Abstract
Introduction
Historically, Candida albicans has represented the most common cause of candidemia. However, the proportion of bloodstream infections due to non-albicans Candida species has increased. Because of the risk for candidemia in intra-abdominal surgical patients, some experts advocate the use of fluconazole prophylaxis. The impact of this practice on the distribution of Candida species isolated in breakthrough fungal infections in this population is unknown. We examined the association of fluconazole prophylaxis with the distribution of Candida species in intra-abdominal surgery patients.
Methods
We retrospectively identified cases with a positive blood culture (BCx) for Candida among hospitalized adult intra-abdominal surgery patients between July 2005 and October 2012. Distribution of Candida species isolated represented our primary endpoint. Qualifying surgical cases were determined based on a review of discharge International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Patients receiving low-dose fluconazole prior to the positive BCx with a known indication for prophylaxis including neutropenia, ICU exposure or history of organ transplantation were classified as prophylaxis. Appropriateness of fungal treatment was determined by the timing and selection of antifungal agent based on fungal isolate.
Results
Among 10,839 intra-abdominal surgery patients, 227 had candidemia. The most common Candida species isolated was C. albicans (n = 90, 39.6%) followed by C. glabrata (n = 81, 35.7%) and C. parapsilosis (n = 38, 16.7%). Non-albicans Candida accounted for 57.7% of isolates among the 194 non-prophylaxis patients and 75.8% among the 33 prophylaxis patients (P = 0.001). C. glabrata, the most common non-C. albicans species, was more prevalent than C. albicans in persons given prophylaxis, but not in those without prophylaxis. A total of 63% of those with candidemia were treated inappropriately based on the timing and selection of antifungal administration.
Conclusions
Selection pressure from fluconazole prophylaxis in at-risk surgical patients may be associated with a drift toward fluconazole-resistant species in subsequent candidemia. Tools are needed to guide appropriate treatment through the prompt recognition and characterization of candidemia.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-014-0590-1) contains supplementary material, which is available to authorized users.
Introduction
With crude mortality approaching 40%, candidemia continues to take a high toll among certain groups of hospitalized patients [1-5]. In the past, the species most frequently responsible for candidemia and invasive Candida infections was Candida albicans [1]. Over time, however, there has been a shift in the epidemiology of invasive Candida infection, with an increasing proportion being due to species other than C. albicans [2,3].
Invasive Candida is of particular concern in specific populations. Intra-abdominal surgery is a well-recognized risk factor for invasive fungal infection [6,7]. The initial choice of an antifungal is critical in the setting of suspected candidemia, as it is strongly associated with the outcomes. Although well-established evidence-based clinical guidelines help in making correct choices, efforts to ensure appropriate and timely initial antifungal therapy have been complicated by the shifting microbiology and antifungal susceptibility patterns. To address this uncertainty, the guidelines recommend empiric therapy with an echinocandin to treat suspected candidemia in the setting of critical illness, as well as in neutropenia with fluconazole prophylaxis, where non-C. albicans isolates are more likely [8]. Unfortunately, candidal microbiology and thus initial coverage decisions are less clear in the setting of intra-abdominal surgery. One factor that may be driving shifts in candidal populations and antifungal susceptibility among these patients is fluconazole prophylaxis, which although controversial is used nevertheless. The concern stems from several studies in nonsurgical cohorts that have detected an association between fluconazole prophylaxis and increased infection with Candida glabrata and Candida krusei, reflecting the impact of selection pressure with fluconazole prophylaxis [3,9-12]. However, it is unclear whether such selection pressure with a drift toward azole-resistant species is a factor among patients undergoing intra-abdominal surgery who develop candidemia.
The primary aim of our study was to examine whether there is an association between fluconazole prophylaxis and the distribution of Candida species found in subsequent breakthrough candidemia among intra-abdominal surgery patients. We also assessed the appropriateness of empiric antifungal therapy among this group of patients with candidemia based on timing, dose, and susceptibility.
Methods
Study design and data source
We conducted a retrospective multicenter cohort study using the Cerner Health Facts® database. Health Facts is a de-identified database built from participating hospitals’ comprehensive medical records, including time-stamped medication orders and laboratory/microbiology data, admission, and billing information from affiliated patient care locations. Data were included from 97 US hospitals with diversity of geographic location, bed size, and teaching status. Cerner Corporation (Kansas City, MO, USA) has established Health Insurance Portability and Accountability Act-compliant operating policies and procedures using statistical methods for de-identification. With the use of an existing de-identified database, institutional review board oversight was deemed inapplicable under Health and Human Services 45 CFR 46.101 (a) (4).
Cohort selection and study definitions
We included all adult (age ≥18 years old) hospitalized patients discharged between July 2005 and October 2012 with International Classification of Disease, Ninth Revision, Clinical Modification codes for primary or secondary diagnosis of intra-abdominal infection and an invasive abdominal surgery (see Additional file 1) [13]. Patients were included if they had candidemia defined by at least one positive blood culture (BCx) for Candida and no BCx with other fungal genera. If patients had more than one candidemia episode during the study window, only the first episode was evaluated for inclusion in the cohort. For purposes of classification and time reference, the draw time of the first positive BCx for Candida was used.
Fluconazole prophylaxis was defined as having: a low dose of fluconazole (200 to 400 mg) prior to positive BCx for Candida with no prior order for a loading dose of fluconazole or other therapeutic antifungal agent; and a known indication for prophylaxis including neutropenia, ICU exposure, or history of organ transplantation. A small portion (approximately 8% of the cohort) required manual assignment by a clinical pharmacist, who also took other available data elements into consideration (dosage, frequency, and timing with respect to other antifungals ordered).
Appropriate treatment was defined as an order for a loading or therapeutic dose of a qualifying antifungal agent from 96 hours before through 24 hours after positive BCx for Candida. The antifungal agents evaluated included azoles, echinocandins, and amphotericin B compounds. Inappropriate treatment was defined as: no antifungal treatment; no order of a qualifying antifungal agent for a therapeutic dose from 96 hours before through 24 hours after positive BCx for Candida; or cultures grew C. glabrata or C. krusei and the patient was treated only with fluconazole. Treatment appropriateness was classified as indeterminate for other scenarios (for example, patients with orders for a therapeutic antifungal dose more than 96 hours prior to positive BCx for Candida).
Time to surgery was defined by calendar days from the admission date based on the first surgical procedure of interest. Encounters were classified as urgent emergent if the admission type was coded urgent/emergent or the source was the emergency room or trauma center. Bacteremia required at least one positive BCx for bacteria (excluding common skin contaminants) at any time during the hospital encounter. Baseline laboratory values were selected as the first value after admission. Respiratory failure prior to index culture was defined based on mechanical ventilation International Classification of Disease, Ninth Revision, Clinical Modification procedure codes and arterial blood gas values; and cardiac dysfunction was indicated by orders for intravenous pressors. ICU exposure was defined based on pharmacy, microbiology, and laboratory care settings. Comorbid conditions were defined by primary or secondary International Classification of Disease, Ninth Revision, Clinical Modification codes during or within 12 months prior to the hospital encounter.
Statistical analyses
Mean and standard deviation were reported for continuous variables, and frequency and percentage were reported for categorical variables. Differences between categorical variables were assessed via chi-squared or Fisher’s exact tests, while those in the continuous variables were examined using the Wilcoxon rank-sum test. The level of statistical significance was set at a two-sided alpha value of 5%. Analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Study population characteristics
A total of 10,839 patients who underwent intra-abdominal surgery were identified, and 227 patients (2.1%) had candidemia (Figure 1), of whom 33 (14.5%) had undergone fluconazole prophylaxis (Table 1). Among the 227 candidemia patients the mean age was 62.4 years, approximately one-half were male, and 74% were Caucasian. Less than 2% of the study cohort had evidence of antifungal therapy within 30 days prior to admission. The majority of candidemia patients had their positive BCx for Candida >96 hours after admission. Approximately one-half of the study population had the first surgical procedure of interest within 2 days of admission, but nearly one-half had the first surgery 3 days or more after admission. Illness severity in the entire cohort was high, with 54% of the cohort being treated with vasopressors and 68% having respiratory failure prior to positive BCx for Candida. Sixty percent of patients were treated in an ICU and 40% of the cohort had bacteremia at some point during the hospital stay.
Figure 1.
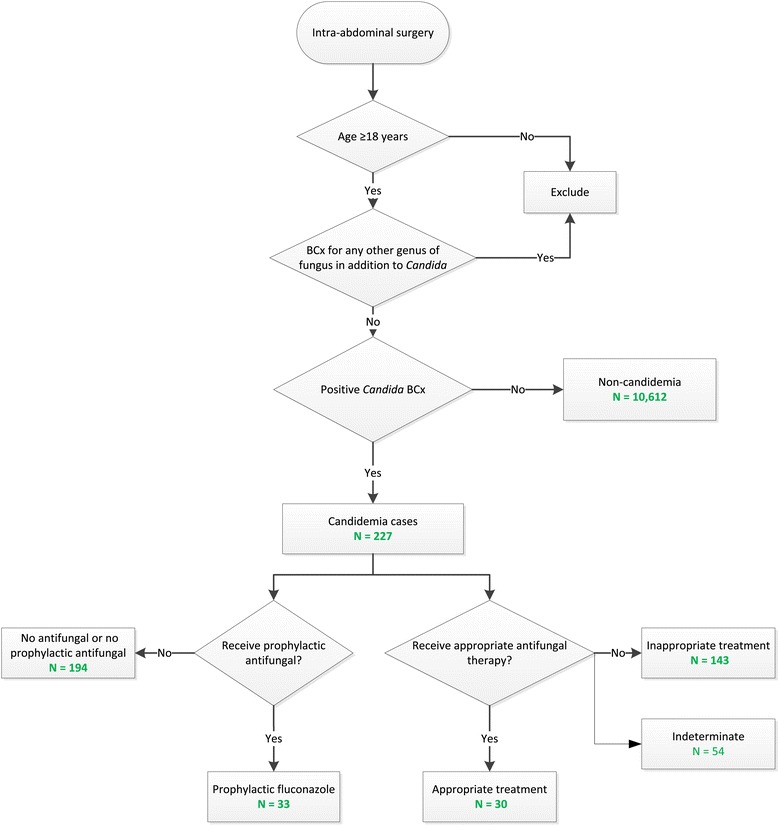
Study population and groups. BCx, blood culture.
Table 1.
Patient demographic and encounter characteristics
| Variable | All candidemia | Fluconazole prophylaxis | No prophylaxis | P value a |
|---|---|---|---|---|
| ( n = 227) | ( n = 33) | ( n = 194) | ||
| Age (years) | 62.4 (15.0) | 61.7 (13.7) | 62.5 (15.2) | 0.6188 |
| Female gender | 118 (52.0%) | 20 (60.6%) | 98 (50.5%) | 0.2835 |
| Race | ||||
| Caucasian | 167 (73.6%) | 24 (72.7%) | 143 (73.7%) | 0.4575 |
| African American | 45 (19.8%) | 9 (27.3%) | 36 (18.6%) | |
| Asian | 2 (0.9%) | 0 (0.0%) | 2 (1.0%) | |
| Other known | 9 (4.0%) | 0 (0.0%) | 9 (4.6%) | |
| Unknown | 4 (1.8%) | 0 (0.0%) | 4 (2.1%) | |
| Admission source | ||||
| Hospital/other care facility | 35 (15.4%) | 5 (15.2%) | 30 (15.5%) | 0.1363 |
| SNF/NH | 3 (1.3%) | 0 (0.0%) | 3 (1.5%) | |
| Emergency room | 102 (44.9%) | 21 (63.6%) | 81 (41.8%) | |
| Other | 78 (34.4%) | 7 (21.2%) | 71 (36.6%) | |
| Unknown | 9 (4.0%) | 0 (0.0%) | 9 (4.6%) | |
| Urgent/emergent admission | 142 (62.6%) | 28 (84.8%) | 114 (58.8%) | |
| Charlson Comorbidity Index score | 2.1 (2.4) | 2.4 (2.9) | 2.0 (2.3) | 0.9624 |
| Comorbid conditions | ||||
| Diabetes | 56 (24.7%) | 10 (30.3%) | 46 (23.7%) | 0.4168 |
| Hypertension | 83 (36.6%) | 10 (30.3%) | 73 (37.6%) | 0.4192 |
| Coronary artery disease | 29 (12.8%) | 7 (21.2%) | 22 (11.3%) | 0.1163 |
| Heart failure | 32 (14.1%) | 6 (18.2) | 26 (13.4%) | 0.4657 |
| Prior stroke/TIA | 12 (5.3%) | 5 (15.2%) | 7 (3.6%) | 0.0062 |
| COPD/bronchiectasis | 36 (15.9%) | 6 (18.2%) | 30 (15.5%) | 0.6927 |
| Encounter events | ||||
| Bacteremia during index encounter | 94 (41.4%) | 18 (54.5%) | 76 (39.2%) | 0.0975 |
| ICU exposure during index encounter | 135 (59.5%) | 30 (90.9%) | 105 (54.1%) | <0.0001 |
| Respiratory failure prior to positive BCx for Candida | 154 (67.8) | 25 (75.8) | 129 (66.5) | 0.2923 |
| IV vasopressor prior to positive BCx for Candida | 123 (54.2) | 24 (72.7) | 99 (51.0) | 0.0207 |
| Baseline laboratory parameters | ||||
| WBC (k/mm3) | 12.8 (8.6) | 13.2 (11.2) | 12.7 (8.1) | 0.597 |
| Neutropenia (ANC <500 cells/mm3) any time | 27 (11.9%) | 9 (27.3%) | 18 (9.3%) | 0.0181 |
| Blood glucose (mg/dl) | 146.5 (72.2) | 145.4 (66.4) | 146.7 (73.3) | 0.8315 |
| eGFR (ml/minute/1.73 m2) | 65.4 (45.8) | 69.8 (69.0) | 64.6 (40.7) | 0.8255 |
| Time from presentation to positive BCx for Candida draw | ||||
| < 48 hours | 16 (7.0%) | 1 (3.0%) | 15 (7.7%) | 0.1914 |
| 48 to 96 hours | 12 (5.3%) | 0 (0%) | 12 (6.2%) | |
| > 96 hours | 199 (87.8%) | 32 (97.0%) | 167 (86.1%) | |
| Time from presentation to initial procedure of interest | ||||
| On the day of admission | 70 (30.8%) | 10 (30.3%) | 60 (30.9%) | 0.5945 |
| 1 to 2 days | 44 (19.4%) | 7 (21.2%) | 37 (19.1%) | |
| ≥ 3 days | 113 (49.8%) | 16 (48.5%) | 97 (50.0%) | |
| Antifungal therapy within 30 days prior to admission | 3 (1.3%) | 1 (3.0%) | 2 (1.0%) | 0.3525 |
Data presented as mean (standard deviation) or number (percentage). ANC, absolute neutrophil count; BCx, blood culture; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IV, intravenous; SNF/NH, skilled nursing facility/nursing home; TIA, transischemic attack; WBC, white blood cell count. a P value for the comparison between fluconazole prophylaxis and nonprophylaxis.
When comparing 33 prophylaxis patients (distributed across 18 institutions) with 194 patients who had not undergone fluconazole prophylaxis (distributed across 47 institutions), illness severity was generally higher in the prophylaxis group as evidenced by the need for vasopressors (73% vs. 51%, P = 0.02) and by having respiratory failure (76% vs. 67%, P >0.05) or hematologic dysfunction (58% vs. 33%, P = 0.007) prior to positive BCx for Candida. Occurrence of bacteremia was also more frequent among patients who received fluconazole prophylaxis compared with those who did not, but this difference did not reach statistical significance (55% vs. 39%, respectively, P = 0.098).
The distribution of Candida species found in positive BCx for Candida is presented in Table 2. Overall, the most common Candida species was C. albicans, followed by C. glabrata and C. parapsilosis. However, within the prophylaxis group, C. glabrata was the most common isolate, followed by C. albicans and C. parapsilosis. The proportion of non-C. albicans species was 76% in the fluconazole prophylaxis group compared with 58% in the nonprophylaxis group (P = 0.001).
Table 2.
Distribution of Candida species based on positive blood culture for total candidemia, prophylaxis and no prophylaxis subgroups
| Candida species | Candidemia | Fluconazole prophylaxis | No prophylaxis | |||
|---|---|---|---|---|---|---|
| ( n = 227) | ( n = 33) | ( n = 194) | ||||
| n | % | n | % | n | % | |
| Albicans | 90 | 39.65 | 8 | 24.24 | 82 | 42.27 |
| Glabrata | 81 | 35.68 | 16 | 48.48 | 65 | 33.51 |
| Parapsilosis | 38 | 16.74 | 6 | 18.18 | 32 | 16.49 |
| Tropicalis | 14 | 6.17 | 2 | 6.06 | 12 | 6.19 |
| Lusitaniae | 3 | 1.32 | 1 | 3.03 | 2 | 1.03 |
| Dubliniensis | 2 | 0.88 | 0 | 0.00 | 2 | 1.03 |
| Krusei | 2 | 0.88 | 1 | 3.03 | 1 | 0.52 |
| Guilliermondii | 1 | 0.44 | 0 | 0.00 | 1 | 0.52 |
| Other | 1 | 0.44 | 0 | 0.00 | 1 | 0.52 |
Appropriateness of therapy
One hundred and forty-three patients (63%) met the criteria for inappropriate therapy based on the timing, dose, and selection of antifungal therapy (Figure 1). Only 30 patients (13%) could be classified as having received appropriate treatment, with antifungal therapy for the remaining patients categorized as indeterminate.
Discussion
Our study has confirmed that C. albicans was the most common species isolate among patients with intra-abdominal surgery and candidemia (39.6%), followed closely by C. glabrata (35.7%). Overall, non-C. albicans species accounted for more than one-half of all isolates and three-quarters of isolates among those who had fluconazole prophylaxis. Most alarmingly, based on timing and selection of empiric coverage, 63% of candidemia patients received treatment categorized as inappropriate based on conservative criteria.
There are several implications to the distribution of non-C. albicans species found among patients with prophylaxis. Admittedly, patients with prophylaxis who developed non-C. albicans candidemia were more severely ill than those without prophylaxis, as evidenced by their need for vasopressors and mechanical ventilation, as well as by their higher likelihood of having bacteremia. One could hypothesize that such patients are more likely to have had prior treatment, and not just prophylaxis, with fluconazole, which may have served as the stimulus for development of resistant species. Our data did not allow us to explore their prior exposure to fluconazole as treatment, an important question to address in future studies. However, the increase in resistant species among patients with prophylaxis also probably points to the selection pressures inherent in antimicrobial prophylaxis in this surgical cohort of patients with intra-abdominal infections, which is not well documented in prior studies. The risks and benefits of fluconazole prophylaxis in this group, although controversial, thus need to continue to be evaluated carefully, given its propensity to drive a switch to resistant species in this deadly disease. Importantly, when faced with a patient who has received prophylaxis, a clinician must consider that such a patient’s risk for a resistant candidemia may be considerably elevated and factor this information into his/her empiric treatment decisions.
Controversy remains regarding antifungal prophylaxis among intra-abdominal surgery patients. Research efforts to resolve these questions are impeded by the need for large sample sizes to demonstrate adjusted differences in clinical outcomes. Changing Candida epidemiology further complicates research and renders some prior studies obsolete. While shown effectively to decrease the incidence of fungal infection in high-risk, critically ill surgical patients [9,14-16], fluconazole prophylaxis does not appear to improve their survival [9,16]. These studies indicate that the benefit to the patient of the decision to use prophylaxis may not exceed the risk of driving the escalation of resistance to existing antifungal treatments. Our data indicate that although only a small proportion of this surgical population received prophylaxis, their risk of a resistant candidal species was high.
While earlier studies reported that non-C. albicans species accounted for one-half or fewer of isolated organisms [17,18], later research uncovered an increase in cases of candidemia involving non-C. albicans [2,3]. We observed a similarly increased proportion of non-C. albicans isolates, most pronounced among patients receiving prophylaxis with fluconazole. This switch in species is concerning, since non-C. albicans species, and in particular C. glabrata, exhibit greater resistance to fluconazole and are associated with worse outcomes when compared with C. albicans [19].
The association of increasingly resistant candidal species causing candidemia and their associated worsened outcomes may be explained by the difficulty of targeting initial empiric therapy amidst the shifting landscape of antifungal susceptibility. Many studies in both candidemia and other serious infections have noted a significant and clinically important rise in the risk of death when the patient does not receive prompt empiric treatment with an agent that covers the culprit pathogen [20-22]. Guidelines for patients with complicated intra-abdominal infections [23] recommend antifungal prophylaxis for healthcare-associated infection and those with severe community-acquired infection, leading with fluconazole. An echinocandin is recommended as initial therapy for critically ill patients, although no guidance is provided with respect to recent fluconazole prophylaxis. The Infectious Disease Society of America Expert Panel favors echinocandins for treating suspected invasive candidiasis in non-neutropenic patients who are moderately or severely ill or had recent azole exposure [8]. Guidelines recommend antifungal agents based on the Candida isolate or suspected isolate, but delaying appropriate selection until culture results are available or until the patient is critically ill may be too late [8,23].
More than 60% of our study population failed to receive appropriate empiric therapy based on timing and coverage (the latter in those whose BCx grew non-C. albicans species). We chose not to evaluate adjusted mortality based on the appropriateness of treatment due to sample size considerations in the context of the low percentage of patients with evidence of appropriate treatment. The challenges that are leading to inappropriate treatment suggest the need for additional improvements to rapidly recognize and characterize candidemia. Risk stratification algorithms and emerging diagnostics such as β-D-glucan may play a role in the future [24-26].
Our study is subject to a number of limitations. As a retrospective cohort, the study is prone to a number of biases, most prominently selection bias. To mitigate this bias, we included all consecutive patients who met our a priori inclusion criteria. Despite the fact that we started out with more than 10,000 surgical patients at risk for candidemia, the final cohort with the infection was relatively small. While it would have been desirable to perform several stratified or adjusted analyses, including those based on dose of fluconazole prophylaxis and year of data with regard to resistance emergence, this limitation precluded such computations. Because physiologic parameters needed to derive such severity of illness scores as the Acute Physiology and Chronic Health Evaluation II score are not available in the study database, we relied on such markers as the need for the ICU, vasopressors, and mechanical ventilation as surrogates. As such, our findings require further exploration in the context of a validated severity of illness system.
Our data may be particularly prone to misclassification of several factors. For example, we used a relatively stringent definition for fluconazole prophylaxis. This was done in order to increase the specificity of the definition. As a consequence, we probably misclassified some patients who did not fit this definition and yet received prophylaxis as not receiving prophylaxis. We chose specificity over sensitivity in order to avoid inflating the magnitude of the differences in the candidal species between the two groups. As a result, the actual differences in the prevalence of potentially azole-resistant species between the groups on and off prophylaxis are probably even greater than what we have observed. On the other hand, it remains possible that at least some of the cases identified as prophylaxis in reality received treatment. However, the stringent nature of our definition of prophylaxis should have minimized such misclassification. The appropriateness of empiric antifungal therapy could not be determined reliably in nearly 25% of the study population based on data elements that were available. However, even in the unlikely event that all of these patients could have been ultimately assigned to the appropriate group, this would not detract from the finding that 63% did not receive appropriate therapy.
A distinct strength of our study, as compared with prior single-center reports, is its multicenter nature, which lends generalizability to our findings.
Conclusions
In summary, our study provides further evidence of the microbiologic shifts in candidemia among a population of patients who are at particular risk for this infection. To the best of our knowledge, this is the first study to examine the association of fluconazole prophylaxis with the candidal species distribution among intra-abdominal surgery patients. The result of this examination points to a likely role for selection pressures in such prophylaxis patients. In turn, the high prevalence of inappropriate treatment detected in our study is an important reminder for clinicians to consider candidemia in a fitting clinical setting, and to be aware of the factors that drive antifungal resistance. Most importantly, in the era of a widening gap between evolving microbial defenses and our abilities to address them, antifungal prophylaxis practices among intra-abdominal surgical patients require a measured re-evaluation.
Key messages
C. albicans was the most common fungal isolate among intra-abdominal surgery patients with candidemia, followed closely by C. glabrata.
The percentage of nonalbicans Candida species was disproportionately high among intra-abdominal surgery patients treated with fluconazole prophylaxis.
The shifting epidemiology of fungal species in intra-abdominal infections and the potential for selection pressure has significant implications to prophylaxis therapy and the empiric treatment of those with suspected infection.
More than 60% of patients did not receive appropriate antifungal therapy based on timing and selection of antifungal agent.
Acknowledgements
The authors gratefully acknowledge assistance from Rob Taylor in data management and Eileen Reyes in project management.
Abbreviation
- BCx
blood culture
Additional file
Is a table presenting the diagnosis of intra-abdominal infection and invasive abdominal surgery by the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code.
Footnotes
Competing interests
MZ has received consulting funding from Astellas in connection with the present project. Unrelated to this project, MZ has received research and/or consulting funding from Cubist, Pfizer, Theravance, ViroPharma, Bard, Forest, and CareFusion. MZ is a stockholder in Johnson & Johnson. MFE and HY are employees and stockholders of Cerner Corporation, which received reimbursement from Astellas for analytic services and data licensing. NK and PC are employees and stockholders of Astellas Pharma US, Inc. AFS has served as a speaker, been a consultant to, and received research support from Astellas. AFS has also served as a speaker, been a consultant to, or received research support from AstraZeneca, Bayer, Bristol-Myers Squibb, Cubist, Forest, Nabriva, Pfizer, and Tetraphase.
Authors’ contributions
MZ, AFS and PC contributed to study conception and design. All authors analyzed and interpreted the data. All authors were members of the writing group and provided input into content approval, draft reviews, and approval of the final version. The authors collectively agree to be accountable to the accuracy and integrity of this study and will ensure that any questions are appropriately investigated and addressed.
Contributor Information
Marya Zilberberg, Email: evimedgroup@gmail.com.
Hsing-Ting Yu, Email: hyu@cerner.com.
Paresh Chaudhari, Email: Paresh.Chaudhari@astellas.com.
Matthew F Emons, Email: MEMONS@CERNER.COM.
Nikhil Khandelwal, Email: Nikhil.Khandelwal@astellas.com.
Andrew F Shorr, Email: andrew.shorr@gmail.com.
References
- 1.Cheng MF, Yang YL, Yao TJ, Lin CY, Liu JS, Tang RB, Yu KW, Fan YH, Hsieh KS, Ho M, Lo HJ. Risk factors for fatal candidemia caused by Candida albicans and non-albicans Candida species. BMC Infect Dis. 2005;5:22. doi: 10.1186/1471-2334-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 3.Kao AS, Brandt ME, Pruitt WR, Conn LA, Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA, Pfaller MA, Pinner RW, Hajjeh RA. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29:1164–1170. doi: 10.1086/313450. [DOI] [PubMed] [Google Scholar]
- 4.Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM. Excess mortality, hospital stay, and cost due to candidemia: a case–control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540–547. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 5.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 7.Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O, Biraghi E, Canton E, Zimmermann K, Seaton S, Grillot R. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis. 2004;23:317–322. doi: 10.1007/s10096-004-1103-y. [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shorr AF, Chung K, Jackson WL, Waterman PE, Kollef MH. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit Care Med. 2005;33:1928–1935. doi: 10.1097/01.CCM.0000178352.14703.49. [DOI] [PubMed] [Google Scholar]
- 10.Tumbarello M, Sanguinetti M, Trecarichi EM, La Sorda M, Rossi M, de Carolis E, de Gaetano DK, Fadda G, Cauda R, Posteraro B. Fungaemia caused by Candida glabrata with reduced susceptibility to fluconazole due to altered gene expression: risk factors, antifungal treatment and outcome. J Antimicrob Chemother. 2008;62:1379–1385. doi: 10.1093/jac/dkn381. [DOI] [PubMed] [Google Scholar]
- 11.Wingard JR, Merz WG, Rinaldi MG, Johnson TR, Karp JE, Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991;325:1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]
- 12.Wingard JR, Merz WG, Rinaldi MG, Miller CB, Karp JE, Saral R. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993;37:1847–1849. doi: 10.1128/AAC.37.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelsberg J, Berger A, Schell S, Mallick R, Kuznik A, Oster G. Economic consequences of failure of initial antibiotic therapy in hospitalized adults with complicated intra-abdominal infections. Surg Infect (Larchmt) 2008;9:335–347. doi: 10.1089/sur.2006.100. [DOI] [PubMed] [Google Scholar]
- 14.Holzknecht BJ, Thorup J, Arendrup MC, Andersen SE, Steensen M, Hesselfeldt P, Nielsen JM, Knudsen JD. Decreasing candidaemia rate in abdominal surgery patients after introduction of fluconazole prophylaxis. Clin Microbiol Infect. 2011;17:1372–1380. doi: 10.1111/j.1469-0691.2010.03422.x. [DOI] [PubMed] [Google Scholar]
- 15.Pelz RK, Hendrix CW, Swoboda SM, Diener-West M, Merz WG, Hammond J, Lipsett PA. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001;233:542–548. doi: 10.1097/00000658-200104000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Playford EG, Webster AC, Sorrell TC, Craig JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother. 2006;57:628–638. doi: 10.1093/jac/dki491. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF, Lazarus DR, Sherner JH, Jackson WL, Morrel M, Fraser VJ, Kollef MH. Do clinical features allow for accurate prediction of fungal pathogenesis in bloodstream infections? Potential implications of the increasing prevalence of non-albicans candidemia. Crit Care Med. 2007;35:1077–1083. doi: 10.1097/01.CCM.0000259379.97694.00. [DOI] [PubMed] [Google Scholar]
- 18.Baran J, Jr, Muckatira B, Khatib R. Candidemia before and during the fluconazole era: prevalence, type of species and approach to treatment in a tertiary care community hospital. Scand J Infect Dis. 2001;33:137–139. doi: 10.1080/003655401750065544. [DOI] [PubMed] [Google Scholar]
- 19.Moran C, Grussemeyer CA, Spalding JR, Benjamin DK, Jr, Reed SD. Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr Infect Dis J. 2009;28:433–435. doi: 10.1097/INF.0b013e3181920ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold HM, Micek ST, Shorr AF, Zilberberg MD, Labelle AJ, Kothari S, Kollef MH. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacotherapy. 2010;30:361–368. doi: 10.1592/phco.30.4.361. [DOI] [PubMed] [Google Scholar]
- 21.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 22.Zilberberg MD, Kollef MH, Arnold H, Labelle A, Micek ST, Kothari S, Shorr AF. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis. 2010;10:150. doi: 10.1186/1471-2334-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 24.Hanson KE, Pfeiffer CD, Lease ED, Balch AH, Zaas AK, Perfect JR, Alexander BD. Beta-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PLoS One. 2012;7:e42282. doi: 10.1371/journal.pone.0042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME. Beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52:750–770. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 26.Tissot F, Lamoth F, Hauser PM, Orasch C, Fluckiger U, Siegemund M, Zimmerli S, Calandra T, Bille J, Eggimann P, Marchetti O. Beta-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med. 2013;188:1100–1109. doi: 10.1164/rccm.201211-2069OC. [DOI] [PubMed] [Google Scholar]


