Abstract
The NineTeen Complex (NTC) of proteins associates with the spliceosome during pre-messenger RNA splicing and is essential for both steps of intron removal. The NTC and other NTC associated proteins are recruited to the spliceosome where they participate in regulating the formation and progression of essential spliceosome conformations required for the two steps of splicing. It is now clear that the NTC is an integral component of active spliceosomes from yeast to humans and provides essential support for the spliceosomal snRNPs. Here we discuss the identification and characterisation of the yeast NTC and review recent work in yeast that supports the essential role for this complex in the regulation and fidelity of splicing.
Keywords: NineTeen Complex (NTC), Prp19, pre-mRNA splicing, spliceosome
Introduction
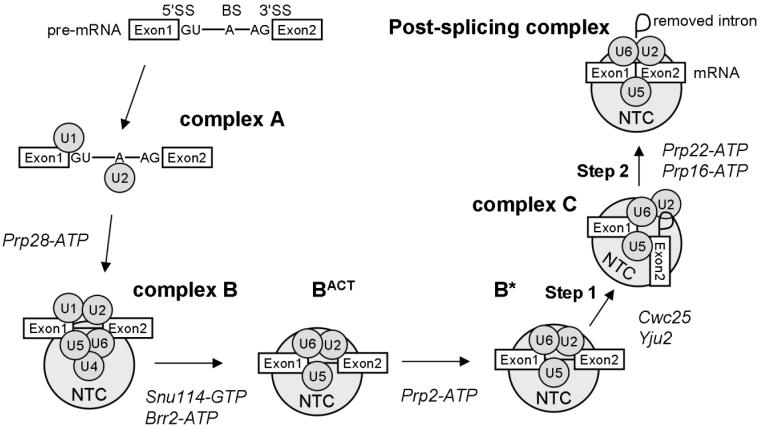
Pre-mRNA (pre-messenger RNA) splicing is the essential process by which introns are removed from pre-mRNA and the exons precisely ligated producing mature mRNAs ready for translation into protein. Splicing occurs by two sequential transesterification reactions, requiring accurate recognition of conserved RNA sequences within introns by a large RNP (ribonucleoprotein) machine called the spliceosome [1]. The spliceosome is composed of five snRNP (small nuclear RNP) particles and numerous protein splicing factors. Each snRNP contains a U-rich snRNA (small nuclear RNA), U1, U2, U4, U5 or U6, and a unique set of proteins. There is a cycle of snRNP assembly and disassembly with intron containing pre-mRNAs that results in the removal of introns (Figure 1). The U1 snRNP first recognises the 5′ splice site with the U1 snRNA base-pairing with a conserved 5′ splice site sequence within the intron. The U2 snRNP then binds the conserved branch site sequence to form complex A. The U2 snRNA forms a helix with the branch site sequence and bulges out a conserved intron adenosine required for the first transesterification reaction. A preformed tri-snRNP composed of the U4, U5 and U6 snRNPs then joins to form complex B. Within the tri-snRNP the U4 and U6 snRNAs are extensively base-paired. To form the active spliceosome (BACT) the U4/U6 base-pairing interaction is unwound allowing U6 to form two mutually exclusive interactions with the 5′ splice site sequence and U2 snRNA, resulting in U1 and U4 destabilisation. These RNA/RNA interactions serve to position the bulged branch site adenosine to attack the 5′ splice site for the first transesterification reaction, producing 5′ exon and intron-3′ exon intermediates. Several rearrangements then occur within the active spliceosome to form complex C for the second transesterification reaction, where the two exons are ligated removing the intron. In addition to the snRNPs, protein splicing factors play key roles in the splicing process [1]. For example, several RNA-dependent ATPases and one GTPase promote the rearrangement of the RNA/RNA and RNA/protein interactions that occur during spliceosome assembly, activation and disassembly [2,3] (Figure 1). Another set of splicing factors, that form a complex called the NTC (NineTeen Complex) in yeast, join the spliceosome before, or during, unwinding of U4 from U6 and stay associated with the spliceosome during the two steps of splicing (Figure 1). In recent years it has become evident that the NTC plays an important role in regulating spliceosome conformations and fidelity. This review will primarily focus on the NTC in the yeast Saccharomyces cerevisiae, however, the NTC is evolutionarily conserved from yeast to humans.
Figure 1.
Complexes formed during spliceosome assembly and activation Intron containing pre-mRNAs with 5′SS (5′ splice site), BS (branch site) and 3′SS (3′ splice site) sequences are recognised by the spliceosomal snRNPs during spliceosome assembly and activation. The U1 and U2 snRNPs interact with the 5′SS and BS, respectively, to form complex A. The ATPase Prp28 then allows the U4/U6.U5 tri-snRNP to assemble with the pre-mRNA along with the NTC proteins to form complex B. The GTPase Snu114 and the ATPase Brr2 induce U4/U6 unwinding with subsequent destabilisation of the U1 and U4 snRNPs to form the complex BACT. The ATPase Prp2 then remodels the spliceosome to the complex B* which is the catalytically active spliceosome. The action of Cwc25 and Yju2 then induce step 1 of splicing and formation of complex C which contains the step 1 intermediates. Finally the ATPases Prp16 and Prp22 induce step 2 of splicing and formation of the post-splicing complex which contains the spliced mRNA and the removed intron. This post-splicing complex is then disassembled and the snRNPs and NTC proteins are recycled for another round of splicing.
Prp19 and the composition of the NTC
The NTC is named after the splicing factor Prp19, which was first identified in 1993 as a splicing factor in the yeast S. cerevisiae [4]. Prp19 is essential for splicing but is not a constituent of any of the individual spliceosomal snRNPs [5,6]. Association of Prp19 with itself to form tetramers provides the basis for the hypothesis that Prp19 provides a scaffold for NTC organisation [7]. Prp19 contains a U-box domain which exhibits E3 ubiquitin ligase activity in vitro [8], however, a target for this activity in the spliceosome is still lacking. Prp19 purified from yeast is associated with a complex of at least ten proteins [9] (Table 1). When the NTC protein Cef1 was used for TAP (Tandem Affinity Purification) a much larger complex was identified containing 27 proteins [10]. Previously unidentified S. cerevisiae proteins in the Cef1-TAP complex were named Cwc (Complexed with Cef1) proteins. However, recent mass spectrometry analysis of different spliceosome complexes suggest that a bona fide yeast NTC consists of eight core proteins (Prp19, Cef1, Syf1, Syf2, Syf3, Snt309, Isy1, and Ntc20) [11] (Table 1). The other NTC associated proteins appear to have a more dynamic association with the NTC and spliceosome. Perhaps the most striking finding of the mass spectrometry analysis was the incorporation of the NTC into the spliceosome B complex, before the release of U1 and U4 [11], because previous immunoprecipitation experiments suggested NTC spliceosome recruitment after B complex formation and upon release of U4 [9,12]. The abundance of NTC proteins is greatly increased in activated spliceosomes suggesting that the NTC may be important for the transition of the spliceosome from the B to BACT complex [11]. Therefore, NTC association with the spliceosome appears to be dynamic and this may reflect the essential roles the NTC proteins play in splicing.
Table 1.
NTC and NTC associated proteins in Saccharomyces cerevisiae
| Yeast gene names | Known domains | Essential | Human protein | |
|---|---|---|---|---|
| Core NTC proteins | PRP19 | U Box, WD40 | Yes | hPRP19 |
| CEF1/NTC85 | c-Myb DNA binding | Yes | CDC5L | |
| CLF1/SYF3/NTC77 | TPR, HAT | Yes | CRNKL1 | |
| SYF1/NTC90 | ROS, HAT, TPR | Yes | hSYF1/XAB2 | |
| SYF2/NTC31 | - | No | GCIP p29 | |
| ISY1/NTC30/UTR3 | - | No | KIAA1160 | |
| SNT309/NTC25 | - | No | SPF27 | |
| NTC20 | - | No | - | |
| NTC associated proteins | CWC2/NTC40 | Zinc finger, RRM | Yes | RBM22 |
| PRP46/NTC50/CWC1 | WD40 | Yes | PRL1 | |
| Bud31/CWC14 | - | No | G10 | |
| CWC15 | - | No | AD-002/HSPC148 | |
| Yju2/CWC16 | - | Yes | CCDC130 | |
| CWC21 | - | No | Srm300 | |
| CWC22 | - | Yes | KIAA1604 | |
| CWC23 | J domain | Yes | - | |
| CWC24 | Zinc finger, RING domain | Yes | RNF113A | |
| CWC25 | - | Yes | CCDC49 | |
| Bud13/CWC26 | - | No | MGC13125 | |
| CWC27 | PPIase cyclophilin-type | No | NY-CO-10 | |
| PRP17/CDC40/SLU4/SLT15 | WD40 | No | hPRP17 | |
| PRP22 | DEAH Box | Yes | hPRP22 | |
| PRP45/FUN20 | SKIP homology | Yes | SKIP1 | |
| SLU7/SLT17 | Zinc finger/knuckle | Yes | hSLU7 | |
| ECM2/SLT11 | Zinc finger, RRM | No | RBM22 | |
| SPP2 | G patch | Yes | GPKOW/T54 |
Regulation by the NTC of spliceosome conformations and fidelity
During spliceosome activation and catalysis the spliceosome adopts specific conformations that promote the two steps of splicing. The two state model of the active spliceosome states that spliceosome conformations that promote either the first or second step of splicing are in kinetic competition [13]. During spliceosome activation the NTC promotes RNA/RNA interactions that form the catalytic core for the first step of splicing [12,14]. For example, the NTC specifies the U6 base-pairing interaction with the 5′ splice site and an interaction of the 3′ end of U6 with the intron. Further, the Lsm proteins are dissociated from the 3′ end of U6 in an NTC dependent manner [12]. The NTC also specifies an interaction of the U5 snRNA with the 5′ exon of the pre-mRNA during spliceosome activation and, in the absence of the NTC, both the U5 and U6 snRNA associations with the active spliceosome are destabilised [12,14]. Therefore, the NTC is essential for specifying snRNA interactions with the pre-mRNA during splicing.
As well as base-pairing with the 5′splice site, U6 also forms helix I with the U2 snRNA, which is proposed to be part of the catalytic sites for both steps of splicing [15]. The ATPase Prp16 has long been proposed to bring about a conformational change after the first step of splicing that is required for the second step of splicing [16]. Recent work suggests that Prp16 promotes unwinding of U2/U6 helix I after the first step of splicing to allow substrate repositioning for the second catalytic reaction [17]. Helix I is then reformed for the second step conformation of the spliceosome. Deletion of the non-essential NTC protein Isy1 (Ntc30) suppresses a mutation in Prp16, suggesting that the NTC may also stabilise the U6 snRNA interaction with U2 for the first step of splicing [18]. The NTC may also be required to stabilise helix I in the second step of splicing as the NTC is associated with the spliceosome throughout the splicing cycle.
Suboptimal pre-mRNA substrates are rejected if they fail to accurately complete the first step of splicing before ATP hydrolysis by Prp16 [16]. This forms the mechanism by which the spliceosome can maintain fidelity by kinetic proofreading [19]. Mutation of the ATPase domain of Prp16 stabilises, and therefore slows exit from, the first step conformation of the spliceosome. This reduces the fidelity of branch site selection in the first transesterification reaction. The role of the NTC in the formation and stabilisation of RNA/RNA interactions in the spliceosome suggests its function may also be required to maintain splicing fidelity. In fact, deletion of Isy1 reduces the fidelity of 3′ splice selection and also restores fidelity of branch site selection when Prp16 is mutated [18].
How the NTC specifies RNA interactions is poorly understood, however, interactions of two proteins place the NTC at the centre of the active spliceosome. The non-essential NTC protein Cwc21 binds directly to the C-terminus of the GTPase Snu114 and the N-terminus of Prp8, two proteins at the heart of the spliceosome [20]. Cwc21 also has a strong genetic interaction with Isy1 so may have a similar function in maintaining the fidelity of splicing [20,21]. Another NTC associated protein, Cwc2/Ntc40, crosslinks directly to U6 in first and second step spliceosomes thus may be involved in specifying/stabilising U6 interactions with the 5′ splice site and/or U2 snRNA in the active spliceosome [22]. Cwc2 is the only essential NTC associated protein with motifs capable of binding RNA, an RRM (RNA Recognition Motif) and zinc finger. Interestingly, Cwc2 also interacts with Isy1 by yeast two-hybrid analysis [23], providing a possible link between Isy1, the NTC, and the U6 snRNA [22]. It is likely that the NTC stabilises conformations and helps maintain splicing fidelity through these direct links with the RNA and proteins at the catalytic core of the spliceosome.
The NTC and the first catalytic reaction
Recent research reveals that the NTC is a dynamic complex with roles in spliceosome activation, disassembly and recycling. The incorporation of the NTC and release of U1 and U4 mark the change from the inactive to the active spliceosome (from B to BACT complex) [12]. However, after NTC mediated activation, several other proteins are also required for the first catalytic reaction to proceed. One of these proteins is the DEAH-box RNA helicase Prp2, which acts to restructure the spliceosome from the activated spliceosome to the pre-catalytic state (BACT to B*) [24] (Figure 1). After Prp2 remodelling, at least two other proteins Yju2 (Cwc16) and the heat resistant factor Cwc25 are required to complete the first catalytic step in an ATP independent manner [25-27] (Figure 1). In vitro splicing assays using purified spliceosomes stalled in the pre-catalytic state, can be complemented with recombinant or native proteins to identify the necessary factors required for the transition to catalysis. These studies have revealed that the essential NTC associated protein Yju2 is required for spliceosome activation [26]. Its function and interaction with the spliceosome appears to be distinct from the NTC. Unlike the NTC, which remains associated through both steps of splicing, Yju2 only associates with the spliceosome upon activation and appears to dissociate after this step [26]. Similar studies have identified Cwc25 as another essential NTC associated protein required for catalysis [25,27]. Similar to Yju2, complementation analysis revealed that Cwc25 allows stalled pre-catalytic spliceosomes to proceed through splicing. Cwc25 is a heat resistant protein, and is believed to be the heat resistant factor (HP-X) responsible for the transition to splicing described in previous complementation analyses [24,25,27]. While complementation of the pre-catalytic spliceosomes with recombinant Cwc25 allows splicing [27], a separate complementation analysis found that addition of recombinant Cwc25 could only restore catalytic activity to a marginal level [25]. This indicates that other factors in addition to Cwc25 may be necessary for catalysis. Furthermore, addition of affinity purified Cwc25-HA improved catalytic activity, indicating that this additional factor may co-purify with Cwc25 [25]. These analyses reveal that essential conformational changes are performed by NTC associated proteins which are not helicases or necessarily require ATP hydrolysis.
Role of NTC in spliceosome biogenesis and recycling
Spliceosome disassembly is catalysed by the NTR (NineTeen complex-Related) proteins, which are Ntr1, Ntr2 and Prp43 [28]. The non-essential NTC associated protein Cwc23 has also been implicated in spliceosome disassembly through its interaction with Ntr1 [29,30]. Cwc23 is one of 13 J proteins found in S. cerevisiae. A partial loss of function Cwc23 mutant displays an increase in unspliced pre-mRNA and an increase in the lariat intron, a phenotype common in mutants which affect spliceosome disassembly [30,31]. Furthermore, deletion of the Cwc23 J domain is synthetically lethal with mutations that compromise the interaction between Ntr1 and Prp43 [30]. These results imply that the J domain is important for the function of the NTR complex however, as the J domain is itself dispensable, its function may be important under different environmental conditions [30].
Reduced expression or deletion of the NTC proteins Prp19, Ntc90, Ntc77, Ntc20, Ntc25 or Ntc30 results in the accumulation of free U4 snRNP [32]. This accumulation of free U4 may be the consequence of reduced spliceosome recycling due to the defective NTC. Deletion of Ntc25 also results in reduced U4/U6 biogenesis, which in turn affects spliceosome recycling [32]. Reduced expression of Cwc2 results in the reduction of U1, U5 and U6 snRNAs and, unlike other NTC proteins, also results in the reduction of free U4 [22]. Although a mechanism is unclear, the evidence suggests that the NTC is required for the disassembly of the spliceosome and its subsequent recycling. Deletion or depletion of NTC proteins may stall the spliceosome at a specific stage where it is then subject to a discard pathway resulting in decreased spliceosome recycling.
The NTC in humans
The role of the NTC in splicing is evolutionarily conserved from yeast to humans. In humans the NTC is found associated with the spliceosome and is called the CDC5L-SNEVPrp19-Pso4 complex or human Prp19/CDC5L complex [33-36]. At the core of the human Prp19/CDC5L complex are hPrp19(SNEVPrp19-Pso4), CDC5L, PRL1(PLRG1), SPF27(BCAS2), AD-002 and Hsp73. Some, but not all, of these core proteins have counterparts in yeast (Table 1). Additionally, as with the yeast NTC, the human Prp19/CDC5L complex appears to associate early with spliceosomes and is remodelled during splicing with its composition and associated factors changing [37-41]. Finally, proteins of the human Prp19/CDC5L complex have been implicated in many non-splicing functions including cellular senescence, DNA damage response, nucleotide excision repair, lipid biogenesis and antibody diversification [42-50].
Summary/future directions
It is now clear that the NTC is an integral component of the active spliceosome. Mass spectrometry analysis of spliceosome complexes has revealed that the NTC core associates early with the spliceosome and additional NTC associated proteins are added as splicing progresses. Recent work has revealed that this dynamic association of the NTC with the spliceosome is involved in setting up spliceosome conformations required for splicing and maintaining the fidelity of splicing. The next step is to determine the exact mechanisms by which the NTC carries out these roles. For example, how do the proteins of the NTC interact with the spliceosome proteins, snRNAs and/or pre-mRNA to regulate spliceosome conformations and fidelity? It is known that the spliceosomal ATPases and GTPase are critically involved in facilitating structural transitions required for splicing. Is the NTC regulated directly or indirectly by the spliceosomal ATPases or GTPase? Finally, the question still remains as to whether there are any targets in the spliceosome for the E3 ubiquitin ligase activity of Prp19 and, if there are, whether their modification has any functional significance to splicing. Now that we have a fully characterised NTC in yeast the door is open for future work into the mechanisms of how the NTC regulates spliceosome conformations and fidelity.
Funding
This work was supported by funding from the Biotechnology and Biological Sciences Research Council and the Wellcome Trust.
Abbreviations
- pre-mRNA
pre-messenger RNA
- RNP
ribonucleoprotein
- snRNP
small nuclear RNP
- snRNA
small nuclear RNA
- NTC
NineTeen Complex
- Cwc
complexed with Cef1
- TAP
tandem affinity purification
- RRM
RNA recognition motif
- NTR
NineTeen complex Related
References
- [1].Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- [2].Frazer LN, Nancollis V, O’Keefe RT. The role of Snu114p during pre-mRNA splicing. Biochem. Soc. Trans. 2008;36:551–553. doi: 10.1042/BST0360551. [DOI] [PubMed] [Google Scholar]
- [3].Smith DJ, Query CC, Konarska MM. “Nought may endure but mutability”: Spliceosome dynamics and the regulation of splicing. Mol. Cell. 2008;30:657–666. doi: 10.1016/j.molcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheng SC, Tarn WY, Tsao TY, Abelson J. PRP19: a novel spliceosomal component. Mol. Cell. Biol. 1993;13:1876–1882. doi: 10.1128/mcb.13.3.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tarn WY, Lee KR, Cheng SC. Yeast precursor mRNA processing protein PRP19 associates with the spliceosome concomitant with or just after dissociation of U4 small nuclear RNA. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10821–10825. doi: 10.1073/pnas.90.22.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tarn WY, Lee KR, Cheng SC. The yeast PRP19 protein is not tightly associated with small nuclear RNAs, but appears to associate with the spliceosome after binding of U2 to the pre-mRNA and prior to formation of the functional spliceosome. Mol. Cell. Biol. 1993;13:1883–1891. doi: 10.1128/mcb.13.3.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohi MD, Vander Kooi CW, Rosenberg JA, Ren L, Hirsch JP, Chazin WJ, Walz T, Gould KL. Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol. Cell. Biol. 2005;25:451–460. doi: 10.1128/MCB.25.1.451-460.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tarn WY, Hsu CH, Huang KT, Chen HR, Kao HY, Lee KR, Cheng SC. Functional association of essential splicing factor(s) with PRP19 in a protein complex. EMBO J. 1994;13:2421–2431. doi: 10.1002/j.1460-2075.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ohi MD, Link AJ, Ren LP, Jennings JL, McDonald WH, Gould KL. Proteomics analysis reveals stable multiprotein complexes in both fission and budding yeasts containing Myb-related Cdc5p/Cef1p, novel pre-mRNA splicing factors, and snRNAs. Mol. Cell. Biol. 2002;22:2011–2024. doi: 10.1128/MCB.22.7.2011-2024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fabrizio P, Dannenberg J, Dube P, Kastner B, Stark H, Urlaub H, Lührmann R. The evolutionarily conserved core design of the catalytic activation step of the yeast spliceosome. Mol. Cell. 2009;36:593–608. doi: 10.1016/j.molcel.2009.09.040. [DOI] [PubMed] [Google Scholar]
- [12].Chan SP, Kao DI, Tsai WY, Cheng SC. The Prp19p-associated complex in spliceosome activation. Science. 2003;302:279–282. doi: 10.1126/science.1086602. [DOI] [PubMed] [Google Scholar]
- [13].Query CC, Konarska MM. Suppression of multiple substrate mutations by spliceosomal prp8 alleles suggests functional correlations with ribosomal ambiguity mutants. Mol. Cell. 2004;14:343–354. doi: 10.1016/s1097-2765(04)00217-5. [DOI] [PubMed] [Google Scholar]
- [14].Chan SP, Cheng SC. The Prp19-associated complex is required for specifying interactions of U5 and U6 with pre-mRNA during spliceosome activation. J. Biol. Chem. 2005;280:31190–31199. doi: 10.1074/jbc.M505060200. [DOI] [PubMed] [Google Scholar]
- [15].Madhani HD, Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992;71:803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- [16].Burgess SM, Guthrie C. A mechanism to enhance mRNA splicing fidelity: The RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell. 1993;73:1377–1391. doi: 10.1016/0092-8674(93)90363-u. [DOI] [PubMed] [Google Scholar]
- [17].Mefford MA, Staley JP. Evidence that U2/U6 helix I promotes both catalytic steps of pre-mRNA splicing and rearranges in between these steps. RNA. 2009;15:1386–1397. doi: 10.1261/rna.1582609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Villa T, Guthrie C. The Isy1p component of the NineTeen complex interacts with the ATPase Prp16p to regulate the fidelity of pre-mRNA splicing. Genes Dev. 2005;19:1894–1904. doi: 10.1101/gad.1336305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Query CC, Konarska MM. Splicing fidelity revisited. Nat. Struct. Mol. Biol. 2006;13:472–474. doi: 10.1038/nsmb0606-472. [DOI] [PubMed] [Google Scholar]
- [20].Grainger RJ, Barrass JD, Jacquier A, Rain J-C, Beggs JD. Physical and genetic interactions of yeast Cwc21p, an ortholog of human SRm300/SRRM2, suggest a role at the catalytic center of the spliceosome. RNA. 2009;15:2161–2173. doi: 10.1261/rna.1908309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khanna M, Van Bakel H, Tang X, Calarco JA, Babak T, Guo G, Emili A, Greenblatt JF, Hughes TR, Krogan NJ, et al. A systematic characterization of Cwc21, the yeast ortholog of the human spliceosomal protein SRm300. RNA. 2009;15:2174–2185. doi: 10.1261/rna.1790509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McGrail JC, Krause A, O’Keefe RT. The RNA binding protein Cwc2 interacts directly with the U6 snRNA to link the nineteen complex to the spliceosome during pre-mRNA splicing. Nucleic Acids Res. 2009;37:4205–4217. doi: 10.1093/nar/gkp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ohi MD, Gould KL. Characterization of interactions among the Cef1p-Prp19p-associated splicing complex. RNA. 2002;8:798–815. doi: 10.1017/s1355838202025050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim SH, Lin RJ. Spliceosome activation by PRP2 ATPase prior to the first transesterification reaction of pre-mRNA splicing. Mol. Cell. Biol. 1996;16:6810–6819. doi: 10.1128/mcb.16.12.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chiu YF, Liu YC, Chiang TW, Yeh TC, Tseng CK, Wu NY, Cheng SC. Cwc25 is a novel splicing factor required after Prp2 and Yju2 to facilitate the first catalytic reaction. Mol. Cell. Biol. 2009;29:5671–5678. doi: 10.1128/MCB.00773-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu YC, Chen HC, Wu NY, Cheng SC. A novel splicing factor, Yju2, is associated with NTC and acts after Prp2 in promoting the first catalytic reaction of pre-mRNA splicing. Mol. Cell. Biol. 2007;27:5403–5413. doi: 10.1128/MCB.00346-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Warkocki Z, Odenwalder P, Schmitzova J, Platzmann F, Stark H, Urlaub H, Ficner R, Fabrizio P, Lührmann R. Reconstitution of both steps of Saccharomyces cerevisiae splicing with purified spliceosomal components. Nat. Struct. Mol. Biol. 2009;16:1237–1243. doi: 10.1038/nsmb.1729. [DOI] [PubMed] [Google Scholar]
- [28].Tsai RT, Fu RH, Yeh FL, Tseng CK, Lin YC, Huang YH, Cheng SC. Spliceosome disassembly catalyzed by Prp43 and its associated components Ntr1 and Ntr2. Genes Dev. 2005;19:2991–3003. doi: 10.1101/gad.1377405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pandit S, Paul S, Zhang L, Chen M, Durbin N, Harrison SM, Rymond BC. Spp382p interacts with multiple yeast splicing factors, including possible regulators of Prp43 DExD/H-Box protein function. Genetics. 2009;183:195–206. doi: 10.1534/genetics.109.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sahi C, Lee T, Inada M, Pleiss JA, Craig EA. Cwc23, an essential J protein critical for pre-mRNA splicing with a dispensable J domain. Mol. Cell. Biol. 2010;30:33–42. doi: 10.1128/MCB.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arenas JE, Abelson JN. Prp43: An RNA helicase-like factor involved in spliceosome disassembly. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11798–11802. doi: 10.1073/pnas.94.22.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen CH, Kao DI, Chan SP, Kao TC, Lin JY, Cheng SC. Functional links between the Prp19-associated complex, U4/U6 biogenesis, and spliceosome recycling. RNA. 2006;12:765–774. doi: 10.1261/rna.2292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI. Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J. 2000;19:6569–6581. doi: 10.1093/emboj/19.23.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jurica MS, Licklider LJ, Gygi SP, Grigorieff N, Moore MJ. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA. 2002;8:426–439. doi: 10.1017/s1355838202021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Makarova OV, Makarov EM, Urlaub H, Will CL, Gentzel M, Wilm M, Lührmann R. A subset of human 35S U5 proteins, including Prp19, function prior to catalytic step 1 of splicing. EMBO J. 2004;23:2381–2391. doi: 10.1038/sj.emboj.7600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;12:1231–1245. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Behzadnia N, Golas MM, Hartmuth K, Sander B, Kastner B, Deckert J, Dube P, Will CL, Urlaub H, Stark H, et al. Composition and three-dimensional EM structure of double affinity-purified, human prespliceosomal A complexes. EMBO J. 2007;26:1737–1748. doi: 10.1038/sj.emboj.7601631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bessonov S, Anokhina M, Will CL, Urlaub H, Luhrmann R. Isolation of an active step I spliceosome and composition of its RNP core. Nature. 2008;452:846–850. doi: 10.1038/nature06842. [DOI] [PubMed] [Google Scholar]
- [39].Deckert J, Hartmuth K, Boehringer D, Behzadnia N, Will CL, Kastner B, Stark H, Urlaub H, Lührmann R. Protein composition and electron microscopy structure of affinity-purified human spliceosomal B complexes isolated under physiological conditions. Mol. Cell. Biol. 2006;26:5528–5543. doi: 10.1128/MCB.00582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hartmuth K, Urlaub H, Vornlocher HP, Will CL, Gentzel M, Wilm M, Lührmann R. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16719–16724. doi: 10.1073/pnas.262483899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Makarov EM, Makarova OV, Urlaub H, Gentzel M, Will CL, Wilm M, Lührmann R. Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science. 2002;298:2205–2208. doi: 10.1126/science.1077783. [DOI] [PubMed] [Google Scholar]
- [42].Beck BD, Park SJ, Lee YJ, Roman Y, Hromas RA, Lee SH. Human Pso4 is a metnase (SETMAR)-binding partner that regulates metnase function in DNA repair. J. Biol. Chem. 2008;283:9023–9030. doi: 10.1074/jbc.M800150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cho SY, Shin ES, Park PJ, Shin DW, Chang HK, Kim D, Lee HH, Lee JH, Kim SH, Song MJ, et al. Identification of mouse Prp19p as a lipid droplet-associated protein and its possible involvement in the biogenesis of lipid droplets. J. Biol. Chem. 2007;282:2456–2465. doi: 10.1074/jbc.M608042200. [DOI] [PubMed] [Google Scholar]
- [44].Conticello SG, Ganesh K, Xue K, Lu M, Rada C, Neuberger MS. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol. Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- [45].Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 2000;35:187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- [46].Kuraoka I, Ito S, Wada T, Hayashida M, Lee L, Saijo M, Nakatsu Y, Matsumoto M, Matsunaga T, Handa H, et al. Isolation of XAB2 complex involved in pre-mRNA splicing, transcription, and transcription-coupled repair. J. Biol. Chem. 2008;283:940–950. doi: 10.1074/jbc.M706647200. [DOI] [PubMed] [Google Scholar]
- [47].Mahajan KN, Mitchell BS. Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10746–10751. doi: 10.1073/pnas.1631060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Voglauer R, Chang MW, Dampier B, Wieser M, Baumann K, Sterovsky T, Schreiber M, Katinger H, Grillari J. SNEV overexpression extends the life span of human endothelial cells. Exp. Cell Res. 2006;312:746–759. doi: 10.1016/j.yexcr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- [49].Zhang N, Kaur R, Akhter S, Legerski RJ. Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint. EMBO Rep. 2009;10:1029–1035. doi: 10.1038/embor.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang N, Kaur R, Lu X, Shen X, Li L, Legerski RJ. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005;280:40559–40567. doi: 10.1074/jbc.M508453200. [DOI] [PubMed] [Google Scholar]