of prenatal atrazine exposure and adverse birth outcomes in the PELAGIE birth cohort. Environ Health Perspect. 2011;119:1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harley KG, Gunier RB, Kogut K, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013;126:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. [DOI] [PubMed] [Google Scholar]
- 54. Trasande L, Attina TM, Trachtman H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013;83:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132:e646–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gerona RR, Woodruff TJ, Dickenson CA, et al. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47:12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Minh TB, Watanabe M, Kajiwara N, et al. Human blood monitoring program in Japan: Contamination and bioaccumulation of persistent organochlorines in Japanese residents. Arch Environ Contam Toxicol. 2006;51:296–313. [DOI] [PubMed] [Google Scholar]
- 58. Gladen BC, Doucet J, Hansen LG. Assessing human polychlorinated biphenyl contamination for epidemiologic studies: lessons from patterns of congener concentrations in Canadians in 1992. Environ Health Perspect. 2003;111:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buck Louis GM, Sundaram R, Schisterman EF, et al. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. Prenatal exposure to persistent organochlorines and childhood obesity in the US collaborative perinatal project. Environ Health Perspect. 2013;121:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim Y, Ha EH, Kim EJ, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children's Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swan SH, Main KM, Liu F, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Whyatt RM, Camann DE, Kinney PL, et al. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2014;101:1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grandjean P, Bellinger D, Bergman A, et al. The Faroes Statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102:73–75. [DOI] [PubMed] [Google Scholar]
- 67. Quinn CL, Wania F, Czub G, Breivik K. Investigating intergenerational differences in human PCB exposure due to variable emissions and reproductive behaviors. Environ Health Perspect. 2011;119:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters–what we have learned from Yusho disease. Environ Res. 2001;86:2–11. [DOI] [PubMed] [Google Scholar]
- 69. Pesatori AC, Consonni D, Bachetti S, et al. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “Seveso Accident.” Ind Health. 2003;41:127–138. [DOI] [PubMed] [Google Scholar]
- 70. Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. [DOI] [PubMed] [Google Scholar]
- 71. Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pravettoni A, Colciago A, Negri-Cesi P, Villa S, Celotti F. Ontogenetic development, sexual differentiation, and effects of Aroclor 1254 exposure on expression of the arylhydrocarbon receptor and of the arylhydrocarbon receptor nuclear translocator in the rat hypothalamus. Reprod Toxicol. 2005;20:521–530. [DOI] [PubMed] [Google Scholar]
- 73. Hany J, Lilienthal H, Sarasin A, et al. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. [DOI] [PubMed] [Google Scholar]
- 74. Lichtensteiger W, Faass O, Ma R, Schlumpf M. Effect of polybrominated diphenylether and PCB on the development of the brain–gonadal axis and gene expression in rats. Organohalog Compd. 2003;61:84–87. [Google Scholar]
- 75. Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011;152:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011;252:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Mol Endocrinol. 2014;28:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ikeda M, Mitsui T, Setani K, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005;205:98–105. [DOI] [PubMed] [Google Scholar]
- 79. Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. [DOI] [PubMed] [Google Scholar]
- 80. Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. [DOI] [PubMed] [Google Scholar]
- 81. Colciago A, Negri-Cesi P, Pravettoni A, Mornati O, Casati L, Celotti F. Prenatal Aroclor 1254 exposure and brain sexual differentiation: effect on the expression of testosterone metabolizing enzymes and androgen receptors in the hypothalamus of male and female rats. Reprod Toxicol. 2006;22:738–745. [DOI] [PubMed] [Google Scholar]
- 82. Shibutani M, Masutomi N, Uneyama C, et al. Down-regulation of GAT-1 mRNA expression in the microdissected hypothalamic medial preoptic area of rat offspring exposed maternally to ethinylestradiol. Toxicology. 2005;208:35–48. [DOI] [PubMed] [Google Scholar]
- 83. Salama J, Chakraborty TR, Ng L, Gore AC. Effects of polychlorinated biphenyls on female reproductive development and estrogen receptor-beta expressionin the anteroventral periventricular nucleus. Environ Health Perspect. 2003;111:1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor alpha transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. [DOI] [PubMed] [Google Scholar]
- 86. Connors SL, Levitt P, Matthews SG, et al. Fetal mechanisms in neurodevelopmental disorders. Pediatr Neurol. 2008;38:163–176. [DOI] [PubMed] [Google Scholar]
- 87. Lein PJ, Yang D, Bachstetter AD, et al. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect. 2007;115:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Willingham EJ. Trenbolone and other cattle growth promoters: need for a new risk-assessment framework. Environ Pract. 2006;8:58–65. [Google Scholar]
- 89. Besse JP, Garric J. Progestagens for human use, exposure and hazard assessment for the aquatic environment. Environ Pollut. 2009;157:3485–3494. [DOI] [PubMed] [Google Scholar]
- 90. Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998;28:319–361 [DOI] [PubMed] [Google Scholar]
- 91. Kang JH, Asai D, Katamaya Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit Rev Toxicol. 2007;37:607–625. [DOI] [PubMed] [Google Scholar]
- 92. Zala SM, Penn DJ. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim Behav. 2004;68:649–664. [Google Scholar]
- 93. Kidd KA, Blanchfield PJ, Mills KH, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci U S A. 2007;104:8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME. Mechanistic basis of resistance to PCBs in Atlantic tomcod from the Hudson River. Science. 2011;331:1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RI. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen Comp Endocrinol. 2008;155:31–62. [DOI] [PubMed] [Google Scholar]
- 96. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose response. Endocr Rev. 2012;33:378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barker DJ. The developmental origins of adult disease. Eur J Epidemiol. 2003;18:733–736. [DOI] [PubMed] [Google Scholar]
- 98. Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. [DOI] [PubMed] [Google Scholar]
- 99. Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. [DOI] [PubMed] [Google Scholar]
- 100. Paris JJ, Brunton PJ, Russell JA, Walf AA, Frye CA. Inhibition of 5α-reductase activity in late pregnancy decreases gestational length and fecundity and impairs object memory and central progestogen milieu of juvenile rat offspring. J Neuroendocrinol. 2011;23:1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. [DOI] [PubMed] [Google Scholar]
- 103. Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. [DOI] [PubMed] [Google Scholar]
- 104. Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog Brain Res. 2002;139:15–29. [DOI] [PubMed] [Google Scholar]
- 105. Lonstein JS, Rood BD, De Vries GJ. Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology. 2005;146:1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54:502–510. [DOI] [PubMed] [Google Scholar]
- 107. Jurkevich A, Grossmann R, Balthazart J, Viglietti-Panzica C. Gender-related changes in the avian vasotocin system during ontogeny. Microsc Res Tech. 2001;55:27–36. [DOI] [PubMed] [Google Scholar]
- 108. Carter DA, Saridaki E, Lightman SL. Sexual differentiation of oxytocin stress responsiveness: effect of neonatal androgenization, castration and a luteinizing hormone-releasing hormone antagonist. Acta Endocrinol. 1988;117:525–530. [DOI] [PubMed] [Google Scholar]
- 109. Auger AP, Olesen KM. Brain sex differences and the organisation of juvenile social play behaviour. J Neuroendocrinol. 2009;21:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. 2001;109:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. [DOI] [PubMed] [Google Scholar]
- 112. Weiss B. Sexually dimorphic nonreproductive behaviors as indicators of endocrine disruption. Environ Health Perspect. 2002;110(Suppl 3):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–439. [DOI] [PubMed] [Google Scholar]
- 114. Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLos One. 2011;6:e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jašarevic E, Sieli PT, Twellman EE, et al. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A. 2011;108:11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Colciago A, Casati L, Mornati O, et al. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat. Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. [DOI] [PubMed] [Google Scholar]
- 118. Engell MD, Godwin J, Young LJ, Vandenbergh JG. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol. 2006;28:103–110. [DOI] [PubMed] [Google Scholar]
- 119. Patisaul HB, Sullivan AW, Radford ME, et al. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLos One. 2012;7:e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. [DOI] [PubMed] [Google Scholar]
- 122. Carbone S, Ponzo OJ, Gobetto N, et al. Antiandrogenic effect of perinatal exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate increases anxiety-like behavior in male rats during sexual maturation. Horm Behav. 2013;63:692–699. [DOI] [PubMed] [Google Scholar]
- 123. Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett. 2010;199:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ehrhardt AA, Meyer-Bahlburg HF. Effects of prenatal sex hormones on gender-related behavior. Science. 1981;211:1312–1318. [DOI] [PubMed] [Google Scholar]
- 125. Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–10. [DOI] [PubMed] [Google Scholar]
- 127. Wizemann TM, Pardue ML. Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: Board on Health Sciences Policy, Institute of Medicine; 2001. [PubMed] [Google Scholar]
- 128. Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–789. [DOI] [PubMed] [Google Scholar]
- 129. Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J Pediatr. 1999;134:33–41. [DOI] [PubMed] [Google Scholar]
- 130. Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to PCBs and infant performance on the fagan test of infant intelligence. Neurotoxicology. 2000;21:1029–1038. [PubMed] [Google Scholar]
- 131. Lai TJ, Guo YL, Guo NW, Hsu CC. Effect of prenatal exposure to polychlorinated biphenyls on cognitive development in children: a longitudinal study in Taiwan. Br J Psychiatry Suppl. 2001;40:s49–s52. [DOI] [PubMed] [Google Scholar]
- 132. Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, Van der Paauw CG, Tuinstra LG, Sauer PJ. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants' mental and psychomotor development. Pediatrics. 1996;97:700–706. [PubMed] [Google Scholar]
- 133. Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol. 2012;34:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Braun JM, Kalkbrenner AE, Just AC, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME Study. Environ Health Perspect. 2014;122:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Shelton JF, Geraghty EM, Tancredi DJ, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE Study. Environ Health Perspect. 2014;122:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Res. 1981;5:129–137. [DOI] [PubMed] [Google Scholar]
- 137. Pfaff DW, Rapin I, Goldman S. Male predominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res. 2011;4:163–176. [DOI] [PubMed] [Google Scholar]
- 138. Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. [DOI] [PubMed] [Google Scholar]
- 139. Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 2006;21:825–845. [DOI] [PubMed] [Google Scholar]
- 140. Khan A, Harney JW, Zavacki AM, Sajdel-Sulkowska EM. Disrupted brain thyroid hormone homeostasis and altered thyroid hormone-dependent brain gene expression in autism spectrum disorders. J Physiol Pharmacol. 2014;65:257–272. [PubMed] [Google Scholar]
- 141. Andersen S, Laurberg P, Wu C, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121:1365–1374. [DOI] [PubMed] [Google Scholar]
- 142. Wudy SA, Dörr HG, Solleder C, Djalali M, Homoki J. Profiling steroid hormones in amniotic fluid of midpregnancy by routine stable isotope dilution/gas chromatography-mass spectrometry: reference values and concentrations in fetuses at risk for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84:2724–2728. [DOI] [PubMed] [Google Scholar]
- 143. Azziz R, Slayden SM. The 21-hydroxylase-deficient adrenal hyperplasias: more than ACTH oversecretion. J Soc Gynecol Investig. 1996;3:297–302. [PubMed] [Google Scholar]
- 144. Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. [DOI] [PubMed] [Google Scholar]
- 145. Hines M, Brook C, Conway GS. Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). J Sex Res. 2004;41:75–81. [DOI] [PubMed] [Google Scholar]
- 146. Hines M. Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Front Neuroendocrinol. 2011;32:170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Knickmeyer R, Baron-Cohen S, Fane BA, et al. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm Behav. 2006;50:148–153. [DOI] [PubMed] [Google Scholar]
- 148. Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychol Sci. 1992;3:203–206. [Google Scholar]
- 149. Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior: rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH). Child Dev. 1994;65:1042–1053. [PubMed] [Google Scholar]
- 150. Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. [DOI] [PubMed] [Google Scholar]
- 151. McGuire LS, Ryan KO, Omenn GS. Congenital adrenal hyperplasia. II. Cognitive and behavioral studies. Behav Genet. 1975;5:175–188. [DOI] [PubMed] [Google Scholar]
- 152. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. [DOI] [PubMed] [Google Scholar]
- 153. Rutter M. Diagnosis and definition of childhood autism. J Autism Child Schizophr. 1978;8:139–161. [DOI] [PubMed] [Google Scholar]
- 154. Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J Autism Dev Disord. 1979;9:11–29. [DOI] [PubMed] [Google Scholar]
- 155. Palomba S, Marotta R, Di Cello A, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf). 2012;77:898–904. [DOI] [PubMed] [Google Scholar]
- 156. Ehrhardt AA, Grisanti GC, Meyer-Bahlburg HF. Prenatal exposure to medroxyprogesterone acetate (MPA) in girls. Psychoneuroendocrinology. 1977;2:391–398. [DOI] [PubMed] [Google Scholar]
- 157. Reinisch JM. Prenatal exposure of human foetuses to synthetic progestin and oestrogen affects personality. Nature. 1977;266:561–562. [DOI] [PubMed] [Google Scholar]
- 158. Middelburg KJ, Heineman MJ, Bos AF, Hadders-Algra M. Neuromotor, cognitive, language and behavioural outcome in children born following IVF or ICSI-a systematic review. Hum Reprod Update. 2008;14:219–231. [DOI] [PubMed] [Google Scholar]
- 159. Maimburg RD, Vaeth M. Do children born after assisted conception have less risk of developing infantile autism? Hum Reprod. 2007;22:1841–1843. [DOI] [PubMed] [Google Scholar]
- 160. Hvidtjørn D, Grove J, Schendel D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2011;65:497–502. [DOI] [PubMed] [Google Scholar]
- 161. Lyall K, Pauls DL, Santangelo SL, Spiegelman D, Ascherio A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. J Autism Dev Disord. 2011;41:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the Nurses' Health Study II. Paediatr Perinat Epidemiol. 2012;26:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kelley RI, Hennekam RC. Smith-Lemli-Opitz syndrome. In: Scriver CR, Beudet AL, Valle D, Sly WS, eds. The Metabolic and Molecular Basis of Inherited Disease. New York, NY: McGraw-Hill; 2001:6183–6201. [Google Scholar]
- 164. Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol-7-reductase deficiency (Smith-Lemli-Opitz syndrome) to neurosteroid production. Steroids. 2004;69:51–60. [DOI] [PubMed] [Google Scholar]
- 165. Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet Part A. 2006;140:1511–1518. [DOI] [PubMed] [Google Scholar]
- 166. Whitehouse AJ, Maybery MT, Hart R, et al. Fetal androgen exposure and pragmatic language ability of girls in middle childhood: implications for the extreme male-brain theory of autism. Psychoneuroendocrinology. 2010;35:1259–1264. [DOI] [PubMed] [Google Scholar]
- 167. Jacklin CN, Maccoby EE, Doering CH. Neonatal sex-steroid hormones and timidity in 6–18-month-old boys and girls. Dev Psychobiol. 1983;16:163–168. [DOI] [PubMed] [Google Scholar]
- 168. Jacklin CN, Wilcox KT, Maccoby EE. Neonatal sex-steroid hormones and cognitive abilities at six years. Dev Psychobiol. 1988;21:567–574. [DOI] [PubMed] [Google Scholar]
- 169. Marcus J, Maccoby EE, Jacklin CN, Doering CH. Individual differences in mood in early childhood: their relation to gender and neonatal sex steroids. Dev Psychobiol. 1985;18:327–340. [DOI] [PubMed] [Google Scholar]
- 170. Hines M, Golombok S, Rust J, Johnston KJ, Golding J. Testosterone during pregnancy and gender role behavior of preschool children: a longitudinal, population study. Child Dev. 2002;73:1678–1687. [DOI] [PubMed] [Google Scholar]
- 171. Finegan JA, Bartleman B, Wong PY. A window for the study of prenatal sex hormone influences on postnatal development. J Genet Psychol. 1989;150:101–112. [DOI] [PubMed] [Google Scholar]
- 172. Finegan JA, Niccols GA, Sitarenios G. Relations between prenatal testosterone levels and cognitive abilities at 4 years. Dev Psychol. 1992;28:1075–1089. [Google Scholar]
- 173. Grimshaw GM, Bryden MP, Finegan JA. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsychology. 1995;9:68–79. [Google Scholar]
- 174. Grimshaw GM, Sitarenios G, Finegan JA. Mental rotation at 7 years: relations with prenatal testosterone levels and spatial play experiences. Brain Cogn. 1995;29:85–100. [DOI] [PubMed] [Google Scholar]
- 175. Escalante-Mead PR, Minshew NJ, Sweeney JA. Abnormal brain lateralization in high-functioning autism. J Autism Dev Disord. 2003;33:539–543. [DOI] [PubMed] [Google Scholar]
- 176. Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behav Dev. 2002;25:327–335. [Google Scholar]
- 177. Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46:198–210. [DOI] [PubMed] [Google Scholar]
- 178. Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K, Hackett G. Fetal testosterone and empathy. Horm Behav. 2006;49:282–292. [DOI] [PubMed] [Google Scholar]
- 179. Auyeung B, Baron-Cohen S, Ashwin E, et al. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci. 2009;20:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and autistic traits. Br J Psychol. 2009;100:1–22. [DOI] [PubMed] [Google Scholar]
- 181. Auyeung B, Taylor K, Hackett G, Baron-Cohen S. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol Autism. 2010;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Auyeung B, Knickmeyer R, Ashwin E, Taylor K, Hackett G, Baron-Cohen S. Effects of fetal testosterone on visuospatial ability. Arch Sex Behav. 2012;41:571–581. [DOI] [PubMed] [Google Scholar]
- 183. White SJ, Saldaña D. Performance of children with autism on the Embedded Figures Test: a closer look at a popular task. J Autism Dev Disord. 2011;41:1565–1572. [DOI] [PubMed] [Google Scholar]
- 184. Baron-Cohen S, Auyeung B, Norgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. doi: 10.1038/mp.2014.48. [Epub ahead of print, June 3, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pfaff DW, Phillips I, Rubin R. Principles of Hormone/Behavior Relations. San Diego, CA: Academic Press/Elsevier; 2004. [Google Scholar]
- 186. Gagnidze K, Pfaff DW. Epigenetic mechanisms: DNA methylation and histone protein modification. In: Pfaff DW, ed. Neuroscience in the 21st Century. New York, NY: Springer; 2013:1–55. [Google Scholar]
- 187. Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. [DOI] [PubMed] [Google Scholar]
- 188. Turner BM. Histone acetylation and an epigenetic code. BioEssays. 2000;22:836–845. [DOI] [PubMed] [Google Scholar]
- 189. Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. [DOI] [PubMed] [Google Scholar]
- 190. Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–849. [DOI] [PubMed] [Google Scholar]
- 191. Koshland D, Strunnikov A. Mitotic chromosome condensation. Annu Rev Cell Dev Biol. 1996;12:305–333. [DOI] [PubMed] [Google Scholar]
- 192. Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991;65:775–783. [DOI] [PubMed] [Google Scholar]
- 193. Wolffe AP, Hayes JJ. Chromatin disruption and modification. Nucleic Acids Res. 1999;27:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. [DOI] [PubMed] [Google Scholar]
- 195. Latham JA, Dent SY. Cross-regulation of histone modifications. Nat Struct Mol Biol. 2007;14:1017–1024. [DOI] [PubMed] [Google Scholar]
- 196. Gagnidze K, Pfaff DW. Hormone-dependent chromatin modifications regulating sexually differentiated animal behavior. In: Pfaff DW, Christen Y, eds. Multiple Origins of Sex Differences in Brain: Neuroendocrine Functions and their Pathologies. New York, NY: Springer; 2013:1–20. [Google Scholar]
- 197. Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Murray EK, Hien A, de Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Murray EK, Varnum MM, Fernandez JL, de Vries GJ, Forger NG. Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J Neuroendocrinol. 2011;23:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Jašarevic E, Geary DC, Rosenfeld CS. Sexually selected traits: a fundamental framework for studies on behavioral epigenetics. ILAR J. 2012;53:253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Auger AP, Auger CJ. Epigenetic turn ons and turn offs: chromatin reorganization and brain differentiation. Endocrinology. 2011;152:349–353. [DOI] [PubMed] [Google Scholar]
- 203. Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav. 2011;59:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011;25:2157–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–1655. [DOI] [PubMed] [Google Scholar]
- 209. Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Saxena A, Carninci P. Long non-coding RNA modifies chromatin: epigenetic silencing by long non-coding RNAs. BioEssays. 2011;33:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Chaumeil J, Le Baccon P, Wutz A, Heard E. A novel role for Xist RNA in the formation of a repressive nuclear compartment into which genes are recruited when silenced. Genes Dev. 2006;20:2223–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Coufal NG, Garcia-Perez JL, Peng GE, et al. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460:1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Baillie JK, Barnett MW, Upton KR, et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature. 2011;479:534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Muotri AR, Marchetto MC, Coufal NG, et al. L1 retrotransposition in neurons is modulated by MeCP2. Nature. 2010;468:443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Singer T, McConnell MJ, Marchetto MC, Coufal NG, Gage FH. LINE-1 retrotransposons: mediators of somatic variation in neuronal genomes? Trends Neurosci. 2010;33:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. [DOI] [PubMed] [Google Scholar]
- 220. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Wong CC, Meaburn EL, Ronald A, et al. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Aldinger KA, Plummer JT, Levitt P. Comparative DNA methylation among females with neurodevelopmental disorders and seizures identifies TAC1 as a MeCP2 target gene. J Neurodev Disord. 2013;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, eds. Hormones, Brain and Behavior. San Diego, CA: Academic Press/Elsevier; 2009. [Google Scholar]
- 227. Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-α and -β knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104:4670–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr Opin Neurobiol. 2013;23:11–16. [DOI] [PubMed] [Google Scholar]
- 230. Gregory SG, Connelly JJ, Towers AJ, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Mamrut S, Harony H, Sood R, et al. DNA methylation of specific CpG sites in the promoter region regulates the transcription of the mouse oxytocin receptor. PLoS One. 2013;8:e56869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci. 2012;6:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rockman MV. The QTN program and the alleles that matter for evolution: all that's gold does not glitter. Evolution. 2012;66:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Crews D, Gore AC. Epigenetic synthesis: A need for a new paradigm for evolution in a contaminated world. F1000 Biol Rep. 2012;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Walker DM, Gore AC. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol. 2011;7:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat Rev Genet. 2011;12:475–486. [DOI] [PubMed] [Google Scholar]
- 237. Cameron NM, Shahrokh D, Del Corpo A, et al. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol. 2008;20:795–801. [DOI] [PubMed] [Google Scholar]
- 238. Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. [DOI] [PubMed] [Google Scholar]
- 239. Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biol Psychiatry. 2002;52:1037–1046. [DOI] [PubMed] [Google Scholar]
- 241. Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. Behavioral and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: impact is dependent on familial factors. Psychoneuroendocrinology. 2007;32:331–349. [DOI] [PubMed] [Google Scholar]
- 242. Yehuda R, Morris A, Labinsky E, Zemelman S, Schmeidler J. Ten-year follow-up study of cortisol levels in aging holocaust survivors with and without PTSD. J Trauma Stress. 2007;20:757–761. [DOI] [PubMed] [Google Scholar]
- 243. Yehuda R, Teicher MH, Seckl JR, Grossman RA, Morris A, Bierer LM. Parental posttraumatic stress disorder as a vulnerability factor for low cortisol trait in offspring of holocaust survivors. Arch Gen Psychiatr. 2007;64:1040–1048. [DOI] [PubMed] [Google Scholar]
- 244. Matthews SG, Phillips DI. Transgenerational inheritance of stress pathology. Exp Neurol. 2012;233:95–101. [DOI] [PubMed] [Google Scholar]
- 245. Gräff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav Brain Res. 2008;192:70–87. [DOI] [PubMed] [Google Scholar]
- 246. Bohacek J, Gapp K, Saab BJ, Mansuy IM. Transgenerational epigenetic effects on brain functions. Biol Psychiatry. 2013;73:313–320. [DOI] [PubMed] [Google Scholar]
- 247. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Roemer I, Reik W, Dean W, Klose J. Epigenetic inheritance in the mouse. Curr Biol. 1997;7:277–280. [DOI] [PubMed] [Google Scholar]
- 249. Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379. [DOI] [PubMed] [Google Scholar]
- 250. Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. [DOI] [PubMed] [Google Scholar]
- 251. Crews D, Gore AC, Hsu TS, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012;109:9143–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology. 2014;155:3853–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Nadeau JH. Transgenerational genetic effects on phenotypic variation and disease risk. Hum Mol Genet. 2009;18:R202–R210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Ho DH, Burggren WW. Epigenetics and transgenerational transfer: a physiological perspective. J Exp Biol. 2010;213:3–16. [DOI] [PubMed] [Google Scholar]
- 261. Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology. 2011;152:2228–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm Behav. 2007;51:597–604. [DOI] [PubMed] [Google Scholar]
- 264. Auyeung B, Ahluwalia J, Thomson L, et al. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol Autism. 2012;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. In: proceedings from the International Meeting for Autism Research, 2012; Toronto, Ontario, Canada Abstract 104.002. [Google Scholar]
- 266. Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161:326–333. [DOI] [PubMed] [Google Scholar]
- 267. Abdallah MW, Larsen N, Grove J, et al. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav Immun. 2012;26:170–176. [DOI] [PubMed] [Google Scholar]
- 268. Crews D, Gore AC. Life imprints: living in a contaminated world. Environ Health Perspect. 2011;119:1208–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120:a258–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Testa C, Nuti F, Hayek J, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect. 2012;120:944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition, 2000.
- 273. Neel BA, Sargis RM. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes. 2011;60:1838–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. [DOI] [PubMed] [Google Scholar]
- 275. Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. [DOI] [PubMed] [Google Scholar]
- 276. Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. [DOI] [PubMed] [Google Scholar]
- 278. O'Carroll D, Schaefer A. General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology. 2013;38:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Winneke G, Ranft U, Wittsiepe J, et al. Behavioral sexual dimorphism in school-age children and early developmental exposure to dioxins and PCBs: a follow-up study of the Duisburg Cohort. Environ Health Perspect. 2014;122:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]