Abstract
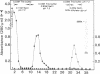
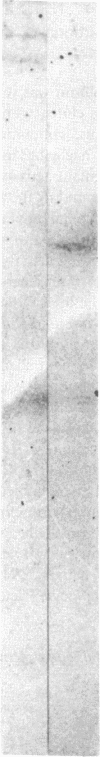
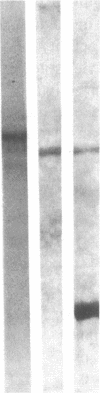
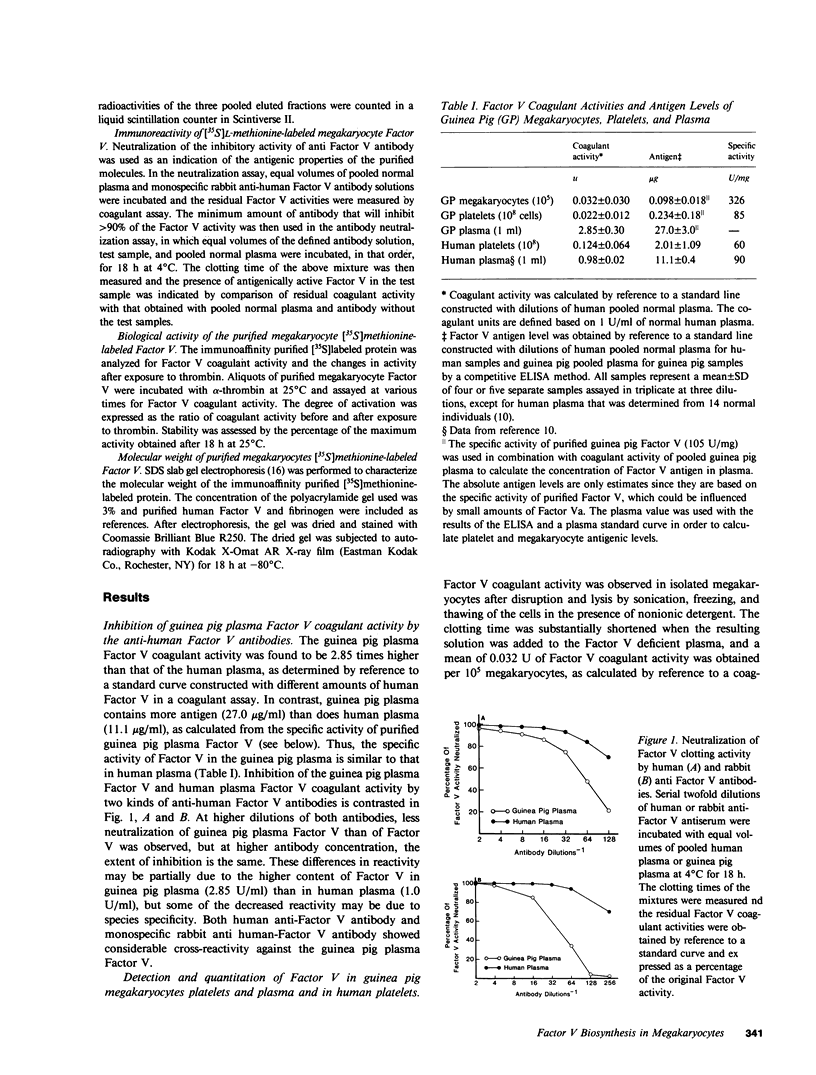
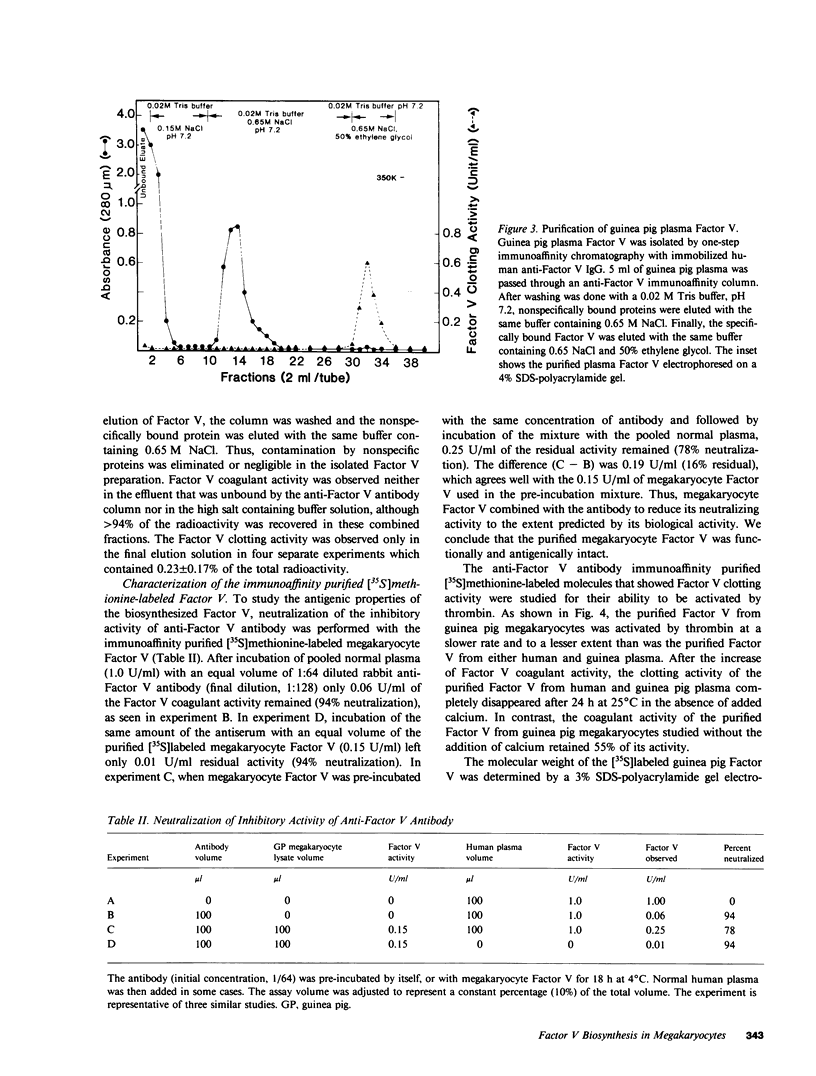
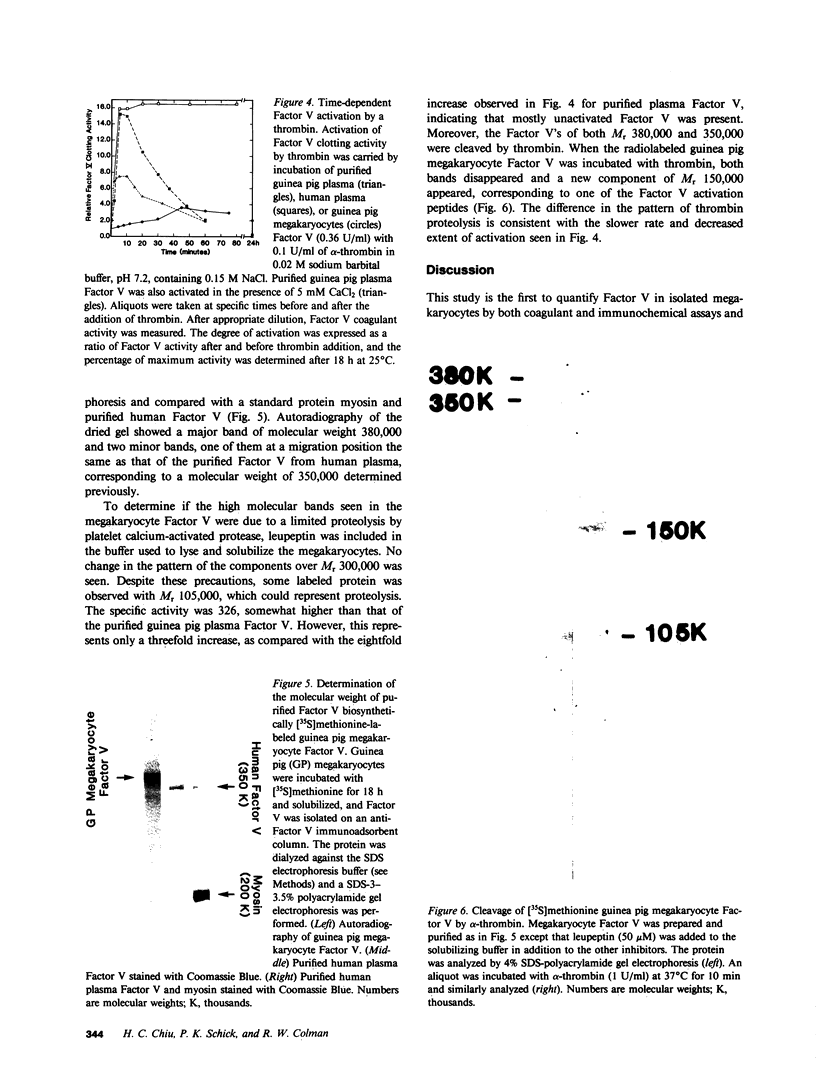
Although platelets contain Factor V, localized primarily in the alpha-granules, the origin of this coagulation cofactor in these cells is not known. We therefore explored whether isolated megakaryocytes could biosynthesize Factor V. Guinea pig plasma Factor V coagulant activity was demonstrated to be neutralized by human monoclonal and rabbit polyclonal antibodies directed monospecifically against human Factor V. These antibodies had been used earlier to purify human Factor V. These antibodies had been used earlier to purify human Factor V and to quantify Factor V antigen concentration, respectively (1983. Chiu, H. C., E. Whitaker, and R. W. Colman. J. Clin. Invest. 72:493-503). As determined by a competitive enzyme-linked immunosorbent assay with guinea pig plasma as a standard, Factor V solubilized from guinea pig megakaryocytes was present at 0.098 +/- 0.018 micrograms/10(5) cells. Each megakaryocyte contained about 500 times as much Factor V as is in a platelet (0.234 +/- 0.180 micrograms/10(8) platelets). The content of Factor V antigen in guinea pig plasma was greater (27.0 +/- 3.0 micrograms/ml) than that of Factor V antigen in human plasma (11.1 +/- 0.4 micrograms/ml). In contrast, human platelets contain ninefold more Factor V antigen (2.01 +/- 1.09 micrograms/10(8) platelets) than do guinea pig were 2.85 +/- 0.30 U/ml plasma, 0.022 +/- 0.012 U/10(8) platelets, and 0.032 +/- 0.03 U/10(5) megakaryocytes, compared with human values of 0.98 +/- 0.02 U/ml plasma and 0.124 +/- 0.064 U/10(8) platelets. Isolated megakaryocytes were found to contain Factor V by cytoimmunofluorescence. The megakaryocytes were incubated with [35S]methionine, and radiolabeled intracellular proteins purified were on a human anti-Factor V immunoaffinity column. The purified protein exhibited Factor V coagulant activity and neutralized the inhibitory activity of a rabbit antihuman Factor V antibody, which suggests that megakaryocyte Factor V is functionally and antigenically intact. These results indicate that Factor V is synthesized by guinea pig megakaryocytes. Nonetheless, megakaryocyte Factor V was more slowly activated by thrombin and in the absence of calcium was more stable after activation than was plasma Factor Va. Electrophoresis in sodium dodecyl sulfate and autoradiography of the purified molecule showed a major band of Mr 380,000 and a minor band of Mr 350,000, as compared with guinea pig and human plasma Factor V, where the protein had an Mr of 350,000. Both forms of Factor V were substrates for thrombin. Possible explanations for the higher molecular weight and different thrombin sensitivity and stability observed are that a precursor of Factor V was isolated or that the megakaryocyte Factor V had not been fully processed before isolation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breederveld K., Giddings J. C., ten Cate J. W., Bloom A. L. The localization of factor V within normal human platelets and the demonstration of a platelet-factor V antigen in congenital factor V deficiency. Br J Haematol. 1975 Mar;29(3):405–412. doi: 10.1111/j.1365-2141.1975.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Pifer D., Colman R. W. Subcellular localization and secretion of factor V from human platelets. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5180–5184. doi: 10.1073/pnas.78.8.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H. C., Whitaker E., Colman R. W. Heterogeneity of human factor V deficiency. Evidence for the existence of antigen-positive variants. J Clin Invest. 1983 Aug;72(2):493–503. doi: 10.1172/JCI110997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtubise P. E., Coots M. C., Jacob D. J., Muhleman A. F., Glueck H. I. A monoclonal IgG4 (lambda) with factor V inhibitory activity. J Immunol. 1979 May;122(5):2119–2121. [PubMed] [Google Scholar]
- Ittyerah T. R., Rawala R., Colman R. W. Factor V binding to human platelets: the role of divalent cations and of platelet preparatory methods. Thromb Res. 1982 Apr 1;26(1):59–71. doi: 10.1016/0049-3848(82)90150-5. [DOI] [PubMed] [Google Scholar]
- Ittyerah T. R., Rawala R., Colman R. W. Immunochemical studies of factor V of bovine platelets. Eur J Biochem. 1981 Nov;120(2):235–241. doi: 10.1111/j.1432-1033.1981.tb05694.x. [DOI] [PubMed] [Google Scholar]
- Kane W. H., Lindhout M. J., Jackson C. M., Majerus P. W. Factor Va-dependent binding of factor Xa to human platelets. J Biol Chem. 1980 Feb 10;255(3):1170–1174. [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. The interaction of human coagulation factor Va with platelets. J Biol Chem. 1982 Apr 10;257(7):3963–3969. [PubMed] [Google Scholar]
- Kiesselbach T. H., Wagner R. H. Demonstration of factor XIII in human megakaryocytes by a fluorescent antibody technique. Ann N Y Acad Sci. 1972 Dec 8;202:318–328. doi: 10.1111/j.1749-6632.1972.tb16344.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. Synthesis of actin by cultured guinea pig megakaryocytes. Complex formation with fibrin. Biochim Biophys Acta. 1978 Sep 21;543(1):91–105. doi: 10.1016/0304-4165(78)90457-9. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Katzmann J. A., Tracy P. B., Mann K. G. Factor V. Methods Enzymol. 1981;80(Pt 100):249–274. doi: 10.1016/s0076-6879(81)80023-7. [DOI] [PubMed] [Google Scholar]
- Rabellino E. M., Levene R. B., Leung L. L., Nachman R. L. Human megakaryocytes. II. Expression of platelet proteins in early marrow megakaryocytes. J Exp Med. 1981 Jul 1;154(1):88–100. doi: 10.1084/jem.154.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E. M., Nachman R. L., Williams N., Winchester R. J., Ross G. D. Human megakaryocytes. I. Characterization of the membrane and cytoplasmic components of isolated marrow megakaryocytes. J Exp Med. 1979 Jun 1;149(6):1273–1287. doi: 10.1084/jem.149.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryo R., Nakeff A., Huang S. S., Ginsberg M., Deuel T. F. New synthesis of a platelet-specific protein: platelet factor 4 synthesis in a megakaryocyte-enriched rabbit bone marrow culture system. J Cell Biol. 1983 Feb;96(2):515–520. doi: 10.1083/jcb.96.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K., Chase P. R. Lipid composition of guinea pig platelets and megakaryocytes. The megakaryocyte as a probable source of platelet lipids. Biochim Biophys Acta. 1981 Jan 26;663(1):239–248. doi: 10.1016/0005-2760(81)90210-1. [DOI] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K., Chase P. R. Lipid composition of guinea pig platelets and megakaryocytes. The megakaryocyte as a probable source of platelet lipids. Biochim Biophys Acta. 1981 Jan 26;663(1):239–248. doi: 10.1016/0005-2760(81)90210-1. [DOI] [PubMed] [Google Scholar]
- Schick B. P., Schick P. K. Cholesterol and phospholipid biosynthesis in guinea pig megakaryocytes. Biochim Biophys Acta. 1981 Jan 26;663(1):249–254. doi: 10.1016/0005-2760(81)90211-3. [DOI] [PubMed] [Google Scholar]
- Schick P. K., Schick B. P., Foster K., Block A. Arachidonate synthesis and uptake in isolated guinea-pig megakaryocytes and platelets. Biochim Biophys Acta. 1984 Sep 12;795(2):341–347. doi: 10.1016/0005-2760(84)90084-5. [DOI] [PubMed] [Google Scholar]
- Schick P. K., Weinstein M. A marker for megakaryocytes: serotonin accumulation in guinea pig megakaryocytes. J Lab Clin Med. 1981 Oct;98(4):607–615. [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Bowie E. J., Mann K. G. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982 Jul;60(1):59–63. [PubMed] [Google Scholar]
- Tracy P. B., Peterson J. M., Nesheim M. E., McDuffie F. C., Mann K. G. Interaction of coagulation factor V and factor Va with platelets. J Biol Chem. 1979 Oct 25;254(20):10354–10361. [PubMed] [Google Scholar]
- Tracy P. B., Rohrbach M. S., Mann K. G. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983 Jun 25;258(12):7264–7267. [PubMed] [Google Scholar]
- Vicic W. J., Lages B., Weiss H. J. Release of human platelet factor V activity is induced by both collagen and ADP and is inhibited by aspirin. Blood. 1980 Sep;56(3):448–455. [PubMed] [Google Scholar]
- Ware A. G., Murphy R. C., Seegers W. H. The Function of Ac-Globulin in Blood Clotting. Science. 1947 Dec 19;106(2764):618–619. doi: 10.1126/science.106.2764.618-a. [DOI] [PubMed] [Google Scholar]