Abstract
Sodium nitroprusside has been used in clinical practice as an arterial and venous vasodilator for 40 years. This prodrug reacts with physiologic sulfhydryl groups to release nitric oxide, causing rapid vasodilation, and acutely lowering blood pressure. It is used clinically in cardiac surgery, hypertensive crises, heart failure, vascular surgery, pediatric surgery, and other acute hemodynamic applications. In some practices, newer agents have replaced nitroprusside, either because they are more effective or because they have a more favorable side-effect profile. However, valid and adequately-powered efficacy studies are sparse and do not identify a superior agent for all indications. The cyanide anion release concurrent with nitroprusside administration is associated with potential cyanide accumulation and severe toxicity. Agents to ameliorate the untoward effects of cyanide are limited by various problems in their practicality and effectiveness. A new orally bioavailable antidote is sodium sulfanegen, which shows promise in reversing this toxicity. The unique effectiveness of nitroprusside as a titratable agent capable of rapid blood pressure control will likely maintain its utilization in clinical practice for the foreseeable future. Additional research will refine and perhaps expand indications for nitroprusside, while parallel investigation continues to develop effective antidotes for cyanide poisoning.
Keywords: Antihypertensives, cyanide, pharmacology, sodium nitroprusside, toxicity
Introduction and History
Sodium nitroprusside (SNP) is a well-known arterial and venous vasodilator used in clinical practice to lower blood pressure. Initially discovered in 1849 by Playfair,[1] SNP's first reported use in a patient was by Johnson in 1922.[2] Its safety and efficacy in lowering blood pressure when given intravenously in severely hypertensive patients was established in 1955.[3] After its successful use as an intraoperative antihypertensive in 1970,[4] it quickly gained acceptance as a fast-acting agent useful to reduce intraoperative hypertension, induce hypotension to minimize surgical blood loss, and decrease afterload and improve cardiac output in heart failure. It has been used clinically in cardiac surgery, hypertensive crises, heart failure, vascular surgery, pediatric surgery, and other acute applications.
However, reports began to surface associating nitroprusside and cyanide toxicity,[5,6,7,8] with the food and drug administration (FDA) issuing new labeling emphasizing this risk in 1991.[9] In some practices newer agents [including nitroglycerin, calcium channel blockers, β-blockers, and dopaminergic agonists, [Table 1] replaced SNP, either because they were recognized to be more arterial selective, or because of a more favorable side-effect profile. Despite the risks, nitroprusside has continued to be used in many of the above settings and others for its potent and fast-acting vasodilatory properties. In addition, the ongoing threat of cyanide as a chemical warfare agent in bioterrorism continues to fuel research to reverse or prevent cyanide poisoning, and thus by association retains an interest in nitroprusside.
Table 1.
Comparison of systemic vasodilators available for the control of perioperative hypertension
The last prominent review of SNP was by Friederich and Butterworth in 1995.[10] Since then, new research has deepened the understanding of its mechanism of action, refined its clinical application by comparing it to newer vasodilators, further elaborated its adverse effects and safety profile, and offered promise for reversing its significant potential toxicity.
Now 40 years since nitroprusside's widespread adoption and almost 20 years since its last thorough review, we summarize the new salient developments for this agent. Our goal is to provide clinicians with a comprehensive, updated benefit-to-risk understanding of the current use of nitroprusside in clinical practice. In addition, we provide newer experimental data of an antidote for cyanide toxicity, which may lead to an expanded role of nitroprusside in the future.
Mechanism of Action and Hemodynamic Effects
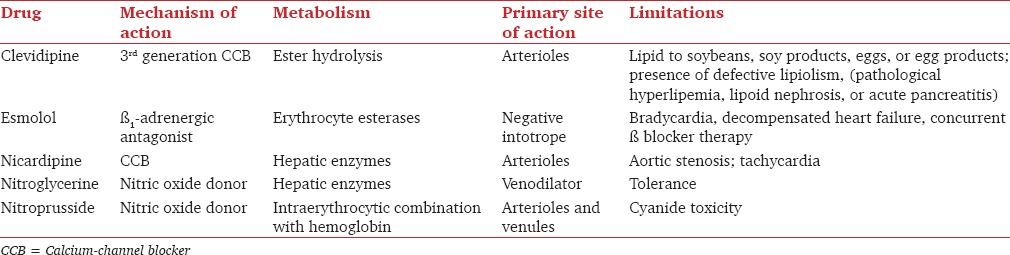
Sodium nitroprusside is a water-soluble sodium salt comprised of Fe2+ complexed with nitric oxide (NO) and five cyanide anions [Figure 1]. In the body it functions as a prodrug, reacting with sulfhydryl groups on erythrocytes, albumin, and other proteins to release NO.[11] NO, or endothelium derived relaxing factor, stimulates guanyl cyclase to produce cyclic GMP, sequestering calcium and inhibiting cellular contraction.[12] At the tissue level, these effects of NO result in reduced vascular tone in muscular conduit arteries.[13] NO released from nitroprusside decreases cerebral vascular resistance, and in a canine study it has been shown to impair brain and myocardial tissue oxygenation due to increase in arterial-venous shunting.[14] It decreases coronary flow reserve, which is the basis for the theory that nitroprusside can cause coronary steal syndrome, discussed further below.[15]
Figure 1.
The sodium nitroprusside molecule is a sodium salt consisting of Fe complexed with five cyanide anions
The role of NO in the coagulation system and platelet function raised the concern that nitroprusside and other NO releasing drugs may affect coagulation, at least in theory.[16] A few studies showed the ability of nitroprusside to inhibit platelet aggregation in vitro[17] and in vivo.[18] One study showed increased intraoperative blood loss in spinal surgery with nitroprusside compared with nicardipine, but the authors did not believe that this was necessarily due to any effect on platelets but rather might be explained by increased venous congestion.[19] The clinical significance, if any, of nitroprusside administration on bleeding remains unproven.
Globally, the net hemodynamic effect of nitroprusside is to cause arterial and venous dilatation, reduce afterload, decrease ventricular filling pressures, lower the systemic blood pressure, and increase cardiac output, without significant lowering of the heart rate. These properties, together with nitroprusside's rapid onset and ability to be titrated to a target blood pressure, make the agent highly effective in situations where rapid blood pressure lowering is indicated.
Clinical Use, Efficacy, and Comparative Advantages of Nitroprusside
Dosing and administration
Sodium nitroprusside is typically started as an intravenous infusion of 0.5 μg/kg/min and titrated to effect, with a maximum dose of 10 μg/kg/min (for short periods to establish blood pressure control). Limited data about pediatric dosing suggests that infusion rates should remain below 2 μg/kg/min, and reserve higher doses for short periods to establish urgent blood pressure control.[20,21] SNP acts within minutes and is effective in clinical situations where urgent lowering of blood pressure is needed. However, this means that it requires vigilant monitoring to avoid the rapid onset of hypo-perfusion or potentially life-threatening hypotension. These properties have traditionally restricted its use to short duration therapy in the operating room, ICU, cardiac care unit, or other areas where continuous close monitoring by experienced providers is available. It must be protected from light to prevent degradation and the subsequent rapid cyanide anion release upon administration.[22] Routine monitoring of cyanide levels may not be necessary.
Cardiac surgery
Perioperative hypertension in cardiac surgery is common, reported in 15-50% of patients depending on the type of surgery performed,[23,24] and is a risk factor for adverse outcomes after surgery.[25] Managing intraoperative hypertension is important because the blood pressure lability in hypertensive patients, due to impaired autoregulation of organ blood flow, confers on them a predilection for hypo-perfusion and subsequent ischemic events and end organ damage.[26] It can also produce vascular anastomotic disruption. Indeed, greater blood pressure variability has been associated with increased 30-day perioperative mortality in cardiac surgery patients.[27] The recommendation is to optimize blood pressure at least 6 weeks prior to noncardiac surgery; this may also be a reasonable strategy in cardiac surgery.[28] The ideal intraoperative agent could be easily and rapidly titrated to effect with minimal swings in blood pressure or risk of hypotension.
Historically, nitroprusside has been a favored agent to control blood pressure intraoperatively, although it carries risk for hypotension in addition to toxicity. Once its efficacy during and after cardiac surgery was established,[29,30] it became the “gold standard” against which newer agents were studied for efficacy and comparative advantages. Nitroglycerin was compared to nitroprusside in a randomized, open-label crossover study of 17 patients by Flaherty et al.[31] While all patients responded to nitroprusside, a subset of patients achieved with nitroglycerin only 50% of the blood pressure reduction achieved with nitroprusside. The time to achieve blood pressure control was not reported. Pulmonary gas exchange parameters were improved during administration of nitroglycerin, while nitroprusside worsened these variables. No significant adverse effects were reported, but nitroprusside was noted to cause tachycardia in four patients.
The B-blockers esmolol and labetalol were compared with nitroprusside in postoperative cardiac surgical patients.[32] Labetalol lowered blood pressure in magnitude similar to nitroprusside, but over a much slower timeframe and with a significantly different hemodynamic profile. Labetalol lowered the heart rate and cardiac index, while central venous pressure was increased. By comparison, patients treated with nitroprusside had significantly greater reductions in diastolic blood pressure (DBP) and mean arterial pressure, and an increased heart rate, stroke volume, and cardiac index. The authors speculated that the higher DBP and lower heart rate might improve coronary perfusion and reduce myocardial oxygen demand in patients treated with labetalol. No complications were noted in either group. Gray et al. compared esmolol with SNP in postcardiac surgical patients and observed similar results. SNP lowered DBP more than esmolol and caused an increase in heart rate. There was a nonsignificant trend of quicker blood pressure control with SNP over esmolol (21 ± 15 vs. 29 ± 14 min, respectively).[33]
The calcium channel blockers nicardipine and clevidipine have been compared with nitroprusside. Nicardipine was compared with nitroprusside by Halpern et al. in cardiac and noncardiac surgical patients.[34] Nicardipine controlled blood pressure more quickly and with less adverse effects, which included tachycardia and hypotension that resulted in discontinuation of the drug in 6 patients. None were discontinued from the nicardipine group. Both drugs exhibited similar effects on circulatory variables. Nitroprusside was shown by Aronson et al. to be inferior to clevidipine in controlling systolic blood pressure after cardiac surgery.[35] They observed greater blood pressure variability and increased mortality with nitroprusside compared to clevidipine. An explanation for this may be because longstanding hypertensive patients with stiff ventricles are more susceptible to reductions in preload from nitroprusside, a direct arterial and venous vasodilator. On the other hand, clevidipine primarily dilates arterial smooth muscle, preserving preload.
Taken together, these studies do not identify a preferred agent in cardiac surgery. In one study, clevidipine appeared superior as a first line agent because it kept blood pressure within predefined ranges better than nitroprusside. However, mortality differences between clevidipine and nitroprusside were explained by sicker patients in nitroprusside patients. In other studies, nitroprusside controlled blood pressure more quickly and was often needed as a second line agent when other drugs failed. While nitroprusside may produce reflex tachycardia in some patients, there were no cases where this was directly attributed to cyanide toxicity.
Hypertensive crises
Hypertensive crises are elevations in systolic blood pressure ≥180 mmHg or DBP ≥110 mmHg and are divided into hypertensive urgencies or hypertensive emergencies, with the latter having clinical evidence of end organ damage.[36] Blood pressure in hypertensive urgencies should be lowered over 24-48 h, while in hypertensive emergencies it should be lowered within minutes to hours. These events have many etiologies and present within a variety of clinical syndromes, and the choice of treatment depends on the target organ affected.[37]
Despite a paucity of definitive comparative, prospective, randomized controlled trials, newer agents have replaced SNP in many of these contexts because of evidence of clinical equipoise, less stringent monitoring requirements, and more favorable side effect profiles. In an analysis of the Special Tertiary Admissions Test registry, investigators found that the most common parental agent given for hypertensive crises in an emergency room or Intensive Care Unit (ICU) setting was labetalol (48%), followed by nicardipine (15%), hydralazine (15%), and nitroprusside (13%). Treatment with nitroprusside and nitroglycerin were associated with a higher mortality, but this was of borderline significance and likely confounded by bias with regard to choice of agent.[38] One study by Immink et al. compared labetalol with nitroprusside in their effects on cerebral hemodynamics in the treatment of malignant hypertension. Nitroprusside preferentially lowered systemic vascular resistance more than cerebral vascular resistance, causing lower middle cerebral blood velocities, presumably by shunting of blood to the low resistance, dilated systemic vascular bed. Labetalol did not produce these effects.[39] Other small, prospective trials have compared nitroprusside to fenoldopam[40] and nicardipine[41,42] with results of similar efficacy and little observable differences in side effects. SNP continues to be used to lower blood pressure in acute aortic dissection and acute pulmonary edema, although the recommendation is to use it only when more preferred intravenous agents are unavailable.[37]
The vasodilatory properties of nitroprusside spurred interest in its use for hypertensive crises associated with cerebrovascular accidents, especially subarachnoid and intracerebral hemorrhage. Early animal studies concluded that nitroprusside could cause vasodilation, prevent vasospasm, and maintain cerebral blood flow immediately following subarachnoid hemorrhage.[43,44,45] One study showed reversal of cerebral vasospasm in humans after nitroprusside administration in three patients who suffered a subarachnoid hemorrhage.[46] Subsequent work conflicted with these results, however, and did not show any increase in cerebral blood flow.[47,48,49] The current American Heart Association guidelines recommend using nitroprusside, labetalol, or nicardipine to treat acute hypertension to a target of a systolic blood pressure below 180 mmHg in patients with intracerebral hemorrhage.[50] There is some evidence to support the use of nicardipine over nitroprusside in this setting, as it was associated with lower in-hospital mortality.[51]
Heart failure
Sodium nitroprusside was first studied as therapy for heart failure in the 1970's. Since then many small studies have shown it to reduce afterload and improve left ventricular filling and cardiac output in acute decompensated heart failure, reviewed thoroughly by Opasich et al.[52] The 2010 Heart Failure Society of America comprehensive heart failure practice guidelines recommend nitroprusside among other vasodilators in the management of acute decompensated heart failure (Grade B recommendation).[53] Nitroprusside infusion should be monitored while its dosing titrated to appropriate clinical effect, observing for hypotension and signs of cyanide toxicity. These requirements have somewhat restricted its use, although at least one study found that with experienced providers, chronic heart failure patients who received low dose nitroprusside therapy showed reduced mortality and adverse outcomes were rare.[54] Another study showed that intermittent low dose nitroprusside infusion reduced mortality in patients with advanced heart failure awaiting transplantation.[55] It has been shown to benefit critically ill patients with left ventricular dysfunction and aortic stenosis as a bridge to valve replacement or oral vasodilator therapy.[56] Elkayam et al. provide an excellent review of the use of nitroprusside and vasodilator therapy in the management of acute decompensated heart failure.[57]
Aortic surgery
Cross-clamping of the aorta is commonly used to repair aortic aneurysms, coarctations, and traumatic injury, among other pathologies. This procedure can have dramatic effects on cardiovascular physiology and regional hemodynamics and oxygenation due to often severe hypertension proximal to the clamping and hypo-perfusion distally, presenting challenges for the anesthesiologist.[58] While outcomes after open and endovascular abdominal aorta surgery have improved dramatically,[59] the survival rate and complications associated with thoracic cross-clamping remain poor.[60]
Few studies directly compare nitroprusside with other intravenous antihypertensives and their effects on surgical outcomes in aortic surgery. Early animal studies showed SNP to be associated with a poorer response of multiple variables in the setting of cross-clamping when compared to other antihypertensive agents or controls, including increased cerebral spinal fluid pressure,[61] lower spinal cord perfusion pressure, and increased neurologic injury[62,63,64] and mortality.[65] In one of the few head to head comparisons of nitroprusside and another antihypertensive agent, fenoldopam, during cross-clamping, no differences were found in intraoperative hemodynamic variables or renal indices. Patients treated with nitroprusside had a higher average heart rate precross clamp.[66] Another study showed decreased mixed venous oxygen saturation in patients controlled with nitroprusside versus amrinone during cross-clamping, but no difference in hemodynamic control.[67] In both studies, the complication rate was the same between nitroprusside and the alternative treatment. Further research is necessary to elucidate a preferred agent in aortic surgery in adults.
Pediatric patients undergoing aortic surgery are particularly susceptible to changes in cerebral oxygenation induced by nitroprusside. One study showed decreased cerebral oxygenation after administration of nitroprusside in two children undergoing cross-clamping for coarctation repair. This decrease was over and above the decrease attributable directly to cross-clamping, as esmolol and ionotrope administration did not result in a similar decrease.[68] Another study showed no differences in cerebral venous oxygenation when nitroprusside was compared with nitroglycerin or sevoflurane.[69] Current practices are not well-described, but they favor control of perioperative hypertension in pediatric aortic surgery with esmolol and nitroprusside or nitroglycerin intravenously.[70]
Emerging applications
Nitroprusside continues to be applied in new ways. In cardiology, the “no-reflow” phenomenon is defined as the lack of blood flow following an intervention to restore patency to coronary vessels.[71] It is estimated to occur in 3.2-4.8% of all percutaneous coronary interventions, more after myocardial infarction, and adversely impacts outcomes.[72] Vasodilation with nitroprusside has offered promise to both prevent and treat this potential complication.[73] In a small, randomized, placebo-controlled trial, nitroprusside was recently shown to improve symptoms of schizophrenia after a single administration.[74] The proposed mechanism for this effect is based on the derangements in cerebral NO regulation observed in schizophrenic patients and nitroprusside's ability to increase NO production in the brain. This NO releasing property of nitroprusside has been shown to increase apoptosis in gastric cancer cells.[75] Table 1 compares the pharmacological profile of SNP to other antihypertensives currently available for the acute control of perioperative hypertension.
Metabolism, Safety, and Toxicity
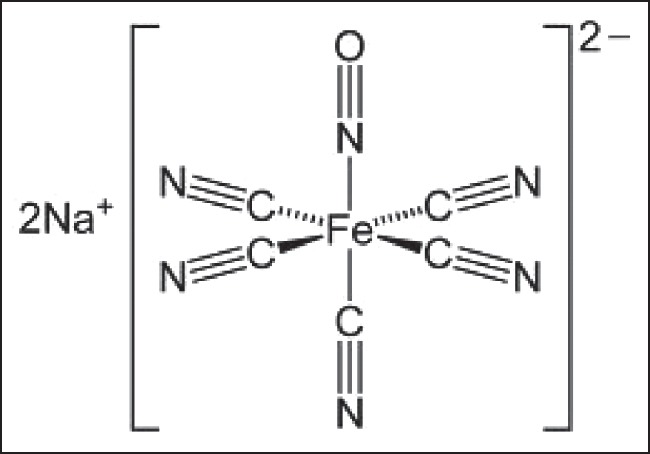
Nitroprusside reacts with oxyhemoglobin to form methemoglobin and release cyanide anions in vivo.[8,76] These ions have multiple possible fates [Figure 2]:
Figure 2.
The possible fates of cyanide anion in the body
They may react again with methemoglobin to form cyanomethemoglobin and accumulate in erythrocytes.
They may be transported to the liver where they react with thiosulfate and cobalamin to form thiocyanate, which is excreted in the kidneys.
They may bind to tissue cytochrome oxidase, inhibiting oxidative phosphorylation.[77]
It is this final pathway that produces “cyanide toxicity,” which has been well-documented in clinical cases and animal studies.[5,6,7,78,79,80] Further research has attempted to characterize these toxic effects in specific tissues. Nitroprusside is toxic to cerebral endothelial cells,[81] hepatocytes,[82] and neural cell lines,[83] generating reactive oxygen species and inducing apoptotic cell death. It is estimated that adults can detoxify 50 mg of nitroprusside (one vial of the traditional commercial formulation), but infusion rates higher than 2 μg/kg/min may lead to toxic cyanide accumulations.[8]
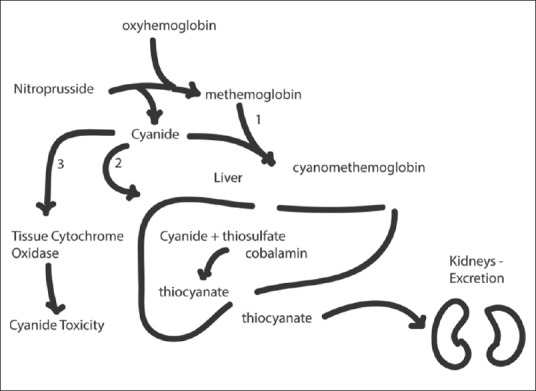
Assessment of cyanide toxicity can be difficult if lab assays measure whole blood cyanide concentrations rather than serum cyanide concentrations, which are better correlated with cyanide toxicity.[84] Elevated lactate concentrations are an excellent surrogate [Figure 3] and serve as a marker of cyanide toxicity in patients; they can be used to support the diagnosis.[85] Unfortunately, many of the clinical signs of cyanide toxicity, such as restlessness, agitation, and sinus tachycardia are difficult to evaluate intraoperatively and lead to a misdiagnosis.
Figure 3.
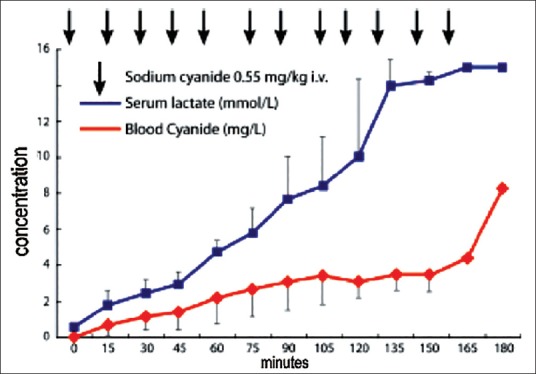
Note the progressive increase in serum lactate levels with the infusion of sodium cyanide in a pig model of cyanide toxicity[96]
Cheung et al. showed that the plasma free hemoglobin concentration correlated positively with time on cardiac bypass and the cyanide anion concentration, suggesting that prolonged exposure to bypass increased a patient's risk for cyanide toxicity because of increased erythrocyte shearing and intracellular cyanide release.[86] Therefore, nitroprusside should be replaced in this setting with newer drugs.
In addition to cyanide toxicity, the concept of “coronary steal” associated with nitroprusside has long been reported. Mann et al. compared regional myocardial blood flow (RMBF) after administration of nitroprusside and nitroglycerin in normal patients and patients with coronary artery disease (CAD).[15] They found some evidence of reduced RMBF in those patients receiving nitroprusside with well-developed collaterals compared to an increase in RMBF in similar patients treated with nitroglycerin. Left ventricular end diastolic pressure was not measured in either group, which may have influenced the myocardial blood flow. In addition, nitroprusside administration was noted to result in a lower average MAP than that achieved with nitroglycerin, further confounding the results because DBP provides the driving force for coronary perfusion pressure. It is postulated that these differences are due to nitroglycerin's preferential effect on larger conductance vessels, while nitroprusside dilates smaller resistance vessels, creating a low pressure system distal to occluded vessels that diverts critical pressure-dependent flow from ischemic areas.[87] The clinical significance of these observations is uncertain, and the true incidence of clinically significant coronary steal remains unknown. The more important clinical consideration in patients with CAD on a nitrovasodilator may be to prevent hypotension, which may be more easily achievable with alternative therapies.[35]
The use of nitroprusside has been associated with increased intracranial pressure (ICP).[88,89] The mechanism for this is due to increased cerebral blood flow and resultant increased blood volume in the setting of impaired autoregulation attributable to nitroprusside.[90] Caution should be taken in patients with intracranial mass lesions, encephalopathy or other reasons for an elevated ICP.
Antidotes: Mechanism and Clinical Application
Knowledge of the metabolic pathways of nitroprusside and mechanism of cyanide toxicity has spurred the investigation of potential agents to reverse or prevent this. A review by Reade et al. of available evidence found both sodium thiosulfate and hydroxocobalamin equally effective in reversal of cyanide poisoning with no significant adverse effects to either.[91] These medicines work by increasing the thiosulfate or hydroxocobalamin substrate normally present in serum to buffer against rising cyanide concentrations and minimize its reaction with mitochondrial cytochromes. The thiosulfate-associated antidotes depend on the enzyme rhodanese to catalyze the conversion of cyanide to the less toxic thiocyanate [Figure 2]. However, since this enzyme is predominantly localized to the liver and red blood cells, important tissues such as the brain and heart remain unprotected.[92] Patients with conditions such as Leber's hereditary optic neuropathy lack adequate rhodanase activity and are especially vulnerable to nitroprusside toxicity. Hydroxocobalamin-based therapies work by binding and trapping cyanide anions as cyanocobalamin which is excreted in urine, but clinical results have been mixed.[93] Only the combination of sodium thiosulfate and sodium nitrite is currently approved by the FDA for treatment of cyanide poisoning. In addition, these treatments can be difficult or expensive to administer or have serious side effects.
Recently however, a new oral cyanide antidote, sulfanegen sodium, a prodrug of 3-mercaptopyruvate, has been developed.[92] It is readily formed from commercially available starting materials [Figure 4] and has additional advantages in that it is available orally and is effective when administered prophylactically up to 1 h before cyanide exposure. The sulfanegen sodium's dimer dissociates nonenzematically in physiologic conditions and pH of 7.4-3-mercaptopyruvate, which through further metabolism ultimately captures and converts cyanide anions into SCN, excreted in the kidneys. The prodrug was shown in initial experiments to be effective in reversing sub lethal cyanide toxicity in murine and rabbit models.[92,94,95]
Figure 4.
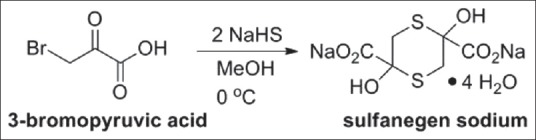
Sulfanegen sodium, prodrug for 3-mercaptopyruvate, is formed from 3-bromopyruvic acid, sodium hydrogen sulfide, and methanol[96]
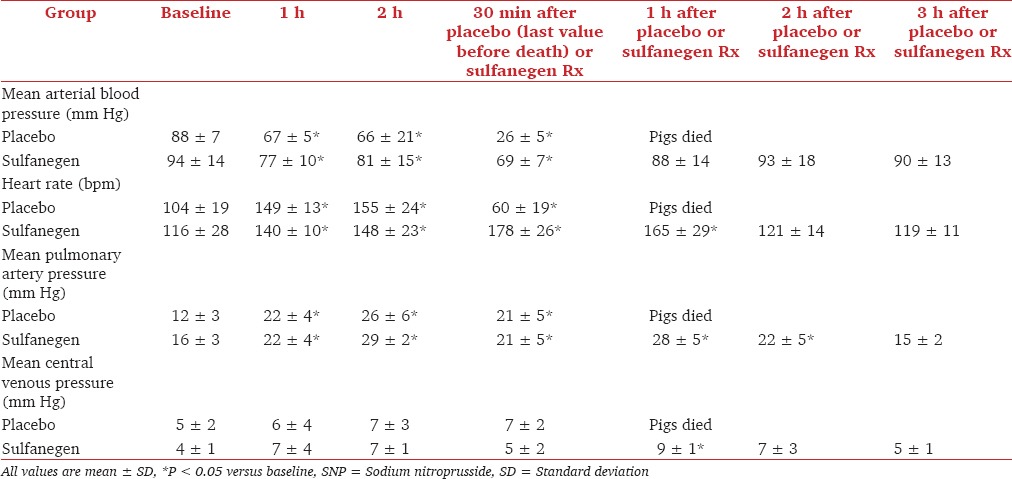
Further experiments have characterized the effect of sulfanegen sodium in juvenile pigs.[96] Lethal injections of SNP were administered, after which either sulfanegen sodium antidote or placebo was given. In the treatment groups, the antidote normalized blood lactate levels and hemodynamic variables, while pigs receiving placebo decompensated and succumbed [Table 2]. Additional research is underway to determine whether this drug may successfully reverse cyanide toxicity in humans.
Table 2.
Results of hemodynamic changes observed during SNP infusion, followed either by placebo or sulfanegen sodium given 2 h after SNP infusion[96]
Summary
Over 150 years since its discovery and 40 years since its widespread adoption into clinical practice, SNP remains a frequently-used vasodilator in the management of acute and severe systemic hypertension and additional applications (such as treatment of cerebral vasospasm) still in development. Due to its ubiquitous availability and widespread use, clinicians must be cognizant about its high potency and potential toxicities, while using this drug, including cyanide toxicity, altered blood flow distribution to and within organs, increased pulmonary shunting, and excessive hypotension. Caution dictates heightened vigilance for worsening confusion, drug tachyphylaxis, and metabolic acidosis with a base deficit — all indicating possible cyanide toxicity. Future antidotes appear to hold promise and may be available for cyanide toxicity; there are no current data about their human efficacy or safety. Therefore, practitioners must balance these factors, while recognizing alternatives that are newer, possibly safer, but usually more expensive. Future prospective, randomized controlled trials that directly compare nitroprusside with other potent vasodilators should facilitate better treatment guidelines. In addition, further research is necessary to develop better ways to detect, prevent, and reverse cyanide toxicity.
Footnotes
Source of Support: Work related to the new cyanide antidote was supported by Grant NIH NINDS/5U01NS058087.
Conflict of Interest: None declared.
References
- 1.Playfair L. On the nitroprusside: A new class of salts. Philos Trans R Soc Lond. 1849;139:41. [Google Scholar]
- 2.Johnson C. Mechanisms of actions and toxicity of nitroprusside. Exp Biol Med. 1928;26:2. [Google Scholar]
- 3.Page IH, Corcoran AC, Dustan HP, Koppanyi T. Cardiovascular actions of sodium nitroprusside in animals and hypertensive patients. Circulation. 1955;11:188–98. doi: 10.1161/01.cir.11.2.188. [DOI] [PubMed] [Google Scholar]
- 4.Taylor TH, Styles M, Lamming AJ. Sodium nitroprusside as a hypotensive agent in general anaesthesia. Br J Anaesth. 1970;42:859–64. doi: 10.1093/bja/42.10.859. [DOI] [PubMed] [Google Scholar]
- 5.Amaranath L, Kellermeyer WF., Jr Tachyphylaxis to sodium nitroprusside. Anesthesiology. 1976;44:345–8. doi: 10.1097/00000542-197604000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Davies DW, Kadar D, Steward DJ, Munro IR. A sudden death associated with the use of sodium nitroprusside for induction of hypotension during anaesthesia. Can Anaesth Soc J. 1975;22:547–52. doi: 10.1007/BF03013407. [DOI] [PubMed] [Google Scholar]
- 7.Cyanide intoxication from sodium nitroprusside in anesthesia. Med Lett Drugs Ther. 1976;18:68. [PubMed] [Google Scholar]
- 8.Vesey CJ, Cole PV, Simpson PJ. Cyanide and thiocyanate concentrations following sodium nitroprusside infusion in man. Br J Anaesth. 1976;48:651–60. doi: 10.1093/bja/48.7.651. [DOI] [PubMed] [Google Scholar]
- 9.Nightingale SL. From the food and drug administration. JAMA. 1991;265:847. [PubMed] [Google Scholar]
- 10.Friederich JA, Butterworth JF., 4th Sodium nitroprusside: Twenty years and counting. Anesth Analg. 1995;81:152–62. doi: 10.1097/00000539-199507000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Ivankovich AD, Miletich DJ, Tinker JH. Sodium nitroprusside: Metabolism and general considerations. Int Anesthesiol Clin. 1978;16:1–29. [PubMed] [Google Scholar]
- 12.Levy JH. Management of systemic and pulmonary hypertension. Tex Heart Inst J. 2005;32:467–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Fok H, Jiang B, Clapp B, Chowienczyk P. Regulation of vascular tone and pulse wave velocity in human muscular conduit arteries: Selective effects of nitric oxide donors to dilate muscular arteries relative to resistance vessels. Hypertension. 2012;60:1220–5. doi: 10.1161/HYPERTENSIONAHA.112.198788. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman WE, Albrecht RF, 2nd, Jonjev ZS. Sodium nitroprusside-induced, but not desflurane-induced, hypotension decreases myocardial tissue oxygenation in dogs anesthetized with 8% desflurane. J Cardiothorac Vasc Anesth. 2002;16:286–9. doi: 10.1053/jcan.2002.124134. [DOI] [PubMed] [Google Scholar]
- 15.Mann T, Cohn PF, Holman LB, Green LH, Markis JE, Phillips DA. Effect of nitroprusside on regional myocardial blood flow in coronary artery disease. Results in 25 patients and comparison with nitroglycerin. Circulation. 1978;57:732–8. doi: 10.1161/01.cir.57.4.732. [DOI] [PubMed] [Google Scholar]
- 16.Clark D, 3rd, Tesseneer S, Tribble CG. Nitroglycerin and sodium nitroprusside: Potential contributors to postoperative bleeding? Heart Surg Forum. 2012;15:E92–6. doi: 10.1532/HSF98.20111109. [DOI] [PubMed] [Google Scholar]
- 17.Hines R, Barash PG. Infusion of sodium nitroprusside induces platelet dysfunction in vitro. Anesthesiology. 1989;70:611–5. doi: 10.1097/00000542-198904000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Butterworth RJ, Cluckie A, Jackson SH, Buxton-Thomas M, Bath PM. Pathophysiological assessment of nitric oxide (given as sodium nitroprusside) in acute ischaemic stroke. Cerebrovasc Dis. 1998;8:158–65. doi: 10.1159/000015842. [DOI] [PubMed] [Google Scholar]
- 19.Hersey SL, O’Dell NE, Lowe S, Rasmussen G, Tobias JD, Deshpande JK, et al. Nicardipine versus nitroprusside for controlled hypotension during spinal surgery in adolescents. Anesth Analg. 1997;84:1239–44. doi: 10.1097/00000539-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Moffett BS, Price JF. Evaluation of sodium nitroprusside toxicity in pediatric cardiac surgical patients. Ann Pharmacother. 2008;42:1600–4. doi: 10.1345/aph.1L192. [DOI] [PubMed] [Google Scholar]
- 21.Thomas C, Svehla L, Moffett BS. Sodium-nitroprusside-induced cyanide toxicity in pediatric patients. Expert Opin Drug Saf. 2009;8:599–602. doi: 10.1517/14740330903081717. [DOI] [PubMed] [Google Scholar]
- 22.Bisset WI, Butler AR, Glidewell C, Reglinski J. Sodium nitroprusside and cyanide release: Reasons for re-appraisal. Br J Anaesth. 1981;53:1015–8. doi: 10.1093/bja/53.10.1015. [DOI] [PubMed] [Google Scholar]
- 23.Estafanous FG, Tarazi RC. Systemic arterial hypertension associated with cardiac surgery. Am J Cardiol. 1980;46:685–94. doi: 10.1016/0002-9149(80)90521-4. [DOI] [PubMed] [Google Scholar]
- 24.Cooper TJ, Clutton-Brock TH, Jones SN, Tinker J, Treasure T. Factors relating to the development of hypertension after cardiopulmonary bypass. Br Heart J. 1985;54:91–5. doi: 10.1136/hrt.54.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335:1857–63. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 26.Aronson S, Fontes ML, Miao Y, Mangano DT Investigators of the Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: Critical dependence on pulse pressure hypertension. Circulation. 2007;115:733–42. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 27.Aronson S, Dyke CM, Levy JH, Cheung AT, Lumb PD, Avery EG, et al. Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? An exploratory analysis of the ECLIPSE trials. Anesth Analg. 2011;113:19–30. doi: 10.1213/ANE.0b013e31820f9231. [DOI] [PubMed] [Google Scholar]
- 28.Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery - Executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2002;105:1257–67. [PubMed] [Google Scholar]
- 29.Lappas DG, Lowenstein E, Waller J, Fahmy NR, Daggett WM. Hemodynamic effects of nitroprusside infusion during coronary artery operation in man. Circulation. 1976;54:III4–10. [PubMed] [Google Scholar]
- 30.Stinson EB, Holloway EL, Derby G, Oyer PE, Hollingsworth J, Griepp RB, et al. Comparative hemodynamic responses to chlorpromazine, nitroprusside, nitroglycerin, and trimethaphan immediately after open-heart operations. Circulation. 1975;52:I26–33. [PubMed] [Google Scholar]
- 31.Flaherty JT, Magee PA, Gardner TL, Potter A, MacAllister NP. Comparison of intravenous nitroglycerin and sodium nitroprusside for treatment of acute hypertension developing after coronary artery bypass surgery. Circulation. 1982;65:1072–7. doi: 10.1161/01.cir.65.6.1072. [DOI] [PubMed] [Google Scholar]
- 32.Cruise CJ, Skrobik Y, Webster RE, Marquez-Julio A, David TE. Intravenous labetalol versus sodium nitroprusside for treatment of hypertension postcoronary bypass surgery. Anesthesiology. 1989;71:835–9. doi: 10.1097/00000542-198912000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Gray RJ, Bateman TM, Czer LS, Conklin C, Matloff JM. Comparison of esmolol and nitroprusside for acute post-cardiac surgical hypertension. Am J Cardiol. 1987;59:887–91. doi: 10.1016/0002-9149(87)91113-1. [DOI] [PubMed] [Google Scholar]
- 34.Halpern NA, Goldberg M, Neely C, Sladen RN, Goldberg JS, Floyd J, et al. Postoperative hypertension: A multicenter, prospective, randomized comparison between intravenous nicardipine and sodium nitroprusside. Crit Care Med. 1992;20:1637–43. [PubMed] [Google Scholar]
- 35.Aronson S, Dyke CM, Stierer KA, Levy JH, Cheung AT, Lumb PD, et al. The ECLIPSE trials: Comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107:1110–21. doi: 10.1213/ane.0b013e31818240db. [DOI] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 37.Marik PE, Varon J. Hypertensive crises: Challenges and management. Chest. 2007;131:1949–62. doi: 10.1378/chest.06-2490. [DOI] [PubMed] [Google Scholar]
- 38.Mayer SA, Kurtz P, Wyman A, Sung GY, Multz AS, Varon J, et al. Clinical practices, complications, and mortality in neurological patients with acute severe hypertension: The Studying the Treatment of Acute hyperTension registry. Crit Care Med. 2011;39:2330–6. doi: 10.1097/CCM.0b013e3182227238. [DOI] [PubMed] [Google Scholar]
- 39.Immink RV, van den Born BJ, van Montfrans GA, Kim YS, Hollmann MW, van Lieshout JJ. Cerebral hemodynamics during treatment with sodium nitroprusside versus labetalol in malignant hypertension. Hypertension. 2008;52:236–40. doi: 10.1161/HYPERTENSIONAHA.108.110395. [DOI] [PubMed] [Google Scholar]
- 40.Panacek EA, Bednarczyk EM, Dunbar LM, Foulke GE, Holcslaw TL. Randomized, prospective trial of fenoldopam vs sodium nitroprusside in the treatment of acute severe hypertension. Fenoldopam Study Group. Acad Emerg Med. 1995;2:959–65. doi: 10.1111/j.1553-2712.1995.tb03122.x. [DOI] [PubMed] [Google Scholar]
- 41.Neutel JM, Smith DH, Wallin D, Cook E, Ram CV, Fletcher E, et al. A comparison of intravenous nicardipine and sodium nitroprusside in the immediate treatment of severe hypertension. Am J Hypertens. 1994;7:623–8. doi: 10.1093/ajh/7.7.623. [DOI] [PubMed] [Google Scholar]
- 42.Yang HJ, Kim JG, Lim YS, Ryoo E, Hyun SY, Lee G. Nicardipine versus nitroprusside infusion as antihypertensive therapy in hypertensive emergencies. J Int Med Res. 2004;32:118–23. doi: 10.1177/147323000403200203. [DOI] [PubMed] [Google Scholar]
- 43.Pluta RM, Oldfield EH, Boock RJ. Reversal and prevention of cerebral vasospasm by intracarotid infusions of nitric oxide donors in a primate model of subarachnoid hemorrhage. J Neurosurg. 1997;87:746–51. doi: 10.3171/jns.1997.87.5.0746. [DOI] [PubMed] [Google Scholar]
- 44.Marshman LA, Morice AH, Thompson JS. Increased efficacy of sodium nitroprusside in middle cerebral arteries following acute subarachnoid hemorrhage: Indications for its use after rupture. J Neurosurg Anesthesiol. 1998;10:171–7. doi: 10.1097/00008506-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Vajkoczy P, Hubner U, Horn P, Bauhuf C, Thome C, Schilling L, et al. Intrathecal sodium nitroprusside improves cerebral blood flow and oxygenation in refractory cerebral vasospasm and ischemia in humans. Stroke. 2000;31:1195–7. doi: 10.1161/01.str.31.5.1194-b. [DOI] [PubMed] [Google Scholar]
- 46.Thomas JE, Rosenwasser RH. Reversal of severe cerebral vasospasm in three patients after aneurysmal subarachnoid hemorrhage: Initial observations regarding the use of intraventricular sodium nitroprusside in humans. Neurosurgery. 1999;44:48–57. doi: 10.1097/00006123-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Joshi S, Duong H, Mangla S, Wang M, Libow AD, Popilskis SJ, et al. In nonhuman primates intracarotid adenosine, but not sodium nitroprusside, increases cerebral blood flow. Anesth Analg. 2002;94:393–9. doi: 10.1097/00000539-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 48.Joshi S, Young WL, Duong H, Aagaard BA, Ostapkovich ND, Connolly ES, et al. Intracarotid nitroprusside does not augment cerebral blood flow in human subjects. Anesthesiology. 2002;96:60–6. doi: 10.1097/00000542-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Joshi S, Hartl R, Sun LS, Libow AD, Wang M, Pile-Spellman J, et al. Despite in vitro increase in cyclic guanosine monophosphate concentrations, intracarotid nitroprusside fails to augment cerebral blood flow of healthy baboons. Anesthesiology. 2003;98:412–9. doi: 10.1097/00000542-200302000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults:2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 51.Suri MF, Vazquez G, Ezzeddine MA, Qureshi AI. A multicenter comparison of outcomes associated with intravenous nitroprusside and nicardipine treatment among patients with intracerebral hemorrhage. Neurocrit Care. 2009;11:50–5. doi: 10.1007/s12028-009-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Opasich C, Cioffi G, Gualco A. Nitroprusside in decompensated heart failure: What should a clinician really know? Curr Heart Fail Rep. 2009;6:182–90. doi: 10.1007/s11897-009-0026-4. [DOI] [PubMed] [Google Scholar]
- 53.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, et al. Heart Failure Society of America. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol. 2008;52:200–7. doi: 10.1016/j.jacc.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 55.Capomolla S, Febo O, Opasich C, Guazzotti G, Caporotondi A, La Rovere MT, et al. Chronic infusion of dobutamine and nitroprusside in patients with end-stage heart failure awaiting heart transplantation: Safety and clinical outcome. Eur J Heart Fail. 2001;3:601–10. doi: 10.1016/s1388-9842(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 56.Khot UN, Novaro GM, Popović ZB, Mills RM, Thomas JD, Tuzcu EM, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003;348:1756–63. doi: 10.1056/NEJMoa022021. [DOI] [PubMed] [Google Scholar]
- 57.Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med. 2008;36:S95–105. doi: 10.1097/01.CCM.0000297161.41559.93. [DOI] [PubMed] [Google Scholar]
- 58.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–60. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 59.Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA. 2012;307:1621–8. doi: 10.1001/jama.2012.453. [DOI] [PubMed] [Google Scholar]
- 60.Gloviczki P. Surgical repair of thoracoabdominal aneurysms: Patient selection, techniques and results. Cardiovasc Surg. 2002;10:434–41. doi: 10.1016/s0967-2109(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 61.Ryan T, Mannion D, O’Brien W, Grace P, Bouchier-Hayes D, Cunningham AJ. Spinal cord perfusion pressure in dogs after control of proximal aortic hypertension during thoracic aortic cross-clamping with esmolol or sodium nitroprusside. Anesthesiology. 1993;78:317–25. doi: 10.1097/00000542-199302000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Simpson JI, Eide TR, Schiff GA, Clagnaz JF, Zisbrod Z, Newman SB, et al. Isoflurane versus sodium nitroprusside for the control of proximal hypertension during thoracic aortic cross-clamping: Effects on spinal cord ischemia. J Cardiothorac Vasc Anesth. 1995;9:491–6. doi: 10.1016/s1053-0770(05)80129-6. [DOI] [PubMed] [Google Scholar]
- 63.Simpson JI, Eide TR, Newman SB, Schiff GA, Levine D, Bermudez R, et al. Trimethaphan versus sodium nitroprusside for the control of proximal hypertension during thoracic aortic cross-clamping: The effects on spinal cord ischemia. Anesth Analg. 1996;82:68–74. doi: 10.1097/00000539-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Marini CP, Grubbs PE, Toporoff B, Woloszyn TT, Coons MS, Acinapura AJ, et al. Effect of sodium nitroprusside on spinal cord perfusion and paraplegia during aortic cross-clamping. Ann Thorac Surg. 1989;47:379–83. doi: 10.1016/0003-4975(89)90377-9. [DOI] [PubMed] [Google Scholar]
- 65.Aadahl P, Aakhus S, Strømholm T, Saether OD, Myhre HO. Effect of sodium nitroprusside on cardiac output during cross-clamping of the descending thoracic aorta in pigs. Eur Surg Res. 1995;27:323–31. doi: 10.1159/000129416. [DOI] [PubMed] [Google Scholar]
- 66.Oliver WC, Jr, Nuttall GA, Cherry KJ, Decker PA, Bower T, Ereth MH. A comparison of fenoldopam with dopamine and sodium nitroprusside in patients undergoing cross-clamping of the abdominal aorta. Anesth Analg. 2006;103:833–40. doi: 10.1213/01.ane.0000237273.79553.9e. [DOI] [PubMed] [Google Scholar]
- 67.Dentz ME, Lubarsky DA, Smith LR, McCann RL, Moskop RJ, Inge W, et al. A comparison of amrinone with sodium nitroprusside for control of hemodynamics during infrarenal abdominal aortic surgery. J Cardiothorac Vasc Anesth. 1995;9:486–90. doi: 10.1016/s1053-0770(05)80128-4. [DOI] [PubMed] [Google Scholar]
- 68.Azakie A, Muse J, Gardner M, Skidmore KL, Miller SP, Karl TR, et al. Cerebral oxygen balance is impaired during repair of aortic coarctation in infants and children. J Thorac Cardiovasc Surg. 2005;130:830–6. doi: 10.1016/j.jtcvs.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 69.Moerman A, Bové T, François K, Jacobs S, Deblaere I, Wouters P, et al. Society of cardiovascular anesthesiologists: The effect of blood pressure regulation during aortic coarctation repair on brain, kidney, and muscle oxygen saturation measured by near-infrared spectroscopy: A randomized, clinical trial. Anesth Analg. 2013;116:760–6. doi: 10.1213/ANE.0b013e31827f5628. [DOI] [PubMed] [Google Scholar]
- 70.Tabbutt S, Nicolson SC, Dominguez TE, Wells W, Backer CL, Tweddell JS, et al. Perioperative course in 118 infants and children undergoing coarctation repair via a thoracotomy: A prospective, multicenter experience. J Thorac Cardiovasc Surg. 2008;136:1229–36. doi: 10.1016/j.jtcvs.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 71.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117:3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 72.Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugelmass AD, et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation. 1994;89:2514–8. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- 73.Pasceri V, Pristipino C, Pelliccia F, Granatelli A, Speciale G, Roncella A, et al. Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. Am J Cardiol. 2005;95:1358–61. doi: 10.1016/j.amjcard.2005.01.082. [DOI] [PubMed] [Google Scholar]
- 74.Hallak JE, Maia-de-Oliveira JP, Abrao J, Evora PR, Zuardi AW, Crippa JA, et al. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: A randomized, double-blind, placebo-controlled trial. JAMA Psychiatry. 2013;70:668–76. doi: 10.1001/jamapsychiatry.2013.1292. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, Lan C, Fang Y, Zhang Y, Wang J, Guo J, et al. Sodium nitroprusside (SNP) sensitizes human gastric cancer cells to TRAIL-induced apoptosis. Int Immunopharmacol. 2013;17:383–9. doi: 10.1016/j.intimp.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Arnold WP, Longnecker DE, Epstein RM. Photodegradation of sodium nitroprusside: Biologic activity and cyanide release. Anesthesiology. 1984;61:254–60. doi: 10.1097/00000542-198409000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Tinker JH, Michenfelder JD. Sodium nitroprusside: Pharmacology, toxicology and therapeutics. Anesthesiology. 1976;45:340–54. [PubMed] [Google Scholar]
- 78.Merrifield AJ, Blundell MD. Letter: Toxicity of sodium nitroprusside. Br J Anaesth. 1974;46:324. [PubMed] [Google Scholar]
- 79.Perschau RA, Modell JH, Bright RW, Shirley PD. Suspected sodium nitroprusside-induced cyanide intoxication. Anesth Analg. 1977;56:533–7. doi: 10.1213/00000539-197707000-00015. [DOI] [PubMed] [Google Scholar]
- 80.Davies DW, Greiss L, Kadar D, Steward DJ. Sodium nitroprusside in children: Observations on metabolism during normal and abnormal responses. Can Anaesth Soc J. 1975;22:553–60. doi: 10.1007/BF03013408. [DOI] [PubMed] [Google Scholar]
- 81.Gobbel GT, Chan TY, Chan PH. Nitric oxide-and superoxide-mediated toxicity in cerebral endothelial cells. J Pharmacol Exp Ther. 1997;282:1600–7. [PubMed] [Google Scholar]
- 82.Niknahad H, O’Brien PJ. Involvement of nitric oxide in nitroprusside-induced hepatocyte cytotoxicity. Biochem Pharmacol. 1996;51:1031–9. doi: 10.1016/0006-2952(96)85086-6. [DOI] [PubMed] [Google Scholar]
- 83.Kanthasamy AG, Ardelt B, Malave A, Mills EM, Powley TL, Borowitz JL, et al. Reactive oxygen species generated by cyanide mediate toxicity in rat pheochromocytoma cells. Toxicol Lett. 1997;93:47–54. doi: 10.1016/s0378-4274(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 84.Alaniz C, Watts B. Monitoring cyanide toxicity in patients receiving nitroprusside therapy. Ann Pharmacother. 2005;39:388–9. doi: 10.1345/aph.1E226. [DOI] [PubMed] [Google Scholar]
- 85.Baud FJ, Borron SW, Mégarbane B, Trout H, Lapostolle F, Vicaut E, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30:2044–50. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 86.Cheung AT, Cruz-Shiavone GE, Meng QC, Pochettino A, Augoustides JA, Bavaria JE, et al. Cardiopulmonary bypass, hemolysis, and nitroprusside-induced cyanide production. Anesth Analg. 2007;105:29–33. doi: 10.1213/01.ane.0000264078.34514.32. [DOI] [PubMed] [Google Scholar]
- 87.Harrison DG, Bates JN. The nitrovasodilators. New ideas about old drugs. Circulation. 1993;87:1461–7. doi: 10.1161/01.cir.87.5.1461. [DOI] [PubMed] [Google Scholar]
- 88.Cottrell JE, Patel K, Turndorf H, Ransohoff J. Intracranial pressure changes induced by sodium nitroprusside in patients with intracranial mass lesions. J Neurosurg. 1978;48:329–31. doi: 10.3171/jns.1978.48.3.0329. [DOI] [PubMed] [Google Scholar]
- 89.Turner JM, Powell D, Gibson RM, McDowall DG. Intracranial pressure changes in neurosurgical patients during hypotension induced with sodium nitroprusside or trimetaphan. Br J Anaesth. 1977;49:419–25. doi: 10.1093/bja/49.5.419. [DOI] [PubMed] [Google Scholar]
- 90.Weiss MH, Spence J, Apuzzo ML, Heiden JS, McComb JG, Kurze T. Influence of nitroprusside on cerebral pressure autoregulation. Neurosurgery. 1979;4:56–9. doi: 10.1227/00006123-197901000-00011. [DOI] [PubMed] [Google Scholar]
- 91.Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC Australian Resuscitation Council. Review article: Management of cyanide poisoning. Emerg Med Australas. 2012;24:225–38. doi: 10.1111/j.1742-6723.2012.01538.x. [DOI] [PubMed] [Google Scholar]
- 92.Nagasawa HT, Goon DJ, Crankshaw DL, Vince R, Patterson SE. Novel, orally effective cyanide antidotes. J Med Chem. 2007;50:6462–4. doi: 10.1021/jm7011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zerbe NF, Wagner BK. Use of vitamin B12 in the treatment and prevention of nitroprusside-induced cyanide toxicity. Crit Care Med. 1993;21:465–7. doi: 10.1097/00003246-199303000-00027. [DOI] [PubMed] [Google Scholar]
- 94.Crankshaw DL, Goon DJ, Briggs JE, DeLong D, Kuskowski M, Patterson SE, et al. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–7. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, et al. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 2010;248:269–76. doi: 10.1016/j.taap.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belani KG, Singh H, Beebe DS, George P, Patterson SE, Nagasawa HT, et al. Cyanide toxicity in juvenile pigs and its reversal by a new prodrug, sulfanegen sodium. Anesth Analg. 2012;114:956–61. doi: 10.1213/ANE.0b013e31824c4eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]


