Abstract
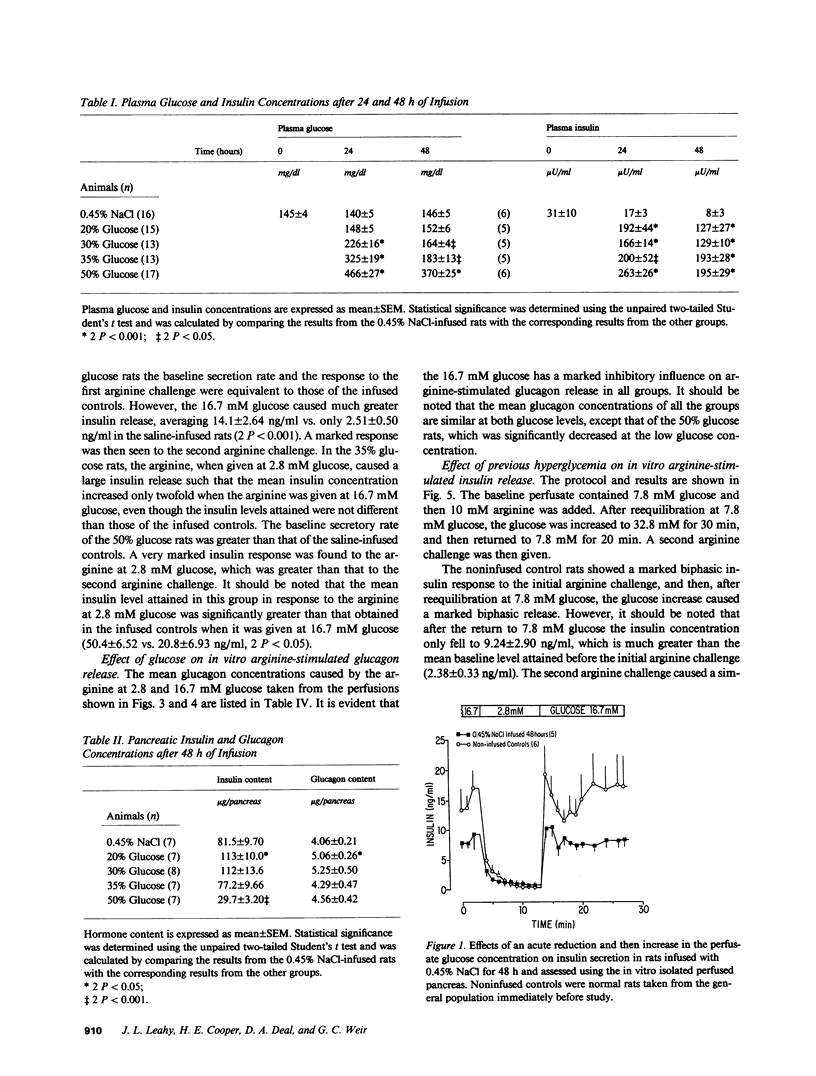
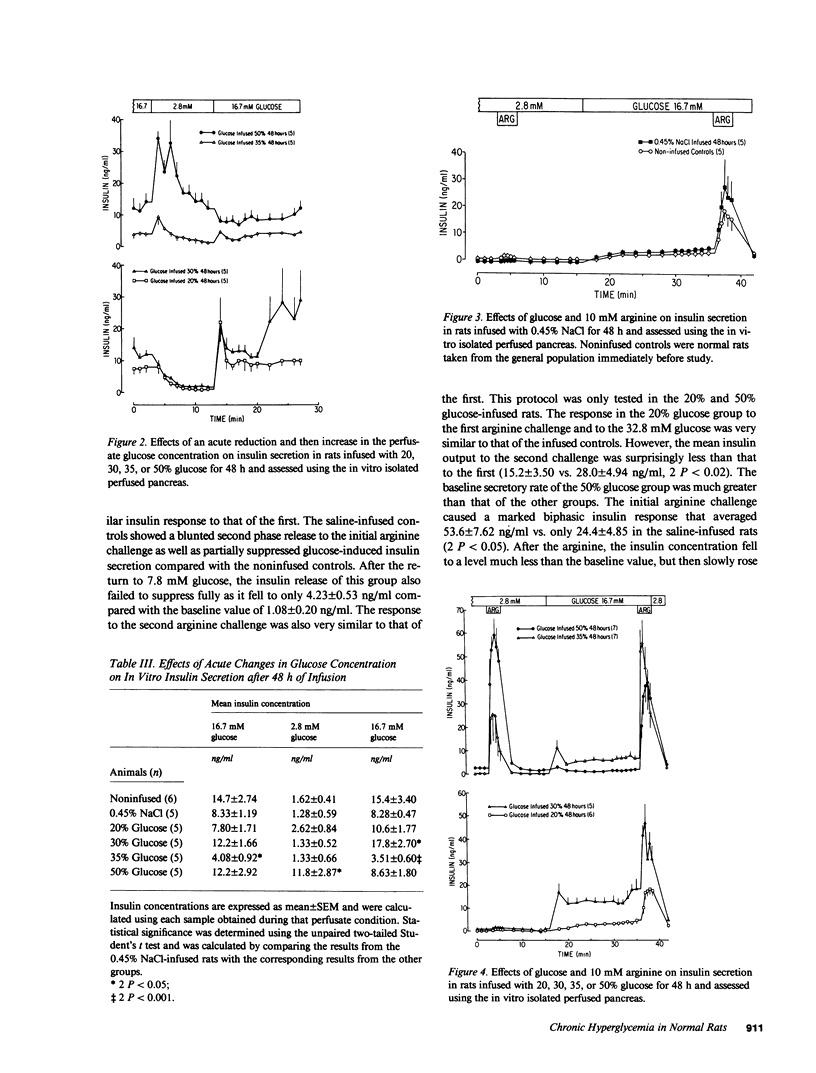
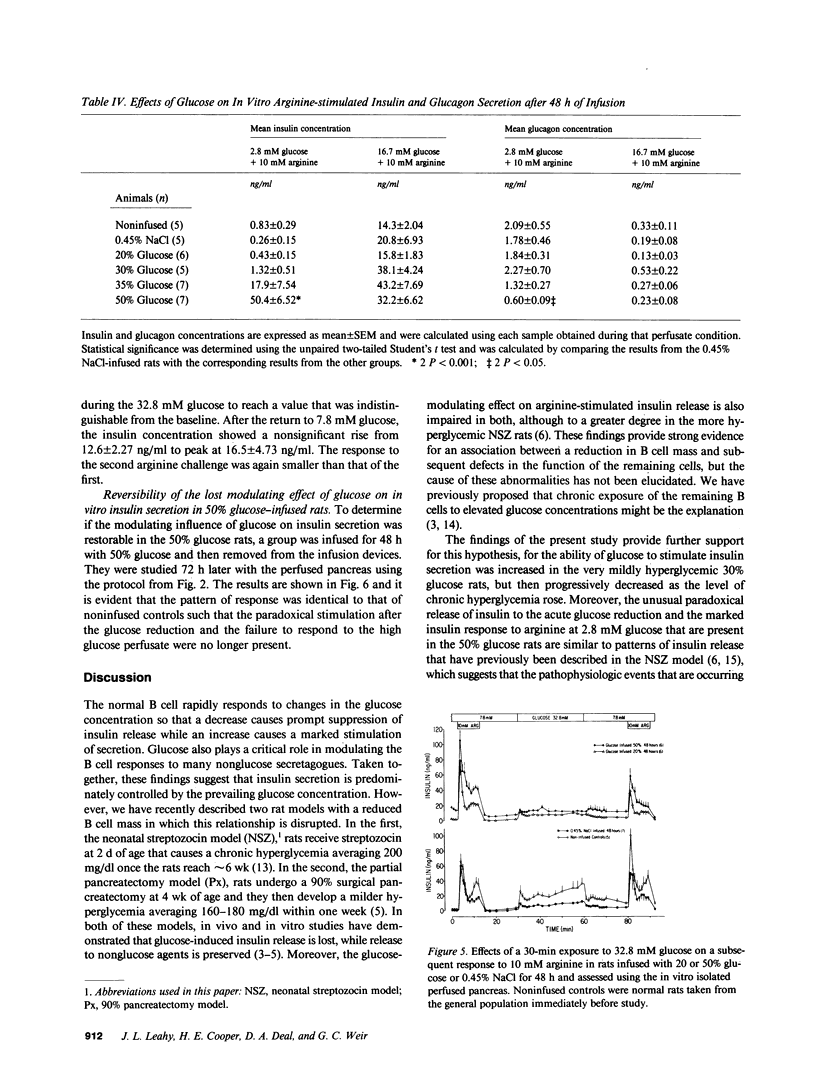
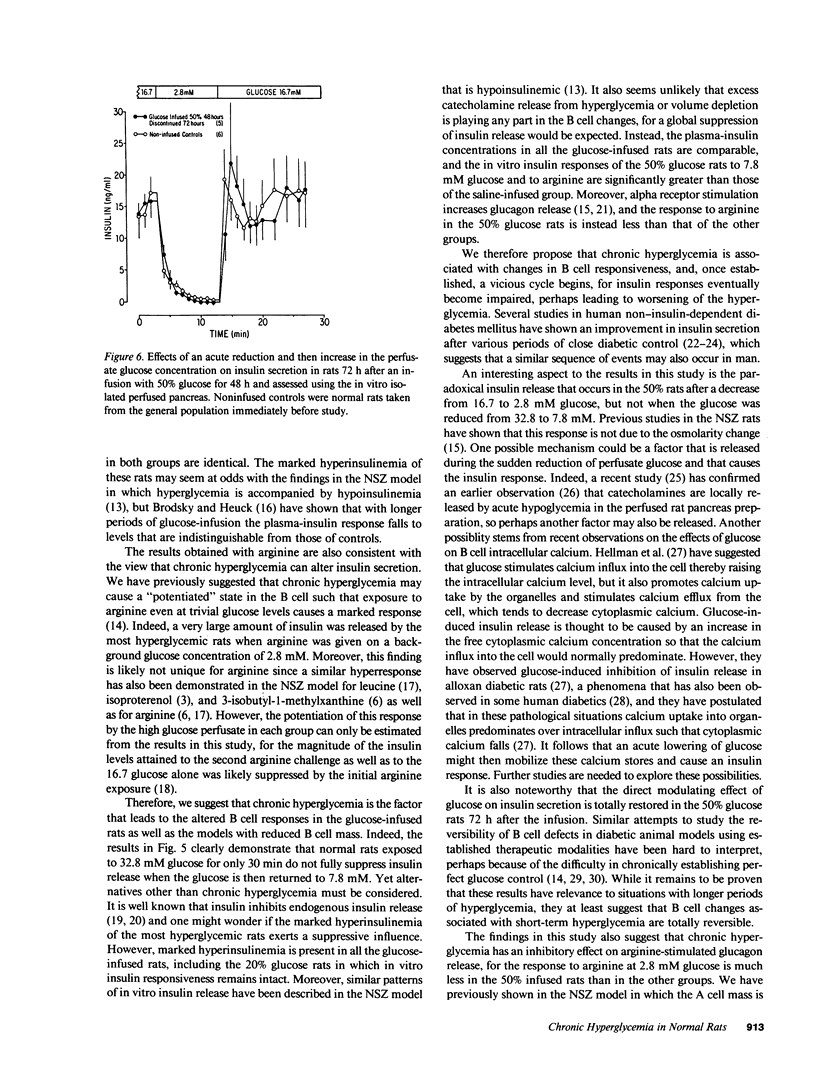
We have proposed that chronic hyperglycemia alters the ability of glucose to modulate insulin secretion, and have now examined the effects of different levels of hyperglycemia on B cell function in normal rats using chronic glucose infusions. Rats weighing 220-300 g were infused with 0.45% NaCl or 20, 30, 35, or 50% glucose at 2 ml/h for 48 h, which raised the plasma glucose by 18 mg/dl in the 30% rats, 37 mg/dl in the 35% rats, and 224 mg/dl in the 50% group. Insulin secretion was then examined using the in vitro isolated perfused pancreas. Glucose-induced insulin secretion remained intact in the normoglycemic 20% glucose rats and it was potentiated in the mildly hyperglycemic 30% glucose rats. However, with even greater hyperglycemia in the 35% glucose group the insulin response to a high glucose perfusate was severely blunted, and it was totally lost in the most hyperglycemic 50% glucose rats. In a second protocol that examined glucose potentiation of arginine-stimulated insulin release, a similar impairment in the ability of glucose to modulate the insulin response to arginine was found with increasing levels of chronic hyperglycemia. On the other hand, the ability of a high glucose concentration to inhibit arginine-stimulated glucagon release was preserved in all glucose-infused rats, but the glucagon levels attained in response to the arginine at 2.8 mM glucose were much less in the 50% glucose rats than in all the other groups. These data clearly show that after 48 h of marked hyperglycemia, glucose influence upon insulin secretion in the rat is severely impaired. This model provides a relatively easy and reproducible method to study the effects of long-term hyperglycemia on B cell function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Honey R. N., Weir G. C. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981 Jan;30(1):64–69. doi: 10.2337/diab.30.1.64. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosky G. M., Heuck C. C. Der Einfluss von Glukoseinfusionen auf die Proinsulinund Insulinsynthese in den Langerhansschen Inseln bei der Ratte. Endokrinologie. 1975 Sep;66(1):46–55. [PubMed] [Google Scholar]
- Christensen N. J., Iversen J. Release of large amounts of noradrenaline from the isolated perfused canine pancreas during glucose deprivation. Diabetologia. 1973 Oct;9(5):396–399. doi: 10.1007/BF01239435. [DOI] [PubMed] [Google Scholar]
- Clark A., Bown E., King T., Vanhegan R. I., Turner R. C. Islet changes induced by hyperglycemia in rats. Effect of insulin or chlorpropamide therapy. Diabetes. 1982 Apr;31(4 Pt 1):319–325. doi: 10.2337/diab.31.4.319. [DOI] [PubMed] [Google Scholar]
- Cole E. H., Logothetopoulos J. Glucose oxidation (14-CO2 production)and insulin secretion by pancreatic islets isolated from hyperglycemic and normoglycemic rats. Diabetes. 1974 May;23(5):469–473. doi: 10.2337/diab.23.5.469. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G., Cryer P., Gerich J. Prolonged hyperglycaemia during infusion of glucose and somatostatin impairs pancreatic A- and B-cell responses to decrements in plasma glucose in normal man: evidence for induction of altered sensitivity to glucose. Diabetologia. 1985 Feb;28(2):63–69. doi: 10.1007/BF00279917. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Schmid F. G., Grodsky G. M. Effect of continuous insulin infusion with an implantable seven-day minipump in the diabetic Chinese hamster. Endocrinology. 1979 May;104(5):1532–1539. doi: 10.1210/endo-104-5-1532. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Giroix M. H., Portha B., Kergoat M., Bailbe D., Picon L. Glucose insensitivity and amino-acid hypersensitivity of insulin release in rats with non-insulin-dependent diabetes. A study with the perfused pancreas. Diabetes. 1983 May;32(5):445–451. doi: 10.2337/diab.32.5.445. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Berry M. N., Williams M. C., Severinghaus E. M. A simple and inexpensive membrane "lung" for small organ perfusion. J Lipid Res. 1974 Mar;15(2):182–186. [PubMed] [Google Scholar]
- Hellman B. Beta-cell cytoplasmic Ca2+ balance as a determinant for glucose-stimulated insulin release. Diabetologia. 1985 Aug;28(8):494–501. doi: 10.1007/BF00281983. [DOI] [PubMed] [Google Scholar]
- Hellman B., Hällgren R., Abrahamsson H., Bergsten P., Berne C., Gylfe E., Rorsman P., Wide L. The dual action of glucose on the cytosolic Ca2+ activity in pancreatic beta-cells. Demonstration of an inhibitory effect of glucose on insulin release in the mouse and man. Biomed Biochim Acta. 1985;44(1):63–70. [PubMed] [Google Scholar]
- Hisatomi A., Maruyama H., Orci L., Vasko M., Unger R. H. Adrenergically mediated intrapancreatic control of the glucagon response to glucopenia in the isolated rat pancreas. J Clin Invest. 1985 Feb;75(2):420–426. doi: 10.1172/JCI111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen J., Miles D. W. Evidence for a feedback inhibition of insulin on insulin secretion in the isolated, perfused canine pancreas. Diabetes. 1971 Jan;20(1):1–9. doi: 10.2337/diab.20.1.1. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Abnormal glucose regulation of insulin secretion in models of reduced B-cell mass. Diabetes. 1984 Jul;33(7):667–673. doi: 10.2337/diab.33.7.667. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Abnormal insulin secretion in a streptozocin model of diabetes. Effects of insulin treatment. Diabetes. 1985 Jul;34(7):660–666. doi: 10.2337/diab.34.7.660. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Weir G. C. Unresponsiveness to glucose in a streptozocin model of diabetes. Inappropriate insulin and glucagon responses to a reduction of glucose concentration. Diabetes. 1985 Jul;34(7):653–659. doi: 10.2337/diab.34.7.653. [DOI] [PubMed] [Google Scholar]
- Levin S. R., Grodsky G. M., Hagura R., Smith D. F., Forsham P. H. Relationships between arginine and glucose in the induction of insulin secretion from the isolated, perfused rat pancreas. Endocrinology. 1972 Mar;90(3):624–631. doi: 10.1210/endo-90-3-624. [DOI] [PubMed] [Google Scholar]
- Logothetopoulos J., Valiquette N. Hormonal and non-hormonal protein biosynthesis in the pancreatic beta cell of the intact rat after prolonged hyperglycaemia. Acta Endocrinol (Copenh) 1984 Nov;107(3):382–389. doi: 10.1530/acta.0.1070382. [DOI] [PubMed] [Google Scholar]
- Nesher R., Waldman L., Cerasi E. Time-dependent inhibition of insulin release: glucose-arginine interactions in the perfused rat pancreas. Diabetologia. 1984 Feb;26(2):146–149. doi: 10.1007/BF00281123. [DOI] [PubMed] [Google Scholar]
- Orland M. J., Chyn R., Permutt M. A. Modulation of proinsulin messenger RNA after partial pancreatectomy in rats. Relationships to glucose homeostasis. J Clin Invest. 1985 Jun;75(6):2047–2055. doi: 10.1172/JCI111924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E., Weir G. C. Adrenergic modulation of pancreatic A, B, and D cells alpha-Adrenergic suppression and beta-adrenergic stimulation of somatostatin secretion, alpha-adrenergic stimulation of glucagon secretion in the perfused dog pancreas. J Clin Invest. 1979 Feb;63(2):230–238. doi: 10.1172/JCI109294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service F. J., Nelson R. L., Rubenstein A. H., Go V. L. Direct effect of insulin on secretion of insulin, glucagon, gastric inhibitory polypeptide, and gastrin during maintenance of normoglycemia. J Clin Endocrinol Metab. 1978 Sep;47(3):488–493. doi: 10.1210/jcem-47-3-488. [DOI] [PubMed] [Google Scholar]
- Trent D. F., Fletcher D. J., May J. M., Bonner-Weir S., Weir G. C. Abnormal islet and adipocyte function in young B-cell-deficient rats with near-normoglycemia. Diabetes. 1984 Feb;33(2):170–175. doi: 10.2337/diab.33.2.170. [DOI] [PubMed] [Google Scholar]
- Vague P., Moulin J. P. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982 Feb;31(2):139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Clore E. T., Zmachinski C. J., Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981 Jul;30(7):590–595. doi: 10.2337/diab.30.7.590. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker P., Logothetopoulos J. Persisting enhanced proinsulin-insulin and protein biosynthesis (3H-leucine incorporation) by pancreatic islets of the rat after glucose exposure. Diabetes. 1975 Feb;24(2):194–200. doi: 10.2337/diab.24.2.194. [DOI] [PubMed] [Google Scholar]



