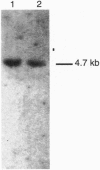
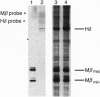
Abstract
Human gamma-globin and delta-globin chains have been previously identified as strong inhibitors of the polymerization of hemoglobin S, in contrast to the beta-globin chain, which exerts only a moderate antisickling effect. However, gamma-globin and delta-globin are normally expressed at very low levels in adult erythroid cells, in contrast to beta-globin. We report the design of a beta-globin/delta-globin hybrid gene, beta/delta-sickle cell inhibitor 1 (beta/delta-SCI1) and its transduction by retrovirus-mediated gene transfer. The beta/delta-SCI1-encoding gene retains the overall structure of the human beta-globin gene, while incorporating specific amino acid residues from the delta chain previously found responsible for its enhanced antisickling properties. To achieve high expression levels of beta/delta-SCI1 in adult erythrocytes, the hybrid gene was placed under the transcriptional control of the human beta-globin promoter and the DNase I hypersensitive site 2 of the human beta locus control region. High-titer retroviruses were generated, and stable proviral transmission was achieved in infected cells. The mRNA expression levels of the beta/delta-SCI1 gene in infected, dimethyl sulfoxide-induced murine erythroleukemia cells approached 85% of the endogenous murine beta maj-globin mRNA, on a per gene basis, evidence that high gene expression levels were achieved in adult erythroid cells. Further evaluation of this strategy in transgenic animal models of sickle cell disease should assess its efficacy for the gene therapy of human patients.
Full text
PDF

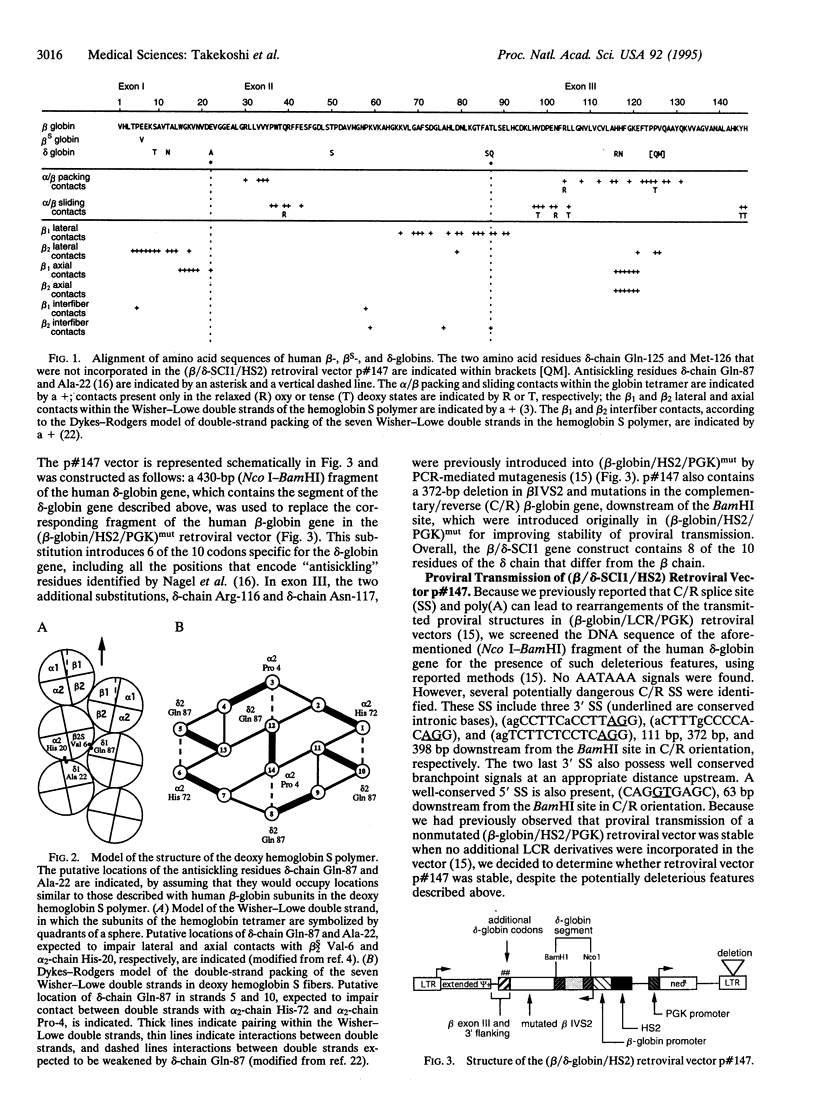


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benesch R. E., Edalji R., Benesch R., Kwong S. Solubilization of hemoglobin S by other hemoglobins. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5130–5134. doi: 10.1073/pnas.77.9.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Liu D., Kan Y. W. A 36-base-pair core sequence of locus control region enhances retrovirally transferred human beta-globin gene expression. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3107–3110. doi: 10.1073/pnas.89.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham R. C., Huehns E. R., Rosemeyer M. A. Participation of haemoglobins A, F, A2 and C in polymerisation of haemoglobin S. J Mol Biol. 1979 Mar 25;129(1):45–61. doi: 10.1016/0022-2836(79)90058-5. [DOI] [PubMed] [Google Scholar]
- Cretegny I., Edelstein S. J. Double strand packing in hemoglobin S fibers. J Mol Biol. 1993 Apr 5;230(3):733–738. doi: 10.1006/jmbi.1993.1195. [DOI] [PubMed] [Google Scholar]
- Curtin P. T., Liu D. P., Liu W., Chang J. C., Kan Y. W. Human beta-globin gene expression in transgenic mice is enhanced by a distant DNase I hypersensitive site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7082–7086. doi: 10.1073/pnas.86.18.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E. Transgenic animal models of sickle cell disease. Experientia. 1993 Jan 15;49(1):28–36. doi: 10.1007/BF01928785. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Kosche K. A., Dobkin C., Bank A. DNA sequences regulating human beta globin gene expression. Nucleic Acids Res. 1985 Nov 11;13(21):7781–7793. doi: 10.1093/nar/13.21.7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboulch P., Huang G. M., Humphries R. K., Oh Y. H., Eaves C. J., Tuan D. Y., London I. M. Mutagenesis of retroviral vectors transducing human beta-globin gene and beta-globin locus control region derivatives results in stable transmission of an active transcriptional structure. EMBO J. 1994 Jul 1;13(13):3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune S. L., Reilly M. P., Chomo M. J., Asakura T., Townes T. M. Recombinant human hemoglobins designed for gene therapy of sickle cell disease. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9852–9856. doi: 10.1073/pnas.91.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Bookchin R. M., Johnson J., Labie D., Wajcman H., Isaac-Sodeye W. A., Honig G. R., Schilirò G., Crookston J. H., Matsutomo K. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci U S A. 1979 Feb;76(2):670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Harris E. A., Forrester W., Groudine M., Gelinas R. High-level beta-globin expression after retroviral transfer of locus activation region-containing human beta-globin gene derivatives into murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3386–3390. doi: 10.1073/pnas.87.9.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavec I., Papayannopoulou T., Maury C., Meyer F. A human beta-globin gene fused to the human beta-globin locus control region is expressed at high levels in erythroid cells of mice engrafted with retrovirus-transduced hematopoietic stem cells. Blood. 1993 Mar 1;81(5):1384–1392. [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol. 1979 Oct 9;133(4):435–467. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D. Y., Solomon W. B., London I. M., Lee D. P. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human "beta-like globin" genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]