Abstract
Splint ligation of RNA, whereby specific RNA molecules are ligated together, can be carried out using T4 DNA ligase and a bridging DNA oligonucleotide complementary to the RNAs. This method takes advantage of the property of T4 DNA ligase to join RNA molecules when they are in an RNA:DNA hybrid. Splint ligation is a useful tool for the introduction of modified nucleotides into RNA molecules, insertion of a radiolabel into a specific position within an RNA and for the assembly of smaller synthetic RNAs into longer RNA molecules. Such modifications enable a wide range of experiments to be carried out with the modified RNA including structural studies, co-immunoprecipitations, and the ability to map sites of RNA:RNA and RNA:protein interactions.
Keywords: RNA modification, RNA ligation, T7 RNA polymerase, in vitro transcription, RNA purification, T4 DNA ligase
1. Introduction
It is becoming increasing clear that RNAs contribute to a diverse range of biological processes. These RNAs exist in ribonuclear protein particles such as the ribosome and spliceosome. Structural probing of these RNAs is difficult and most of the advances into the structure of these RNAs have been made by inserting modified nucleotides into these RNAs. Due to size limitation, and the high cost, of synthesizing large RNA molecules containing modified nucleotides, methods of RNA ligation have been developed.
The method described here, using T4 DNA ligase, is based on the method developed by Moore and Sharp (1). Originally this method was used to create a nuclear pre-messenger RNA substrate where the 2′-hydroxyl group at either splice site was substituted for a single hydrogen or O-methyl group (1). The authors used this pre-mRNA substrate to show that the 2′-hydroxyl at the 3′ splice site is important for the second step of splicing. Subsequently this ligation method has been widely used to investigate all aspects of RNA biology including the spliceosome (2) and ribosome (3). Our experience in RNA ligation has evolved from studying the RNAs associated with the spliceosome. By inserting photo-crosslinkable groups at specific positions into the pre-mRNA or snRNAs the RNA:RNA or protein:RNA interactions within the spliceosome can be probed (2-8). RNA ligation can also be a useful tool for NMR spectroscopy. Producing segmentally labelled full-length RNA from one modified and one unmodified RNA can help in structural determination by NMR (9). Preparation of isotopically labelled rNTPs is described in Chapters 17 and 18.
The method described here utilizes T4 DNA ligase to ligate the 3′ end of one RNA to the 5′ end of another. The RNA ligase ability of T4 bacteriophage DNA ligase has been extensively investigated (10-12) and has several advantages in comparison to RNA ligase of T4 Bacteriophage. RNA ligase is not as efficient as T4 DNA ligase, it is capable of circularising RNA and producing RNA oligomers, both of which would be undesirable side products to the RNA ligation reaction. A DNA oligonucleotide bridge complementary to the RNAs either side of the site of ligation is required to facilitate the process of ligation with T4 DNA ligase. This bridging DNA oligonucleotide is hybridized to the RNAs prior to ligation to tether them in the correct orientation and form a double stranded nucleic acid molecule for the T4 DNA ligase to act on. When using T4 DNA ligase the bridging DNA oligonucleotide specifically designates the site of ligation and prevents the production of undesirable ligation products. This specificity of ligation is also useful as in vitro transcribed RNA produced using T7 RNA polymerase will have a population of 3′ ends with additional nucleotides. These +1 transcripts would be ligated by T4 RNA ligase but not by the T4 DNA ligase method removing the requirement for 3′ end processing by a ribozyme. Once ligated the RNA is purified by polyacrylamide gel electrophoresis and recovered from the gel fragment.
2. Materials
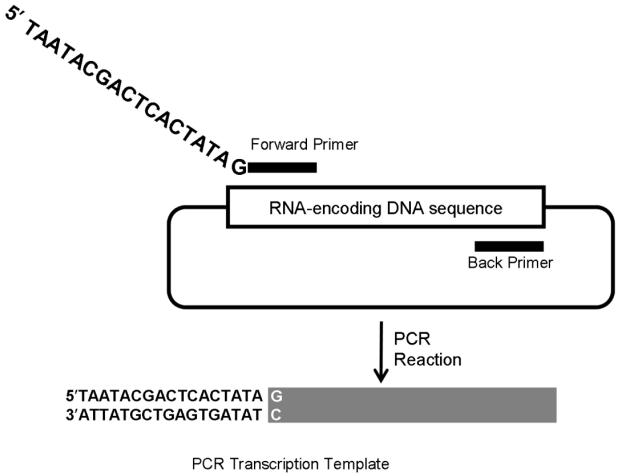
As RNA is susceptible to degradation all solutions must be RNase free. It is also advisable to use filter tips when working with both RNA and radiolabelled nucleotides. In vitro transcription requires a DNA template and certain considerations must be taken into account when designing the DNA template. DNA templates can either be plasmid DNA or DNA produced by PCR. For plasmid DNA templates the sequence to be transcribed must be cloned into a plasmid downstream of a phage RNA polymerase promoter like the T3 or T7 promoter. Once cloned into a plasmid, the plasmid DNA must then be cleaved completely with a restriction enzyme that leaves a blunt end or 5′ overhang to allow for run-off transcription. A more convenient and controllable method for producing specific DNA templates of any sequence cloned in a plasmid is PCR. A forward primer is designed that incorporates a phage RNA polymerase promoter, like T7, upstream of the first 18-20 nucleotides of sequence to be transcribed. The only restriction is that the first nucleotide to be transcribed must be a G. A back primer is then designed that will produce the specific 3′ end of the DNA template required (Figure 1). Production of DNA templates by PCR obviates the need for cloning to produce plasmid DNA templates.
Fig. 1.
Production of transcription template DNA by PCR amplification of a specific sequence from any gene cloned into a plasmid. Two PCR primers are designed, a forward primer that contains a T7 RNA polymerase promoter and a back primer. The resulting PCR transcription template will contain a T7 promoter and be used for run-off transcription.
2.1 In vitro transcription of RNA components
RNase-free water. This can be achieved by filtering deionized water through a cellulose nitrate filter with a 2 μm pore size or treatment with diethylpyrocarbonate (DEPC), which is also called diethyl dicarbonate. To treat water with DEPC, add to a final concentration of 0.1 % v/v, stir overnight on a stir plate at room temperature and autoclave to degrade the DEPC.
Transcription buffer (5×): 200 mM Tris-HCl pH 8.0, 10 mM spermidine, 50 mM dithiothreitol (DTT), 100 mM MgCl2. This can be made by mixing 400 μl of 0.5 M Tris-Cl pH 8, 100 μl of 1 M spermidine, 50 μl of 1 M DTT, 100 μl of 1M MgCl2 and 350 μl of RNase free water. Alternatively the 5× transcription buffer and DTT supplied with a commercial T7 RNA polymerase can be used.
DNA template, either PCR product or digested plasmid, at a final concentration of 0.05 μg/μl.
Nucleotide triphosphate (NTP) mixes (5×): 5 mM of each of ATP, CTP, GTP and UTP. For incorporation of XpG dinucleotide, GMP or guanosine as the first nucleotide, use NTP mix with GTP adjusted to 2 mM.
50 mM of ApG, GMP or 200 mM guanosine for priming transcription. Guanosine is not soluble in water and must be dissolved in 0.33 M sodium hydroxide (NaOH). The 0.33 M NaOH is made by dissolving 0.132 g of NaOH in 10 ml RNase-free water. The 200 mM guanosine stock is then made by adding 0.17 g of guanosine to 3 ml of 0.33 M NaOH.
T7 RNA polymerase (at least 18 units/μl).
Ribonuclease inhibitor, e.g. RNasin (Promega), 40 units/μl
RNase-free DNase
0.5 M disodium ethylenediamine tetraacetate (EDTA) pH 8. Made by dissolving 93.06 g of EDTA in 350 ml of water and altering the pH to 8 using sodium hydroxide pellets. Once the pH is adjusted to 8 bring the total volume of the solution to 500 ml.
Sodium acetate buffered phenol:chloroform:isoamyl alcohol (25:24:1) made by mixing 25 ml of sodium acetate buffered phenol, 24 ml of chloroform and 1 ml of isoamyl alcohol.
5 M ammonium acetate in water made by dissolving 77.085 g of ammonium acetate in 200 ml of RNase-free water.
3 M sodium acetate pH 5.3 in water is made by dissolving 24.609 g of sodium acetate in 80ml of RNase-free water, adjusting the pH to 5.3 and bringing solution to a final volume of 100 ml.
Absolute ethanol
2.2 Purification of in vitro transcribed RNA and synthetic RNA by polyacrylamide gel electrophoresis
Synthetic RNAs can be obtained commercially from several companies.
Formamide gel loading dye: 80 % v/v formamide, 10 mM EDTA pH 8, 1 mg/ml bromophenol blue, 1 mg/ml xylene cyanol.
Urea
Sequencing grade 40 % acrylamide solution (19:1 acrylamide:bis-acrylamide mixture), alternatively premixed polyacrylamide denaturing gel solutions are commercially available (Sequagel, National Diagnostics, Atlanta, Georgia).
RNase-free water (see section 2.1)
N,N,N,N’-tetramethyl-ethylenediamine (TEMED). Store at 4°C.
Ammonium persulfate 25 % in water (see Note 1)
Tris-borate EDTA buffer (TBE; 5×): 0.45 M Tris-borate, 100 mM EDTA, pH 8. Made by dissolving 54 g of Tris, 27.5 g of orthoboric acid in 980ml water and adding 20 ml of 0.5 M EDTA pH 8. This stock is then diluted to 1× TBE prior to use.
Polyacrylamide gel running apparatus (e.g. Cambridge Scientific): front and back gel plates measuring 185 × 200 mm, 0.5 mm spacers, Tesa 4120 UPVC packaging tape, Repelcote VS water repellent, a 50:50 water/ethanol solution, bulldog clips and a gel tank of sufficient dimensions.
Passive elution solution: 300 mM sodium acetate pH 5.3, 1 mM EDTA pH 8. Made by mixing 898 μl of RNase free water, 100 μl of 3M sodium acetate pH 5.3 and 2 μl of 0.5 M EDTA pH 8. Alternative: electroelution using Schleicher and Schuell Elutrap electropreparation system (Whatman)
Absolute ethanol
3 M sodium acetate pH 5.3
1 mg/ml glycogen
2.3 End labelling and ligation of RNA components
20 μM of 3′ RNA (T7 RNA polymerase transcribed or synthetic), 20 μM of 5′ RNA (T7 transcribed or synthetic)
DNA bridge at 25 μM with at least 20 base pairs complementarity to each RNA, resulting in an double-stranded RNA/DNA hybridization region of at least 40 base-pairs in length.
Polynucleotide kinase buffer (10×): 700 mM Tris-HCl (pH 7.6), 100 mM MgCl2, 50 mM DTT)
γ-32P-ATP (3000 Ci/mmole)
T4 polynucleotide kinase (T4 PNK)
Ligase buffer (5×): 250 mM Tris-HCl (pH 7.6), 50 mM MgCl2, 50 mM DTT, 5 mM ATP. 6. High concentration T4 DNA ligase (2,000,000 units/ml)
RNasin (at least 40 units/μl)
RNase-free water
0.5 M EDTA pH 8
Phenol:chloroform:isoamyl alcohol (25:24:1)
5 M ammonium acetate
10 mg/ml tRNA or 10 mg/ml glycogen
Absolute ethanol.
2.4 Purification of ligated RNA product
Formamide loading dye (see section 2.2)
Urea
Sequencing grade 40 % acrylamide solution (see section 2.2)
RNase-free water (see section 2.1)
N,N,N,N’-tetramethyl-ethylenediamine (TEMED). Store at 4°C.
Ammonium persulfate 25 % in water (see Note 1)
TBE buffer (1×; see section 2.2).
Saran Wrap
Glogos II Autorad Markers (Stratagene/Agilent)
Passive elution solution or electroelution system (see section 2.2)
3. Methods
Warning
When working with radioactivity the local rules on safe practice, recording the use of and disposal of radioactive materials must be followed.
3.1 In vitro transcription of 5′ or 3′ RNA with initiation of RNA transcript with GTP, XpG, GMP or guanosine
Depending upon the downstream use of the in vitro transcribed RNA, initiators may need to be incorporated during the in vitro transcription reaction to provide the correct 5′ end of RNA transcript to allow ligation and/or end labelling. When transcribing 5′ RNA GTP can be used in equimolar amounts (a final concentration of 1 mM) with ATP, CTP and UTP. However, to enable the ligation to the 3′ end of an RNA requires that the 3′ RNA possess a single phosphate at its 5′ end. Therefore, when transcribing 3′ RNA it must be primed with GMP, or either XpG or guanosine, which will allow the addition of a 32P or cold phosphate. In these three cases an NTP mix with reduced (a final concentration of 0.4 mM) GTP concentration must be used to favour incorporation of either GMP, XpG or guanosine at the beginning of the transcript.
-
In vitro transcribe RNA from the DNA template using one of the following three options depending on the desired target:
Option A. To produce 5′ RNA without priming: mix 40 μl of 5× transcription buffer with 86 μl of RNase-free water, 40 μl of 5× NTPs and 24 μl of DNA template in a microcentrifuge tube. Then add 5 μl (200 units) of RNasin and 5 μl (90 units) of T7 RNA polymerase. Incubate at 37 °C for three hours.
Option B. To produce RNA primed with either XpG or GMP: mix 40 μl of 5× transcription buffer with 78 μl of RNase-free water, 40 μl of 5× NTPs with 2 mM of GTP, 8 μl of 50 mM XpG or GMP and 24 μl of DNA template in a microcentrifuge tube. Then add 5 μl (200 units) of RNasin and 5 μl (90 units) of T7 RNA polymerase. Incubate at 37 °C for three hours.
Option C. To produce RNA primed with guanosine: mix 40 μl of 5× transcription buffer with 66 μl of RNase-free water, 40 μl of 5× NTPs with 2 mM of GTP, 20 μl of 200 mM guanosine and 24 μl of DNA template in a microcentrifuge tube. Then add 5 μl (200 units) of RNasin and 5 μl (90 units) of T7 RNA polymerase. Incubate at 37 °C for three hours.
Add 10 μl (10 units) of RNase-free DNase to the reaction and incubate at 37 °C for 15 minutes to digest the DNA template.
After the DNase incubation the RNA must be purified. To the reaction add 20 μl of 0.5 M EDTA pH 8 (see Note 2) and 220 μl of phenol:chloroform:isoamyl alcohol. Phenol extract the reaction by vortexing for two minutes and centrifugation in a bench-top microcentrifuge for 2 minutes at maximum rpm. Remove 200 μl of the upper aqueous layer to a new microcentrifuge tube and add 40 μl of 5 M ammonium acetate and 600 μl of absolute ethanol then mix by vortexing.
Precipitate RNA for at least 30 minutes at −20°C.
Centrifuge for 5 minutes at maximum rpm in a bench-top centrifuge, carefully remove the ethanol and discard leaving a small white pellet of RNA behind (see Note 3). Resuspend this RNA pellet in 10 μl of RNase-free water and 20 μl of formamide loading dye.
3.2 Purification of in vitro transcribed and synthetic RNAs
In vitro transcribed and synthetic RNAs should be gel purified to obtain RNA of the desired size. This is performed by purifying the RNA in a denaturing acrylamide gel, excising the full-length RNA and eluting the RNA from the gel.
To assemble the gel casting apparatus, coat one gel plate (usually notched plate) with Repelcote VS water repellent to encourage the gel to stick to the opposite gel plate when the gel plates are separated in step 5. Remove all excess repellent with a 50:50 water/ethanol solution. Ensure the gel plates are clean and then, after placing the spacers between the glass plates, surround the bottom and the sides of the plates with Tesa 4120 UPVC packaging tape or equivalent product.
-
a) For in vitro transcribed RNA make 20 ml of 6 % polyacrylamide gel solution (see Table 1 for gel constituents), add 40 μl TEMED and 40 μl 25 % ammonium persulfate. Act quickly to transfer the gel solution into the gel plates taking care not to introduce bubbles into the gel solution (see Note 4). Insert the comb and hold the gel together with bulldog clips. Remove the excess gel solution from around the top of the gel and allow the gel to polymerize for at least 30 minutes.
b) For synthetic RNAs resuspend the RNA in RNase-free water and add equal volume of formamide loading dye. Make a high percentage acrylamide (12 – 19%),.the volume of acrylamide and water is dependent upon the gel percentage required to resolve the RNA (Table 1). To prepare the gel solution, dissolve 10.5 g of urea in 5 ml of 5× TBE (see Note 5) and add 40 % acrylamide and RNase-free water for the desired gel percentage (see Table 1 for volumes) resulting in a final volume of 25 ml. These gel solution volumes are sufficient to pour a gel of 185 × 200 mm with 0.5 mm spacers. Once the urea is dissolved filter the gel solution through a 0.45 μm syringe filter using a 50 ml syringe. Once filtered add 40 μl of TEMED and 40 μl of 25 % ammonium persulfate, stir and pour into gel casting apparatus as described in 2a above. Leave to set for at least 30 minutes.
Once the gel has set remove the bulldog clips then the comb. Immediately wash out the wells with 1× TBE buffer to remove any unpolymerized gel solution. This is performed by attaching a 19 gauge needle to a clean 50ml syringe and washing out the wells after the comb has been removed. Remove the packaging tape before assembling gel in gel apparatus.
Run the gel at a constant Wattage depending on the gel apparatus used and for the time required to allow the RNA to migrate approximately 2/3 of the way through the gel using the dyes as indicators.
Carefully pry apart the gel plates with a spatula and sandwich the gel between two pieces of Saran Wrap then visualize the RNA by UV shadowing (see Note 6).
Excise the band from the gel with a clean scalpel or razor blade and either passive elute or electroelute the RNA from the gel slice following the manufacturer’s instructions for the electroelution device.
To passively elute the RNA from the gel slice cut the gel slice into pieces measuring roughly 4 × 4 mm, transfer into a microcentrifuge tube and cover completely with sufficient passive elution solution. Incubate overnight at room temperature. Remove elution solution from the tube making sure to leave behind any gel slices.
To electroelute the RNA from the gel slice follow the manufacturer’s instructions for the electroelution device.
Precipitate the RNA by adding 1μl of glycogen and 2.5× volumes of absolute ethanol. Briefly vortex to mix.
Precipitate for at least 30 minutes at −20 °C.
Centrifuge for 5 minutes at maximum rpm in a bench-top centrifuge, carefully remove the ethanol and discard leaving a small white pellet of RNA/glycogen behind (see Note 3).
Resuspend the RNA in a small volume (10μl) RNase-free water and quantify by measuring the OD260 (see Note 7).
Table 1.
Solutions for acrylamide gels to resolve RNAs.
Dissolve 10.5 g of urea in 5 ml of 5× TBE, 40% acrylamide and RNase free water:
| Gel percentage | 40% Acrylamide (× ml) | RNAse free water (× ml) | Size separation | Xylene cyanol (light blue dye) | Bromophenol blue (Dark blue dye) |
|---|---|---|---|---|---|
| 6% | 3.9 | 8.6 | 100 or above | ~106 | ~26 |
| 12% | 7.8 | 4.7 | 40 – 100 | ~40 | ~15 |
| 15% | 9.8 | 2.7 | 25 – 40 | ~30 | ~9 – 10 |
| 19% | 12.5 | 0 | 25 or less | ~22 | ~6 |
3.3 Preparation of the 5′ end of the 3′ RNA used for a typical two-way RNA ligation
Successful ligation requires the 3′ RNA to possess a single phosphate at its 5′ end. This single phosphate can be a 32P or an unlabelled phosphate. Depending on how the 3′ RNA was produced for the ligation reaction there are a number of different treatments that may, or may not, need to be carried out before the 3′ RNA is capable of being ligated to a 5′ RNA. A 3′ RNA that contains a single phosphate, for example an RNA from an in vitro transcription reaction primed with GMP, can be directly ligated to a 5′ RNA. A synthetic 3′ RNA or a 3′ RNA primed with XpG or guanosine requires a 5′ phosphate group added to it to allow ligation. 3′ RNA molecules that contain a single or tri-phosphate 5′ end can also be dephosphorylated (see Note 8) to then allow a single phosphate to be added. The protocol below describes a small scale reaction where a 32P is introduced at the ligation junction, but other variations on this protocol are possible (see Note 9).
To 5′-end label the 3′ RNA with a single 32P, mix 1 μl of 20 μM 3′ RNA without a 5′ phosphate with 1 μl of RNase-free water, 1 μl of 10× PNK buffer, 6 μl of γ-32P-ATP (3000 Ci/mmole; see Note 10), 0.5 μl (20 units) of RNasin and 0.5 μl (5 units) of T4 PNK in either a 0.2 ml, a 0.5 ml PCR tube (dependent upon thermocycler block size locally available, see Note 11) or a microcentrifuge tube. Incubate at 37 °C for 1 hour.
Heat to 65 °C for 20 minutes to inactivate the T4 PNK.
Next, hybridize the DNA oligonucleotide bridge to the 5′ and 3′ RNAs. To the radiolabelling reaction add 1.1 μl of 5′ RNA at 20 μM, 0.8 μl of 25 μM DNA oligonucleotide bridge and 8.1 μl of RNase-free water. Heat to 90 °C and cool slowly to 25 °C (see Note 11).
To ligate the 5′ and 3′ RNAs together add 1 μl of 10× ligase buffer, 0.5 μl (20 units) of RNasin and 1μl (2000 units) of high-concentration T4 DNA ligase to the hybridization mixture. Incubate at 37 °C for at least 1 hour. Longer incubations of 2-4 hours may result in a slight increase in ligation efficiency but risks RNA degradation.
After incubation add 1 μl of 0.5 M EDTA pH 8 to stop the reaction. If the reaction has been performed in a 0.2 or 0.5 ml PCR tube transfer it to a 1.7 ml microcentrifuge tube. Add 80 μl of RNase-free water and 100 μl of phenol:chloroform:isoamyl alcohol then vortex for 2 minutes and centrifuge at maximum rpm in a bench-top centrifuge.
Remove 100 μl of the upper, aqueous phase into a new 1.7 ml microcentrifuge and add 20 μl of 5 M ammonium acetate, 1 μl of 1 mg/ml tRNA and 300 μl of absolute ethanol. Briefly vortex and precipitate at −20°C for at least 30 min.
3.4 Purification of ligated RNA
Make a gel of the appropriate percentage (see Table 1) as described in section 3.2 (steps 1 and 2)
Centrifuge the microcentrifuge tube containing the precipitated ligated RNA for 5 minutes at maximum rpm in a bench-top centrifuge. Remove ethanol and discard in the correct manner if this ethanol is radioactive. Resuspend in 6 μl of formamide loading dye and heat the sample at 80 °C for 2 – 5 minutes prior to loading onto the acrylamide gel.
Run the gel at constant Wattage depending on the gel apparatus used and for the time required to allow the RNA to migrate approximately 2/3 of the way through the gel (see Note 12).
If the ligated RNA is radiolabeled then it is possible to visualise the 3′ RNA and ligated RNA using X-ray film. Remove one gel plate then cover the gel with Saran Wrap, attach two Glogo Autorad Markers to Saran Wrap on both sides of gel and place gel plate in an autoradiography cassette. Expose gel to X-ray film. The exposure time is dependent on the activity of the radiolabelled RNA, if highly active then a five minute exposure will suffice. Develop the film and cut around the band on the film representing the ligated RNA. Align the film to the gel using the two Glogo markers and using the film as a mask, excise the ligated RNA.
If the ligated RNA is not radiolabeled then visualize by UV shadowing (see Note 5)
Electroelute or passively elute the ligated RNA as described in section 3.2 (steps 7 to 12).
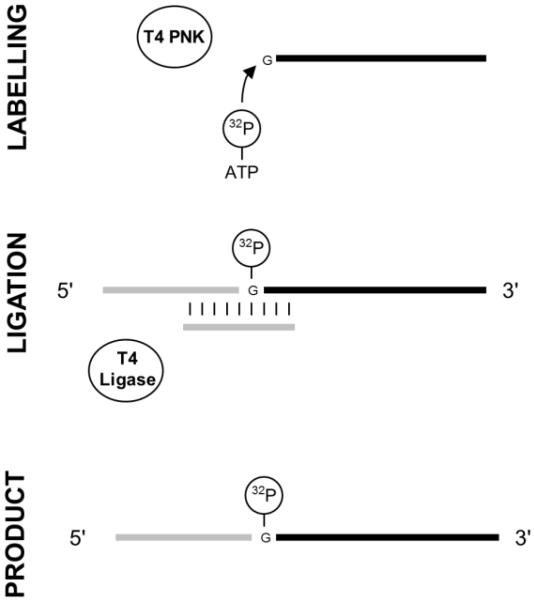
Fig. 2.
Diagram of a typical splint ligation with two RNA molecules. A 3′ RNA is first labelled at its 5′ end with 32P by T4 polynucleotide kinase (PNK). The labelled 3′ RNA is then mixed with a 5′ RNA and hybridized to a bridging DNA oligonucleotide. T4 DNA ligase can then catalyse the ligation of the two RNAs in the RNA:DNA hybrid. Finally the ligation product is purified from the other molecules in the reaction.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and the Wellcome Trust.
Footnotes
Ammonium persulfate works best fresh but it can be stored for up to one week at 4 °C.
EDTA is added following the transcription reaction as in some cases the transcription reaction becomes cloudy as it contains precipitated pyrophosphates released during the incorporation of NTPs by the polymerase. The EDTA solubilizes these pyrophosphates.
It may be difficult to see this pellet so it helps to take note of the side of the tube the pellet is likely to be on after centrifugation. This can be easily done by centrifuging with the hinge of the centrifuge tube placed to the outside of the centrifuge and therefore the pellet will stick to the wall of the tube on the same side as the hinge of the tube.
When casting the gel using a 50 ml syringe, tilt the gel slightly away from you, holding the glass plates in one hand and the syringe in your dominant hand. Gently compress the syringe to slowly fill the casting apparatus. As you pour the gel it is possible to tap the glass where bubbles have formed and encourage them to move up and out of the gel, this works as long as the gel front is less than 10 mm away from the bubble.
Dissolving urea is an endothermic reaction. It will therefore take some time to completely dissolve as all the urea will not go into solution until the mixture has reached room temperature. Although it is tempting to heat the mixture to encourage the urea to dissolve this is inadvisable as this will greatly accelerate the rate at which the gel sets and it is possible for the gel solution to set before it has been introduced into the casting apparatus. Allow time in your protocol to dissolve the urea at room temperature.
To identify the position of the RNA, the gel, sandwiched between Saran Wrap, is placed on an intensifying screen or TLC plate and short wave UV light is shined directly on the gel from above. The outline of the shadow of RNA is then marked on the Saran Wrap with a Sharpie ultra fine point permanent marker pen.
The concentration of RNA in μM can be roughly calculated by using the equation: OD260 divided by length of RNA multiplied by 0.01.
An in vitro transcribed RNA can be dephosphorylated by inserting additional steps into the protocol in Section 3.1. You will lose a small amount of the total RNA by adding these steps. During step 5, resuspend the RNA in 87 μl of RNase-free water, add 10 μl of New England Biolabs restriction enzyme Buffer 3 (10×: 500 mM Tris-HCl pH 7.9, 100 mM MgCl2, 1 M NaCl, 10 mM dithiothreitol), 2 μl (80 units) of RNasin and 1 μl (10 units) of calf intestinal alkaline phosphatase. Incubate for 1 hour at 37 °C. Add 100 μl of phenol:chloroform:isoamyl alcohol (25:24:1) then vortex for two minutes and centrifugation in a bench top microcentrifuge for 2 minutes at maximum rpm. Remove 95 μl of the upper aqueous layer to a new microcentrifuge tube and add 19 μl of 5 M ammonium acetate, 285 μl of absolute ethanol then mix by vortexing. Resuspend this RNA pellet in 10 μl of RNase-free water and 20 μl of formamide loading dye. Purify RNA as described in section 3.2.
The ligation protocol can be varied to ligate more than one RNA by the method described. In addition, if the concentrations of the RNAs to be ligated are increased 25 times with a corresponding increase in bridging oligonucleotide concentration the ligation reaction can be visualised by UV shadowing and remove the requirement for labelling with 32P.
Although this quantity of radioactivity provides the highest specific activity, and will yield the greatest amount of radiolabelled RNA, lower amounts of radioactive γ-32P-ATP between 1 and 5 μl can be used.
Using a thermocycler (PCR machine) to perform the labelling, hybridization and ligation enables close temperature control. During the hybridization step it is possible, using a thermocycler, to reduce the temperature of the reaction slowly from 90 to 25 °C (0.1°C per second) in a highly controlled manner.
When attempting a new ligation it is usually helpful to run a labelled 3′ RNA on its own to distinguish unligated from ligated RNA and to determine what gel % is appropriate for separation of unligated from ligated product. A good efficiency of ligation is 50 % or above, however lower than this is useable.
References
- 1.Moore MJ, Sharp PA. Site-specific modification of pre-mRNA: the 2′-hydroxyl groups at the splice sites. Science. 1992;256:992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe RT, Newman AJ. Functional analysis of the U5 snRNA loop 1 in the second catalytic step of yeast pre-mRNA splicing. EMBO. 1998;17:565–574. doi: 10.1093/emboj/17.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juzumiene DI, Wollenzien P. Arrangement of the central pseudoknot region of 16S rRNA in the 30S ribosomal subunit determined by site-directed 4-thiouridine crosslinking. RNA. 2001;7:71–84. doi: 10.1017/s1355838201001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Keefe RT, Norman C, Newman AJ. The invariant U5 snRNA loop 1 sequence is dispensable for the first catalytic step of pre-mRNA splicing in yeast. Cell. 1996;86:679–689. doi: 10.1016/s0092-8674(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 5.Dix I, Russel CS, O’Keefe RT, Newman AJ, Beggs JD. Protein-RNA interactions in the U5 snRNP of Saccharomyces cerevisiae. RNA. 1998;4:1675–1686. doi: 10.1017/s1355838298412998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvi RK, Lund M, O’Keefe RT. ATP-dependent interaction of yeast U5 snRNA loop1 with the 5′ splice site. RNA. 2001;7:1013–1023. doi: 10.1017/s135583820101041x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrail JC, Tatum E, O’Keefe RT. Mutation in the U2 snRNA influences exon interactions of the U5 snRNA loop 1 during pre-mRNA splicing. EMBO. 2006;25:3813–3822. doi: 10.1038/sj.emboj.7601258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrail JC, O’Keefe RT. The U1, U2 and U5 snRNAs crosslink to the 5′ exon during yeast pre-mRNA splicing. Nucleic Acids Research. 2008;36:814–825. doi: 10.1093/nar/gkm1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzakos AG, Easton LE, Lukavsky PJ. Perparation of large RNA oligonucleotides wth complementary isotope-labeled segments for NMR structural studies. Nature Protocols. 2007;2:2139–2147. doi: 10.1038/nprot.2007.306. [DOI] [PubMed] [Google Scholar]
- 10.Kleppe K, van de Sande JH, Khorana HG. Polynucleotide ligase-catalyzed joining of deoxyribo-oligonucleotides on ribopolynucleotide templates and of ribo-oligonucleotides on deoxyribopolynucleotide templates. PNAS. 1970;67:68–73. doi: 10.1073/pnas.67.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fareed GC, Wilt EM, Richardson CC. Enzymatic breakage and joining of deoxyribonucleic acid. J Biol Chem. 1971;246:925–932. [PubMed] [Google Scholar]
- 12.Sano H, Feix G. Ribonucleic acid ligase activity of deoxyribonucleic acid ligase from phage T4 infacted Escherichia coli. Biochemistry. 1974;13:5110–5115. doi: 10.1021/bi00722a009. [DOI] [PubMed] [Google Scholar]