Abstract
Background and Aims:
Intravenous agents such as propofol are commonly used to maintain adequate depth of anesthesia. Dexmedetomidine which has an anesthetic sparing effect is being considered for maintaining intraoperative depth of anesthesia. We hypothesized to compare the effect of dexmedetomidine on depth of anesthesia with propofol and evaluated whether dexmedetomidine can be used as sole anesthetic agent in maintaining depth of anesthesia.
Materials and Methods:
Sixty patients of ASA PS I, 18-65 years of age, scheduled for laparotomy under general anesthesia were randomly divided into two groups of 30 each. Group A received propofol 1 mg/kg bolus followed by infusion (50 mcg/kg/min) and Group B received dexmedetomidine 1 mcg/kg bolus followed by infusion (0.5 mcg/kg/h). Both the groups were administered standard general anesthesia with routine monitoring along with Bispectral index (BIS) and values were recorded at intervals of 10 min. In all patients Ramsay sedation score was recorded after extubation and they were assessed for recall of intraoperative events using Modified Brice questionnaire.
Results:
Heart rate and mean arterial pressure were less in Group B than Group A. Intraoperative BIS values were significantly lower in Group B (P < 0.0001). Although sedation score was more in Group B it did not prolong recovery. No recall was found in any patient.
Conclusion:
Dexmedetomidine was comparable with propofol in maintaining anesthesia and it can produce better control of hemodynamics and BIS value. Thus dexmedetomidine can be used as the sole maintenance anesthetic agent.
Keywords: BIS, Bispectral index, dexmedetomidine, propofol, recall, sedation score
Introduction
Balanced general anesthesia comprises amnesia, analgesia, hypnosis, muscle relaxation, and obtundation of reflexes. To achieve all these components, we use different combinations of drugs. However muscle relaxants may confound our evaluation of depth of anesthesia due to lack of motor activity and may make the patient aware of the intraoperative events. This false impression about depth of sedation may lead to severe posttraumatic stress disorder. Bispectral index (BIS) is a widely used quantitative parameter for evaluating depth of anesthesia and sedation.[1] It is a continuous noninvasive electroencephalographic method that has been proposed to monitor the hypnotic state during sedation and anesthesia.[1]
Inhalational agents have been employed to avoid intraoperative awareness. Among intravenous agents, propofol is commonly used for intraoperative sedation. The alpha-2 receptor agonist dexmedetomidine has potent sedative properties and has received Food and Drug Administration approval for use in the intensive care unit (ICU) for sedation.[2] Dexmedetomidine has analgesic sparing properties and is not associated with respiratory depression in therapeutic doses.[3,4]. It has been used as an anesthetic sparing agent but not as a sole anesthetic agent.[5] We have proposed to evaluate the effect of dexmedetomidine on depth of anesthesia and compared it with that of propofol by using hemodynamic variables and BIS values, with the aim of studying the feasibility of dexmedetomidine as a sole anesthetic agent in maintaining depth of anesthesia.
Materials and Methods
Approval for the study was obtained from our institution's ethics committee and written informed consent was obtained from the patients. Sixty male and female patients, 18-65 years of age, ASA physical status of I, scheduled for laparotomy under general anesthesia were considered for enrolment randomly into two groups (30 patients in each) by a computer-generated randomization scheme. Patients having heart block, hypertension, diabetes mellitus, renal dysfunction, psychiatric illness, or patients taking antipsychotic drugs or sedatives were excluded from the study. The patients were received at the operation room in a calm and quiet environment. Demographic data were recorded. Baseline hemodynamic variables were noted by attaching monitors [noninvasive blood pressure, heart rate (HR), oxygen saturation, end-tidal carbon dioxide, electrocardiogram, and temperature]. BIS monitor was also attached. Intravenous cannulation was achieved and infusion of study drugs was initiated. Group A patients received bolus dose of propofol (1 mg/kg for 10 min) followed by an infusion of propofol (50 mcg/kg/min) and Group-B received bolus dose of dexmedetomidine (1 mcg/kg for 10 min) followed by an infusion of dexmedetomidine (0.5 mcg/kg/h). After preoxygenation for 3 min, anesthesia was induced with intravenous (I.V) thiopentone sodium (3-5 mg/kg). Intubation was achieved with I.V succinylcholine (1-1.5 mg/kg). Fentanyl (2 mcg/kg) was administered as analgesic intravenously. Intraoperative muscle relaxation was maintained with vecuronium (0.1 mg/kg). Neuromuscular monitoring could not be done due to unavailability of instrument. Sedation and anesthesia was maintained with infusion of study drugs (propofol or dexmedetomidine).
Depth of anesthesia was evaluated by monitoring intraoperative hemodynamic values (HR, blood pressure) and BIS value. BIS was recorded by Aspect monitor with surface electrodes. Sensors were placed diagonally on the forehead after wiping skin with alcohol and drying. Sensors were placed as follows: One at the center of forehead approximately 2 inches above the bridge of nose; second one directly above forehead, and third one on temple between the corner of the eye and hairline. Data were collected at regular 10-min intervals. Administration of drug and data collection were done by a separate anesthesiologist. Target BIS value was 40-65. At emergence, sedation score was assessed by Ramsay sedation scale[6] in the immediate postoperative period. All the patients were asked about recall of intraoperative events using modified Brice questionnaire.[7]
The statistical analysis was done using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows. Parameters [age, gender, weight, mean arterial pressure (MAP), HR, BIS value, sedation score] were compared between the two groups by unpaired t test. All data with P < 0.05 were considered as significant. Abouleish and Taylor modification of the Brice questionnaire:[7]

Ramsay Sedation Score

Results
Sixty patients were enrolled as per inclusion criteria. They were randomly divided into two groups. Group A received propofol and Group B received dexmedetomidine. These two groups were comparable in respect to demographic variables (age, weight, gender, ASA PS I, type of surgery, and duration of anesthesia) [Table 1 and Figure 1].
Table 1.
Demographic data
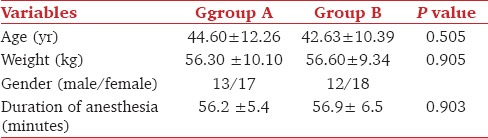
Figure 1.
Demographic data of two groups
The two groups were also comparable with respect to their baseline MAP and HR. Postintubation rise in MAP and HR was noted. Subsequently, MAP and HR were decreased in both the groups. Postintubation rise was less in Group B. Subsequent MAP and HR were also less in Group B. But the difference between the two groups was statistically significant only for MAP at 20 min after infusion (P = 0.039) [Figures 2 and 3].
Figure 2.
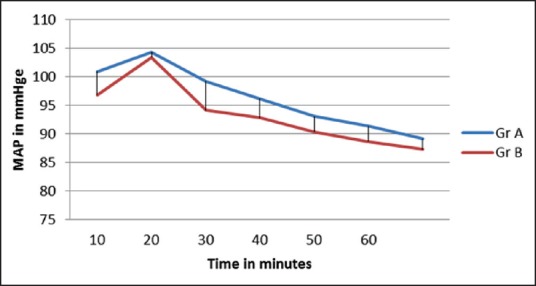
Trend of mean arterial pressure in two groups
Figure 3.
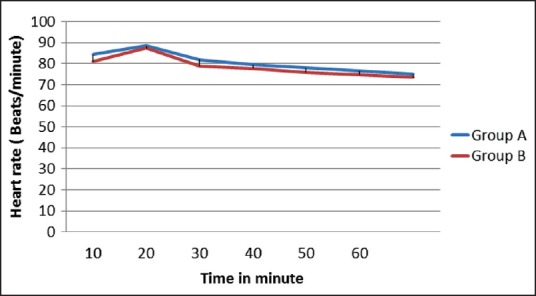
Trend of heart rate in two groups
Baseline BIS value was comparable between the two groups. BIS value was recorded at every 10 min interval. BIS value decreased after induction in both the groups but patients getting dexmedetomidine infusion showed statistically significant lower values of BIS when compared with propofol (P < 0.0001) [Table 2 and Figure 4].
Table 2.
Intraoperative BIS value
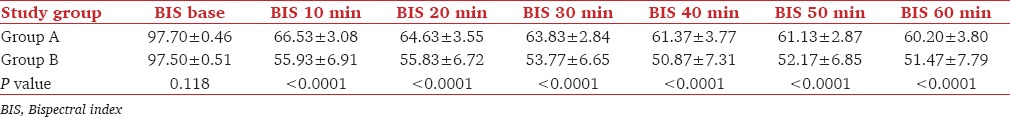
Figure 4.
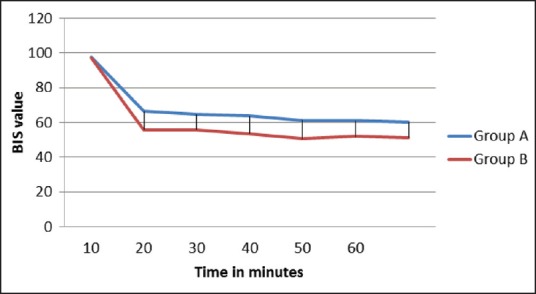
Trend of bispectral index in the two groups
Sedation score was noted in the immediate postoperative period by Ramsay sedation scale. It was seen that patients recieving dexmedetomidine were more sedated in the postoperative period, but it did not impair ventilation though the difference between the two groups was statistically significant (P < 0.0001) [Table 3 and Figure 5]. None of the patients in both the groups was able to recall any intraoperative events.
Table 3.
Sedation score in immediate postoperative period

Figure 5.
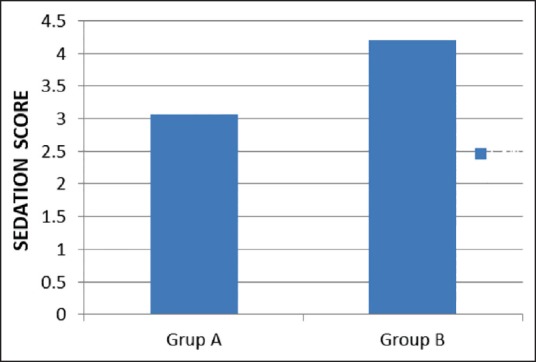
Sedation score of both groups
Discussion
General anesthesia has several components, namely, amnesia, hypnosis, analgesia, immobility, and blunting of autonomic reflexes. Often in operating rooms anesthetic induction has been referred to as “going to sleep.” But all the components of general anesthesia may not be achieved as muscle relaxants may confound our evaluation of depth of anesthesia due to lack of motor activity and may make patients aware of the intraoperative events. For achieving adequate depth of anesthesia, use of inhalational or intravenous anesthetic agents in hypnotic doses are recommended which ensures amnesia in patients. However, it has been observed that certain anesthetic drugs in subhypnotic concentrations produce amnesia, (eg, propofol, midazolam) due to engrams (physical alterations in neural tissues thought to be substrate for memory) impaired by these drugs. In these cases of episodic amnesia, memory is lost before consolidation.[8] In our study, we have similarly used subhypnotic equisedative doses of propofol and dexmedetomidine and observed episodic amnesia in these cases. Similar dosing pattern of bolus and infusion were used by previous authors who used a higher loading dose of dexmedetomidine.[9] In the current study, a minimum dose of both the drugs was chosen that ensured amnesia but caused least side effects such as hypotension, bradycardia, or arrhythmia Alpha-2 agonist dexmedetomidine is a sedative and analgesic agent, which has been used for ICU sedation upto 24 h after surgery.[9] Dexmedetomidine provides hemodynamic stability and appears to have no clinically important adverse effect on respiration.[10] Compared with propofol and midazolam, dexmedetomidine was as effective in maintaining adequate sedation for prolonged mechanical ventilation.[11] Dexmedetomidine has also been used for sedation for short surgical procedures and as an adjunct resulting in reduced intraoperative requirements of anesthetics.[12,13] It has been used as sole anesthetic agent only in a few occasions,[14,15] whereas propofol has been used to maintain the depth of anesthesia routinely. The results of our study validate the use of dexmedetomidine as a maintenance anesthetic agent.
Loading dose in this study has been reduced as maximum adverse effects were observed immediately after administration of the loading dose. Target BIS is delayed when loading dose is reduced but in this study target BIS (40-65) was achieved with the addition of an induction agent.[13] Dexmedetomidine even in lower loading dose produced better amnesia for procedural sedation compared with remifentanil.[13] Ramsay and his co-workers reported the use of dexmedetomidine at a higher dose for intubation in difficult airway scenarios without any induction agent or neuromuscular blocker and observed hypotension, bradycardia, and upper airway obstruction.[14] In the current study, along with study drug, induction agent and neuromuscular blockers were used to facilitate endotracheal intubation and much less dose could be used in contrast to the cases reported by Ramsay.
BIS is a continuous noninvasive electroencephalographic method that has been proposed to monitor the hypnotic state during sedation and anesthesia.[16,17,18] Its use has been documented to be associated with lesser chance of intraoperative awareness.[19] In a study on volunteers, Yusuke and his co-workers compared BIS value with a clinical scoring [Observer Assessment of Alertness Sedation Score (OAA/S)] both with propofol and dexmedetomidine.[1] They found that equivalent doses of dexmedetomidine produced lower BIS value and the BIS value of 46 with dexmedetomidine was comparable to BIS value of 67 with propofol both of which produced OAA/S score ≤2 (OAA/S ≤2 denotes adequate sedation). In the current study patients getting dexmedetomidine infusion showed statistically significant lower values of BIS when compared with propofol (BIS value 60-65 in propofol as opposed to 50-55 with dexmedetomidine). Despite the difference in BIS equal depth was achieved with both the drugs with no recall in any patient as assessed by modified Brice questionnaire. This may be explained by the different mechanisms of sedation by the two study drugs. Dexmedetomidine produces sleep by hyperpolarization of noradrenergic locus ceruleus neurons as opposed to GABA agonism by propofol.[20]
A rise in MAP and HR was noted after intubation with subsequent fall of both in the two groups. Postintubation rise was less in Group B. Subsequent MAP and HR were also less in Group B. But the difference between the two groups was statistically significant only for MAP at 20 min of infusion (P = 0.039). Earlier study has also shown that dexmedetomidine attenuates stress response to intubation by decreasing central sympathetic outflow.[5] Similar hemodynamic response was found by various other studies.[21,22,23] Another study done in bariatric surgery found dexmedetomidine produces better control of hemodynamic parameters compared with fentanyl.[24] Sedation score was calculated in both the groups in immediate postoperative period by Ramsay sedation scale. It was seen that patients receiving dexmedetomidine were more sedated during postoperative period (P value < 0.0001), but without any impairment of ventilation. It is consistent with the result of the study done by Venn and Grounds who also found more sedation with dexmedetomidine postoperatively but without any delay in extubation.[9] The longer sedation with dexmedetomidine could be explained by longer elimination half-life of the drug.[25] But incidence of delayed recovery and longer discharge time with dexmedetomidine were observed by other investigators.[26,27,28] The observations of the present study could have been more conclusive with larger sample size, multicenter trial and measurement of plasma levels of drugs.
Conclusion
From our study we can conclude that dexmedetomidine is comparable with propofol as maintenance anesthetic agent and it can produce better control of hemodynamic variables and BIS value. So, dexmedetomidine may be used as sole anesthetic agent for maintenance of depth of anesthesia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anaesth Analg. 2009;109:1811–5. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine--a novel alpha 2-adrenoceptor agonist — in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 4.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative hemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56:542–6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abouleish E, Taylor FH. Effect of morphine-diazepam on signs of anesthesia, awareness and dreams of patients under N2O for cesarian section. Anesth Analg. 1976;55:702–6. doi: 10.1213/00000539-197609000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller DR. 7th ed. Philadelphia, Pennsylvania: Churchill Livingstone; 2010. Text book of Miller's anaesthesia; p. 1229. [Google Scholar]
- 9.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 10.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 11.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012;307:1151–60. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–81. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Cattano D, Lam NC, Ferrario L, Seitan C, Vahdat K, Wilcox DW, et al. Dexmedetomidine versus remifentanil for sedation during awake fibre optic intubation. Anaesth Res Pract. 2012;10:753–60. doi: 10.1155/2012/753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Shukry M, Kennedy K. Dexmedetomidine as a total intravenous anesthetic in infants. Pediatr Anaesth. 2007;17:581–3. doi: 10.1111/j.1460-9592.2006.02171.x. [DOI] [PubMed] [Google Scholar]
- 16.Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Singh H, White PF. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth Analg. 1997;84:185–9. doi: 10.1097/00000539-199701000-00033. [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: The B-Aware randomised controlled trial. Lancet. 2004;363:1757–63. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 20.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992;75:940–6. [PubMed] [Google Scholar]
- 22.Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997;52:736–44. doi: 10.1111/j.1365-2044.1997.169-az0303.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97:658–65. doi: 10.1093/bja/ael220. [DOI] [PubMed] [Google Scholar]
- 24.Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006;18:24–8. doi: 10.1016/j.jclinane.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: A meta-analysis. Am J Med. 2003;114:742–52. doi: 10.1016/s0002-9343(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 26.Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: A comparative study with remifentanil in gynecologic videolaparoscopic surgery. J Clin Anesth. 2007;19:280–5. doi: 10.1016/j.jclinane.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Salman N, Uzun S, Coskun F, Salman MA, Salman AE, Aypar U. Dexmedetomidine as a substitute for remifentanil in ambulatory gynecologic laparoscopic surgery. Saudi Med J. 2009;30:77–81. [PubMed] [Google Scholar]
- 28.Turgut N, Turkmen A, Ali A, Altan A. Remifentanil-propofol vs dexmedetomidine-propofol — anesthesia for supratentorial craniotomy. Middle East J Anesthesiol. 2009;20:63–70. [PubMed] [Google Scholar]


