Abstract
Background
Non-communicable diseases (NCDs) are rapidly becoming leading causes of morbidity and mortality in low and middle-income countries, including those in sub-Saharan Africa. By contrast to high-income countries, the sociodemographic distribution, including socioeconomic inequalities, of NCDs and their risk factors is unclear in sub-Saharan Africa, particularly among rural populations.
Methods
We undertook a cross-sectional population-based survey of 7809 residents 13 years or older in the General Population Cohort in south-western rural Uganda. Information on behavioural, physiological, and biochemical risk factors was obtained using standardised methods as recommended by the WHO STEPwise Approach to Surveillance. Socioeconomic status (SES) was determined by principal component analysis including household features, ownership, and occupation and education of the head of household.
Results
SES was found to be associated with NCD risk factors in this rural population. Smoking, alcohol consumption (men only), and low HDL-cholesterol were more common among those of lower SES. For example, the prevalence of smoking decreased fourfold from the lowest to highest SES groups, from 22.0% to 5.7% for men and 2.2% to 0.4% for women. By contrast, overweight, raised blood pressure, raised HbA1c (women only), and raised cholesterol were more common among those of higher SES. For example the prevalence of overweight increased fivefold from 2.1% to 10.1% for men and twofold from 12.0% to 23.4% for women from the lowest to highest SES groups. However, neither low physical activity nor fruit, vegetable, or staples consumption was associated with SES. Furthermore, associations between NCD risk factors and SES were modified by age and sex.
Conclusions
Within this rural population NCD risk factors are common and vary both inversely and positively across the socioeconomic status gradient. A better understanding of the determinants of the sociodemographic distribution of NCDs and their risk factors in rural sub-Saharan African populations will help identify populations at most risk of developing NCDs and help plan interventions to reduce their burden.
Medical Subject Headings: epidemiology, public health, sub-Saharan Africa, Africa, Uganda, sociodemographic, socioeconomic status, non-communicable diseases, NCDs, chronic diseases, risk factors, epidemiological transition
Introduction
Sub-Saharan Africa (SSA) is undergoing epidemiological transition. Recent estimates and projections suggest that SSA is one of the regions with the highest proportion of premature deaths due to non-communicable diseases (NCDs) in the world.1 In 2004, around 1.2 million deaths in Africa were thought to be attributable to cardiovascular disease (CVD).2 This figure is expected to double by 2030.3 In the same context, the number of people with diabetes in SSA almost doubled from seven million in 2000 to 12.1 million in 2010. This number is expected to double again to 23.9 million by 2030.4
The increase in NCDs and their risk factors in low- and middle-income countries (LMICs) is often considered to be due to an increase in urbanisation, a transition from a ‘traditional healthy’ to ‘modern unhealthy’ diet and lifestyle, and also an ageing society.5-7 These individual transitions are thought to be factors in determining an overall epidemiological transition. The epidemiological transition describes a theory for which countries transition from a burden of mostly infectious diseases to mostly NCDs as they undergo economic and social development.7 Many African populations are experiencing the early stages of the epidemiological transition, as the sub-continent continues to develop, lower child mortality, and treat HIV.
However, the impact of these transitions on health is not the same for all sectors of the population. Social inequalities, and their relationship with the distribution and treatment of NCDs and their risk factors, have been well described in high-income countries.8 9 However, less is known about the socioeconomic distribution of NCDs and their risk factors in LMICs, especially among rural SSA populations.10 Given that the majority of Africans still live in rural areas,11 it is important to understand the heterogeneity and social inequalities which may exist within these rural populations. Variation in diet, education, types of occupation, housing, exposure to globalised media and products, access to healthcare and treatment, and the prevalence of infection, are just examples of heterogeneity which may affect risk of NCDs. A clearer understanding of the sociodemographic distribution of NCD risk factors may help towards identifying the underlying determinants of NCDs, NCD risk factors, and health inequality.8 9 We therefore assessed the sociodemographic distribution of NCD risk factors in a rural Ugandan population.
Methods
We conducted a cross-sectional population based survey of participants 13 years and older within the Medical Research Council/Uganda Virus Research Institute (MRC/UVRI) General Population Cohort (GPC) Round 22 in 2011. Full details on the cohort have been published elsewhere.12 Briefly, the cohort comprises all residents (52% aged ≥13years, men and women in equal proportions) within one-half of a rural sub-county, not far from Lake Victoria. Houses are mostly scattered across the county-side in villages defined by administrative boundaries, rather than socio-economic centres. There are no tarmacked roads and buildings are mostly semi-permanent structures built from locally available materials.13 Participants are mostly subsistence farmers, literacy levels are comparatively low, and the main income-earning activity is trading in bananas, coffee, beans, and fish. The main documented change in health status over the past 20 years has been due to the impact of HIV.
Data collection
Interviewer-administered questionnaires were used to collect demographic data on individual and household socio-economic indicators. Lifestyle and health history data were collected using an adapted World Health Organisation (WHO) STEPwise Approach to Surveillance questionnaire.14
Standardised procedures were used for all biophysical measurements. Blood pressure was measured in the sitting position three times with resting intervals of 5 minutes, using the Omron M4-I, for participants who had been resting for at least 15 minutes before measurement. Blood pressure was taken as the mean of the second and third reading. Height and weight were measured using the Leicester Stadiometer to the nearest 0.1 cm and the Seca 761 mechanical scales to the nearest 1kg, respectively. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist and hip circumferences were measured twice over one layer of light clothing using the Seca 201 Ergonomic Circumference Measuring Tape to the nearest 0.1 cm. A third measurement was taken if the first two measurements differed by more than 3 cm. Waist and hip circumferences were taken as the mean of two (or three where applicable) measurements. Women in their second or third trimester of pregnancy were excluded from physical measurement.
Biochemical analysis was performed using the Cobas Integra 400 plus chemistry analyser to determine HbA1c from whole blood samples and lipid profiles for total cholesterol, high density lipoprotein (HDL) cholesterol, and triglycerides from serum samples. The MRC/UVRI Entebbe laboratories were enrolled in National Health Laboratory Service (NHLS) and College of American Pathologists (CAP) external quality control programmes.
Statistical analysis
Data were collected on ultra-mobile personal computers using a tool designed in Microsoft Access, and stored in Microsoft Access. Data were analysed using the Stata11 software package (StataCorp: College Station, TX, USA) for Windows and Macintosh. Analyses were restricted to participants with complete data, with the exception of 11.8% of participants missing data on diet staples. In total 12.1% of participants were excluded from analyses. A list of the source of missing data is outlined in supplemental table S1. Briefly, missing data on socioeconomic status (7.0%) was the main contributor to missing data and 3.3% of participants were missing data on BMI and/or waist circumference. Otherwise, the amount of missing data per variable was small (<1%). Supplemental table S2 presents a sensitivity analysis for those excluded compared to those included in the analysis.
SES was constructed on a household level using principal component analysis (PCA).15 The first component of the PCA output was taken to be the continuous SES variable. This continuous SES variable was then categorised into quintiles to produce a discrete SES variable with categories defined as lowest, lower-middle, middle-higher, highest SES. The household socioeconomic variables included in the PCA were: roof material type, roof quality, wall material type, ratio of number of rooms in a house to number of people living in that household, ownership of house and land, employment of workers for household or land, level of education reached by the head of household, and occupation type of the head of household. All individuals who identified as being part of the same household were assigned the same SES value. The distribution of SES variables included in the PCA by SES quintiles is outlined in supplemental table S3.
The distribution of each risk factor was examined by age, SES, and sex. We calculated prevalences with 95% confidence intervals for binary variables and means with 95% confidence intervals for continuous variables. Prevalences and means of NCD risk factors were adjusted for multi-level mixed-effects, including random-effects for data clustered into households and villages. Prevalences and means by age categories were further adjusted by SES as a continuous measure. Prevalences by SES categories were further adjusted for age as a continuous measure.
We assessed trends in age and SES, and sex differences for each outcome by performing Poisson regression for binary variables and linear regression for continuous variables. The normality of distribution of continuous variables was assessed before analysis, and where necessary, skewed distributions were log transformed. Fully adjusted models were multi-level mixed-effects Poisson and linear regression models, including random-effects for data clustered into households and villages. There was a total of 2983 household, with an average of 2.3 participants per household (range 1-11) and 119.3 households per village (range 44-256), across 25 villages.
To assess whether demographic factors modify the association between NCD risk factors and SES, a Poisson regression model was performed for each NCD risk factor and an interaction term for sex with SES or age (model A). A second Poisson model was performed without an interaction term (model B). Models A and B were then compared using a likelihood ratio test with one degree of freedom, and p-values were reported.
Definitions
Low physical activity was defined as achieving less than 5 days a week of any combination of walking, moderate- or vigorous-intensity activities and less than 600 minutes of physical activity per week.16 Insufficient fruit and vegetable consumption was defined as <5 servings of fruit or vegetables a day. Staples included food such as posho (maize), matooke (banana), cassava, sweet potato, and rice. High staple consumption was defined as above the 75th percentile of the population. Raised blood pressure was defined as systolic ≥140 or diastolic ≥ 90 mmHg or reported treatment for raised blood pressure. Abdominal obesity was defined as a waist circumference ≥94cm for men and ≥80cm for women. Those with a BMI ≥25 kg/m2 were categorised as overweight. Abnormal lipids were defined as follows: raised total cholesterol >5.2 mmol/l, low HDL-cholesterol <1.0 (men) or <1.3 (women) mmol/l, raised triglycerides >1.7 mmol/l.14,17,18 Raised HbA1c was defined as HbA1c>6.5%.19
Ethics
The study was approved by the Science and Ethics Committee of the Uganda Virus Research Institute (UVRI), the Ugandan National Council for Science and Technology, and the East of England-Cambridge South (formerly Cambridgeshire 4) NHS Research Ethics Committee UK.
Results
A total of 7809 participants were surveyed. Nine hundred and forty two were excluded from analysis due to incomplete data, leaving 6867 (55% women) for the present analysis, representing 83% (6867/8309) of the population invited to partake in the study (supplemental tables S1 and S2 outline the distribution of missing data). The sociodemographic characteristics of the study population are shown in table 1. This study population was young, with a mean age of 34.4 years (32.8 years in men and 35.6 years in women). The majority (75.2%) of participants were from the Baganda tribe, the main tribal group in the region. Forty percent of participants had less than complete primary education. Farming was the most common primary source of livelihood. Furthermore, table 2 outlines the distribution of household socioeconomic indicators for men and women by age group. Overall SES decreased with age for both men and women (p-value <0.001) and men had only slightly higher SES than women (p-value 0.088).
Table 1. Sociodemographic characteristics of participants of the General Population Cohort Study, 2011.
| Variable | Men (n=3071) n (%) | Women (n=3796) n (%) | Total (n=6867) n (%) |
|---|---|---|---|
| Age groups (years) | |||
| 13-19 | 1085 (35.3) | 1013 (26.7) | 2098 (30.6) |
| 20-29 | 558 (18.2) | 711 (18.7) | 1269 (18.5) |
| 30-39 | 455 (14.8) | 679 (17.9) | 1134 (16.5) |
| 40-49 | 407 (13.3) | 551 (14.5) | 958 (14.0) |
| ≥50 | 566 (18.4) | 842 (22.2) | 1408 (20.5) |
| Education | |||
| Still in education | 938 (30.5) | 897 (23.6) | 1835 (26.7) |
| Less than or incomplete primary | 1130 (36.8) | 1654 (43.6) | 2784 (40.5) |
| Completed primary only | 472 (15.4) | 584 (15.4) | 1056 (15.4) |
| Above primary | 531 (17.3) | 661 (17.4) | 1192 (17.4) |
| Primary source of livelihood 1 | |||
| Subsistence farmers | 1016 (47.6) | 1754 (60.5) | 2770 (55.0) |
| Cash crop farmer | 446 (20.9) | 406 (14.0) | 852 (16.9) |
| Looked after by others | 96 (4.5) | 344 (11.9) | 440 (8.74) |
| Other | 575 (27.0) | 396 (13.7) | 971 (19.3) |
| Occupation type of head of household | |||
| Unemployed | 1905 (62.0) | 2618 (69.0) | 4523 (65.9) |
| Unskilled labour | 111 (3.6) | 68 (1.8) | 179 (2.6) |
| Skilled labour | 145 (4.7) | 103 (2.7) | 248 (3.6) |
| Traders | 733 (23.9) | 761 (20.1) | 1494 (21.8) |
| Property owners | 30 (1.0) | 41 (1.1) | 71 (1.0) |
| Professionals | 147 (4.8) | 205 (5.4) | 352 (5.1) |
| Marital status 2 | |||
| Never married | 1485 (48.4) | 1186 (31.3) | 2671 (38.9) |
| Currently married | 1287 (42.0) | 1649 (43.5) | 2936 (42.8) |
| Divorced/widowed | 295 (9.6) | 956 (25.2) | 1251 (18.2) |
| Religion 3 | |||
| Christian | 2,322 (76.5) | 2,826 (75.3) | 5,148 (75.8) |
| Muslim | 712 (23.5) | 929 (24.7) | 1,641 (24.2) |
| Other/none | 2 (0.1) | 3 (0.1) | 5 (0.1) |
| Tribe 4 | |||
| Baganda | 2,248 (74.1) | 2,854 (76.0) | 5,102 (75.2) |
| Banyarwanda (Rwandan origin) | 454 (15.0) | 545 (14.5) | 999 (14.7) |
| Other | 330 (10.9) | 356 (9.5) | 686 (10.1) |
n=5034 for total population, n=2133 for men, and n=2900 for women
n=6858 for total population, n=3067 for men, and n=3791 for women
n=6794 for total population, n=3036 for men, and n=3758 for women
n=6787 for total population, n=3032 for men, and n=3755 for women
Table 2. Distribution of socioeconomic indicators by age category for men and women.
| Variable | Men’s age group | Women’s age group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13-19 (n=1,085) | 20-29 (n=558) | 30-39 (n=455) | 40-49 (n=407) | 50+ (n=566) | 13-19 (n=1,013) | 20-29 (n=711) | 30-39 (n=679) | 40-49 (n=551) | 50+ (n=842) | |
| SES | 0.04 (1.66) | 0.02 (1.74) | 0.79 (1.73) | −0.9 (1.69) | −0.32 (1.66) | 0.10 (1.61) | 0.15 (1.53) | 0.09 (1.71) | 0.09 (1.71) | −0.24 (1.55) |
| Roof type/material (%) | ||||||||||
| Plastic sheeting | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.10 | 0.00 | 0.15 | 0.00 | 0.00 |
| Thatch | 5.25 | 8.24 | 9.89 | 10.81 | 8.83 | 6.32 | 8.58 | 7.51 | 5.99 | 5.34 |
| Mixed iron/thatch | 0.18 | 0.00 | 0.44 | 0.74 | 0.71 | 0.30 | 0.14 | 0.15 | 0.36 | 0.48 |
| Mixed iron/tile | 0.09 | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 0.00 | 0.15 | 0.18 | 0.24 |
| Iron sheets | 93.64 | 91.40 | 88.79 | 87.47 | 89.22 | 92.20 | 91.00 | 91.61 | 62.38 | 92.16 |
| Tile | 0.83 | 0.36 | 0.66 | 0.98 | 0.88 | 1.09 | 0.28 | 0.44 | 1.09 | 1.78 |
| Roof quality (%) | ||||||||||
| Poor | 12.17 | 15.05 | 16.70 | 17.69 | 18.90 | 10.66 | 10.83 | 14.29 | 12.52 | 13.18 |
| Fair | 32.17 | 30.65 | 27.47 | 35.63 | 30.39 | 31.98 | 28.69 | 30.19 | 33.76 | 33.25 |
| Good | 55.67 | 54.30 | 55.82 | 46.68 | 50.71 | 57.35 | 60.48 | 55.52 | 53.72 | 53.56 |
| Wall type/material (%) | ||||||||||
| Mud and pole | 17.79 | 23.12 | 18.46 | 20.88 | 31.63 | 17.18 | 16.32 | 17.38 | 20.69 | 23.28 |
| Mud and brick | 1.29 | 0.72 | 0.66 | 0.98 | 1.06 | 0.99 | 0.70 | 0.88 | 0.91 | 1.78 |
| Brick | 48.11 | 41.76 | 52.31 | 50.12 | 38.52 | 46.50 | 45.29 | 49.93 | 44.83 | 43.59 |
| Brick and concrete | 23.87 | 26.16 | 22.86 | 20.64 | 19.08 | 25.96 | 29.68 | 23.42 | 24.50 | 22.45 |
| Concrete | 8.94 | 8.24 | 5.71 | 7.37 | 9.72 | 9.38 | 8.02 | 8.39 | 9.07 | 8.91 |
| Mean number of rooms per person (SD) | 1.16 (0.58) | 1.38 (0.89) | 1.55 (0.96) | 1.47 (0.99) | 1.64 (1.22) | 1.15 (0.63) | 1.58 (1.05) | 1.55 (1.06) | 1.49 (1.10) | 1.78 (1.24) |
| Own their own house (%) | ||||||||||
| No | 4.61 | 23.30 | 18.46 | 15.48 | 9.36 | 7.80 | 25.18 | 18.11 | 10.89 | 5.94 |
| Yes | 95.39 | 76.70 | 81.54 | 84.52 | 90.64 | 92.20 | 74.82 | 81.89 | 89.11 | 94.06 |
| Own property for renting (%) | ||||||||||
| No | 92.26 | 93.37 | 92.97 | 91.40 | 95.58 | 91.51 | 92.97 | 92.78 | 90.56 | 93.59 |
| Yes | 7.74 | 6.63 | 7.03 | 8.60 | 4.42 | 8.49 | 7.03 | 7.22 | 9.44 | 6.41 |
| Status of household land (%) | ||||||||||
| Borrowed | 1.57 | 4.84 | 3.52 | 6.88 | 6.18 | 3.36 | 4.50 | 4.42 | 3.81 | 2.73 |
| Rented | 2.95 | 18.46 | 16.70 | 9.34 | 3.71 | 5.23 | 21.94 | 13.99 | 7.62 | 3.80 |
| Owned | 95.48 | 76.70 | 79.78 | 83.78 | 90.11 | 91.41 | 73.56 | 81.59 | 88.57 | 93.47 |
| Own additional land (other than land with house on it) (%) | ||||||||||
| No | 50.14 | 55.02 | 45.71 | 50.37 | 58.13 | 47.98 | 50.21 | 47.57 | 47.91 | 60.33 |
| Yes | 49.86 | 44.98 | 54.29 | 49.63 | 41.87 | 52.02 | 49.79 | 52.43 | 52.09 | 39.67 |
| Size of other land owned (acres) (SD) | 1.31 (3.99) | 1.12 (3.91) | 0.96 (1.38) | 1.02 (1.76) | 1.09 (2.24) | 1.32 (3.83) | 0.91 (1.76) | 1.08 (3.69) | 1.27 (3.34) | 0.88 (2.00) |
| Type of workers employed (%) | ||||||||||
| None | 75.39 | 75.99 | 75.82 | 75.43 | 78.98 | 73.54 | 77.22 | 74.96 | 72.60 | 75.18 |
| Temporary workers | 20.83 | 19.53 | 22.42 | 22.11 | 18.02 | 23.49 | 19.55 | 22.53 | 23.41 | 21.73 |
| Permanent workers | 3.78 | 4.48 | 1.76 | 2.46 | 3.00 | 2.96 | 3.23 | 2.50 | 3.99 | 3.09 |
| Mean number of workers employed (SD) | 0.70 (1.60) | 0.66 (1.6) | 0.78 (2.00) | 0.63 (1.41) | 0.62 (1.56) | 0.68 (1.54) | 0.59 (1.38) | 0.67 (1.52) | 0.79 (1.79) | 0.59 (1.47) |
| Occupation type of head of household (%) | ||||||||||
| Unemployed | 67.10 | 55.20 | 53.41 | 59.21 | 68.02 | 71.27 | 60.06 | 64.06 | 70.78 | 76.48 |
| Unskilled labour | 1.20 | 5.02 | 6.15 | 3.93 | 4.59 | 1.18 | 3.52 | 2.06 | 1.63 | 0.95 |
| Skilled labour | 3.13 | 9.32 | 8.13 | 2.70 | 1.94 | 2.37 | 3.94 | 3.24 | 2.18 | 2.02 |
| Traders | 24.79 | 22.04 | 22.20 | 26.29 | 23.50 | 20.04 | 21.24 | 21.50 | 19.78 | 18.05 |
| Property owners | 0.74 | 1.61 | 1.98 | 0.74 | 0.18 | 0.49 | 2.53 | 1.62 | 1.09 | 0.12 |
| Professionals | 3.04 | 6.81 | 8.13 | 7.13 | 1.77 | 4.64 | 8.72 | 7.51 | 4.54 | 2.38 |
| Mean education level of head of household (SD) | 8.04 (4.45) | 9.18 (4.83) | 9.44 (4.87) | 8.70 (4.62) | 7.61 (4.79) | 8.30 (4.60) | 9.96 (4.91) | 9.18 (4.98) | 7.88 (4.60) | 6.60 (4.66) |
P-values were determined by fitting multi-level mixed-effects Poisson regression models with random effects to adjust for clustering at household and village levels.
Among self-reported lifestyle risk factors in this population we found low consumption of fruit and vegetables to have the highest prevalence (75.8%), followed by low physical activity (29.8%), weekly consumption of alcohol (11.4%), and current daily smoking (6.5%) (table 3). The prevalences of overweight and abdominal obesity were found to be 11.8% and 17.7%, respectively. Raised blood pressure was found among 16.5% of the population. The most common lipid risk factor was low HDL-cholesterol, which had a prevalence of 71.8%. By contrast only 5.2% and 13.2% of the population had raised total cholesterol and raised triglycerides, respectively. Raised HbA1c was found in only 0.8% of the population.
Table 3. Prevalences of NCD risk factors and healthcare factors by sex.
| Variable | Total (n=6,867) % (95% CI) | Men (n=3,071) % (95% CI) | Women (n=3,796) % (95% CI) | P-value* | Adjusted P-value** |
|---|---|---|---|---|---|
| Lifestyle risk factors | |||||
| Current smoker | 6.5 (5.8-7.1) | 13.1 (11.7-14.5) | 1.3 (9.5-16.8) | <0.001 | <0.001 |
| Alcohol user | 34.6 (30.6-38.6) | 40.2 (35.3-45.2) | 30.3 (26.6-34.0) | <0.001 | <0.001 |
| Weekly drinker | 11.4 (9.6-13.3) | 19.1 (15.9-22.3) | 5.7(4.6-6.8) | <0.001 | <0.001 |
| Low physical activity | 29.8 (28.2-31.5) | 20.8 (19.0-22.6) | 36.7 (34.5-39.1) | <0.001 | <0.001 |
| Low fruit and vegetable consumption | 75.8 (72.4-79.2) | 74.5 (70.4-78.6) | 76.9 (723.0-80.8) | 0.260 | 0.256 |
| High staple consumption1 | 17.2 (10.3-24.1) | 18.3 (10.9-25.7) | 16.3 (9.7-22.9) | 0.039 | 0.029 |
| Physical risk factors | |||||
| Overweight | 11.8 (10.6-13.1) | 5.2 (4.3-6.1) | 16.9 (15.0-18.8) | <0.001 | <0.001 |
| Abdominal obesity | 17.7 (16.4-19.0) | 1.5 (1.1-2.0) | 30.0(27.8-32.3) | <0.001 | <0.001 |
| Raised blood pressure | 16.5 (15.3-17.6) | 16.9 (15.3-18.5) | 16.1 (14.8-17.5) | 0.532 | 0.443 |
| Cardiometabolic risk factors | |||||
| Raised total cholesterol | 5.2 (4.3-6.1) | 3.1 (2.4-3.9) | 6.7 (5.5-8.0) | <0.001 | <0.001 |
| Low HDL-cholesterol | 71.8 (69.5-74.1) | 60.7 (57.8-63.6) | 81.0 (77.9-84.1) | <0.001 | <0.001 |
| Raised triglycerides | 13.2 (12.2-14.2) | 12.4(11.0-13.7) | 13.9 (12.6-15.2) | 0.091 | 0.092 |
| Raised HbA1c | 0.8 (0.4-1.1) | 0.6(0.3-1.0) | 0.9 (0.4-1.3) | 0.121 | 0.166 |
| Health screening | |||||
| Blood pressure screen | 49.4 (46.6-52.3) | 43.4(40.3-46.5) | 54.1 (50.7-57.5) | <0.001 | <0.001 |
| Diabetes screen | 23.2 (19.7-26.7) | 21.3 (17.8-24.8) | 24.6 (20.8-28.5) | 0.005 | 0.004 |
| Cholesterol screen | 1.0 (0.7-1.4) | 1.1 (0.6-1.5) | 1.0 (0.7-1.4) | 0.907 | 0.876 |
| Diagnosis | |||||
| Diagnosed with raised blood pressure | 5.9 (5.1-6.8) | 3.9 (3.1-4.7) | 7.4 (6.3-8.6) | <0.001 | <0.001 |
| Diagnosed with diabetes | 1.2 (0.5-1.9) | 1.2 (0.4-2.0) | 1.2 (0.5-2.0) | 0.794 | 0.811 |
| Diagnosed with raised cholesterol | 0.4 (0.2-0.6) | 0.4(0.2-0.7) | 0.4(0.2-0.6) | 0.747 | 0.750 |
| Treatment among those diagnoses | |||||
| On treatment for raised blood pressure | 47.5 (41.0-54.1) | 42.1 (30.4-53.7) | 49.7 (41.8-57.5) | 0.297 | 0.310 |
| On treatment for diabetes | 29.2 (18.0-40.4) | 35.1 (16.0-54.2) | 25.0(11.4-38.6) | 0.391 | 0.391 |
| On treatment for raised cholesterol | 23.5 (7.2-39.8) | 18.6 (-0.0-39.9) | 28.0 (3.1-5.3) | 0.659 | 0.584 |
Prevalences are adjusted for multi-level mixed-effects, with random effects to account for clustering at household and village levels.
P-values are for the difference between men and women and were determined by fitting Poisson regression models and adjusting for age.
Adjusted p-values were determined by fitting multi-level mixed-effects Poisson regression models adjusted for age and SES and with random effects to adjust for clustering at household and village levels.
n=6056 for total population, n=2701 for men, and n=3355 for women
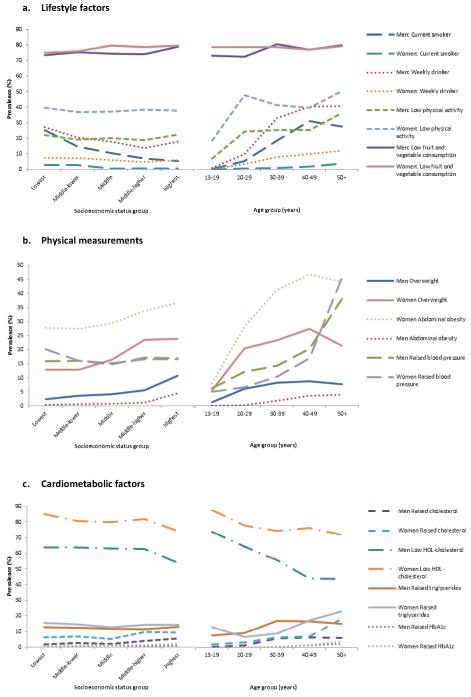
Marked differences were seen in the prevalence of risk factors between men and women (table 3). Smoking and weekly alcohol consumption were more common in men than women (13.1% versus 1.3% and 19.1% versus 5.7%, respectively, p-value <0.001 for all). However, low physical activity, overweight, and abdominal obesity were much more common in women than men (36.7% versus 20.8%, 16.9% versus 5.2%, and 30.0% versus 1.5%, respectively, p-value <0.001 for all). Consistent with the differences in overweight and abdominal obesity between men and women, lipid profiles were worse in women. By contrast, no marked difference between men and women was found in the prevalence of raised blood pressure (16.9% versus 16.1%, p-value 0.443) or raised HbA1c (0.6% versus 0.9%, p-value 0.166).
We found the levels of most NCD risk factors to be higher among older than younger age groups (p-value <0.001) (table 4). Notable exceptions were low fruit and vegetable consumption, which showed no clear relationship with age for women (p-value 0.757) and a weak relationship with age for men (p-value 0.033), and high staple consumption which showed no evidence for being related to age for men (p-value 0.289) or women (p-value 0.203). HDL-cholesterol, which is considered a protective factor, increased across age groups (p-value <0.001).
Table 4. Distribution of SES-adjusted prevalences of lifestyle risk factors and SES-adjusted means of physical and cardiometabolic risk factors by age and sex.
| Variable | Men | Women | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13-19 (n=1,085) |
20-29 (n=558) |
30-39 (n=455) |
40-49 (n=407) |
50+ (n=566) |
Unadjusted p-value |
P value* | Adjusted P-value** |
13-19 (n=1,013) |
20-29 (n=711) |
30-39 (n=679) |
40-49 (n=551) |
50+ (n=842) |
Unadjusted p-value |
P value* | Adjusted P-value** |
|
| Lifestyle risk factors, % (95% CI) | ||||||||||||||||
| Current smoker | 0.1 (−0.1-0.3) | 5.2 (3.3-7.2) | 18.9 (14.8-23.1) | 30.1 (24.5-35.4) | 25.1 (21.0-29.2) | <0.001 | <0.001 | <0.001 | 0.0 (0.0-0.0) | 0.4 (−0.1-1.0) | 0.9 (0.2-1.6) | 1.8 (0.7-3.0) | 3.5 (2.3-4.7) | <0.001 | <0.001 | <0.001 |
| Alcohol user | 8.5 (6.6-10.4) | 31.5 (26.1-36.9) | 58.7 (49.9-67.5) | 65.2 (55.5-74.9) | 70.2 (60.9-79.4) | <0.001 | <0.001 | <0.001 | 6.4 (4.7-8.1) | 24.0 (19.6-28.3) | 41.4 (34.9-47.9) | 42.3 (35.3-49.2) | 51.7 (44.4-59.0) | <0.001 | <0.001 | <0.001 |
| Weekly drinker | 0.7 (0.2-1.2) | 9.4 (6.7-12.1) | 30.6 (24.5-36.8) | 38.5 (31.2-45.9) | 38.6 (32.0-45.3) | <0.001 | <0.001 | <0.001 | 0.3 (−0.0-0.6) | 2.9 (1.6-4.2) | 6.6 (4.4-8.9) | 8.8 (5.9-11.7) | 11.1 (8.1-14.1) | <0.001 | <0.001 | <0.001 |
| Low physical activity | 6.9 (5.3-8.5) | 23.8 (19.5-28.0) | 24.6 (19.9-29.4) | 24.8 (19.8-29.8) | 35.8 (30.6-41.1) | <0.001 | <0.001 | <0.001 | 18.4 (15.7-21.0) | 47.1 (42.1-52.2) | 40.9 (36.1-45.8) | 39.3 (34.1-44.5) | 49.9 (45.1-54.7) | <0.001 | <0.001 | <0.001 |
| Low fruit and vegetable consumption | 71.9 (66.1-77.6) | 70.7 (63.2-78.2) | 78.3 (69.6-87.0) | 75.2 (66.3-84.1) | 79.1 (71.1-87.1) | 0.049 | 0.039 | 0.033 | 77.5 (71.8-83.1) | 77.5 (70.8-84.31) | 77.2 (70.4-84.0) | 76.0 (68.5-83.4) | 78.6 (72.3-84.8) | 0.840 | 0.773 | 0.757 |
| High staple consumption1 | 16.4 (10.1-22.7) | 20.3 (12.3-28.4) | 21.8 (13.1-30.5) | 21.6 (12.8-30.4) | 18.2 (10.8-25.5) | 0.272 | 0.289 | 0.203 | 13.5 (8.3-18.8) | 19.2 (11.7-26.6) | 19.6 (12.0-27.2) | 18.8 (11.3-26.3) | 16.6 (10.1-23.1) | 0.692 | 0.669 | 0.442 |
| Physical risk factors, mean (95% CI) | ||||||||||||||||
| Body mass index (BMI) (kg/m2) | 18.5 (18.3-18.7) | 21.3 (21.0-21.5) | 21.3 (21.0-21.6) | 20.9 (20.6-21.2) | 20.2 (19.9-20.5) | <0.001 | <0.001 | <0.001 | 20.0 (19.7-20.3) | 22.6 (22.3-22.9) | 23.0 (22.7-23.3) | 23.2 (22.8-23.5) | 22.3 (22.0-22.6) | <0.001 | <0.001 | <0.001 |
| Waist circumference (cm) | 67.3 (66.9-67.7) | 74.1 (73.6-74.6) | 76.5 (75.9-77.1) | 77.2 (76.6-77.8) | 77.9 (77.3-78.4) | <0.001 | <0.001 | <0.001 | 70.2 (69.5-70.8) | 76.3 (75.6-77.1) | 78.9 (78.2-79.7) | 80.3 (79.5-81.2) | 80.0 (79.3-80.7) | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure (mmHg) | 118.1 (117.0-19.1) | 123.9 (122.5-25.3) | 123.2 (121.7-24.7) | 124.8 (123.2-26.3) | 133.4 (132.1-34.8) | <0.001 | <0.001 | <0.001 | 117.7 (116.6-18.8) | 116.5 (115.3-17.8) | 117.6 (116.3-18.8) | 120.8 (119.4-22.2) | 136.9 (135.7-38.1) | <0.001 | <0.001 | <0.001 |
| Diastolic blood pressure (mmHg) | 68.9 (68.1-69.6) | 71.8 (70.8-72.7) | 75.5 (74.5-76.4) | 77.9 (76.9-78.9) | 79.7 (78.8-80.6) | <0.001 | <0.001 | <0.001 | 71.2 (70.5-72.0) | 72.6 (71.7-73.4) | 75.4 (74.5-76.2) | 77.0 (76.0-77.8) | 80.0 (79.2-80.7) | <0.001 | <0.001 | <0.001 |
| Cardiometabolic risk factors, mean (95% CI) | ||||||||||||||||
| Total cholesterol (mmol/l) | 2.9 (2.9-3.0) | 3.1 (3.0-3.2) | 3.5 (3.4-3.6) | 3.6 (3.5-3.7) | 3.7 (3.6-3.7) | <0.001 | <0.001 | <0.001 | 3.3 (3.3-3.4) | 3.5 (3.4-3.5) | 3.7 (3.6-3.8) | 3.8 (3.7-3.9) | 4.2 (4.1-4.3) | <0.001 | <0.001 | <0.001 |
| HDL-cholesterol (mmol/l) | 0.8 (0.8-0.8) | 0.9 (0.8-0.9) | 1.0 (0.9-1.0) | 1.1 (1.1-1.2) | 1.1 (1.1-1.2) | <0.001 | <0.001 | <0.001 | 0.9 (0.8-0.9) | 1.0 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.1-1.2) | <0.001 | <0.001 | <0.001 |
| LDL-cholesterol (mmol/l) | 1.7 (1.6-1.7) | 1.8 (1.7-1.8) | 2.0 (1.9-2.0) | 1.9 (1.9-2.0) | 2.0 (1.9-2.1) | <0.001 | <0.001 | <0.001 | 1.9 (1.9-2.0) | 2.0 (1.9-2.1) | 2.1 (2.1-2.2) | 2.2 (2.1-2.3) | 2.5 (2.4-2.6) | <0.001 | <0.001 | <0.001 |
| Log triglycerides (mmol/l) | −0.03 (−0.06-0.01) | −0.02 (−0.06-0.01) | 0.13 (0.09-0.17) | 0.14 (0.10-0.18) | 0.10 (0.06-0.14) | <0.001 | <0.001 | <0.001 | 0.08 (0.05-0.10) | −0.15 (−0.18-0.11) | −0.04 (−0.07-−0.00) | 0.12 (0.08-0.16) | 0.25 (0.22-0.29) | <0.001 | <0.001 | <0.001 |
| HbA1c (%) | 5.1 (5.0-5.2) | 5.1 (5.0-5.2) | 5.1 (5.0-5.3) | 5.2 (5.1-5.3) | 5.3 (5.2-5.4) | <0.001 | <0.001 | <0.001 | 5.1 (5.0-5.2) | 5.1 (5.0-5.2) | 5.2 (5.1-5.3) | 5.3 (5.1-5.4) | 5.4 (5.3-5.5) | <0.001 | <0.001 | <0.001 |
Prevalences are adjusted for multi-level mixed-effects, with random effects to account for clustering at household and village levels, apart from smoking among women as the model could not complete due to values equalling zero.
P values were determined by fitting linear regression models for continuous dependent variables (i.e. physical risk factors) and fitting Poisson regression models for categorical dependent variables (i.e. lifestyle risk factors). Regressions were adjusting for SES and the independent variable was age as a continuous variable.
Adjusted p-values were determined by fitting multi-level mixed-effects Poisson regression models adjusted for SES and with random effects to adjust for clustering at household and village levels.
n=6056 for total population, n=2,701 for men, and n=3355 for women
Whereas smoking, alcohol consumption (men only), and low HDL-cholesterol were more common in the lower SES group, we found that overweight, abdominal obesity, raised blood pressure, raised total cholesterol, and raised HbA1c (women only) were more common in the higher SES group (table 5). Although overweight was more common among those of higher SES (p-value <0.001 for men and women), low physical activity (p-value 0.230 for men, 0.397 for women), low fruit and vegetable consumption (p-value 0.499 for men, 0.299 for women) and high staples consumption (p-value 0.365 for men, 0.088 for women) showed no clear trend with SES.
Table 5. Distribution of age-adjusted prevalences of NCD risk factors and healthcare factors by SES quintiles and sex.
| Men’s socioeconomic status | Women’s socioeconomic status | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lowest (n=655) |
Lower-middle (n=614) |
Middle (n=617) |
Middle-high (n=579) |
Highest (n=606) |
Unadjusted p-value |
P value* | Adjusted P-value** |
Lowest (n=719) |
Lower-middle (n=759) |
Middle (n=757) |
Middle-high (n=795) |
Highest (n=766) |
Unadjusted p-value |
P value* | Adjusted P-value** |
|
| Lifestyle risk factors, % (95% CI) | ||||||||||||||||
| Current smoker | 22.0 (18.4-25.6) | 13.7 (10.7-16.7) | 11.1 (8.3-13.9) | 7.6 (5.1-9.9) | 5.7 (3.7-7.7) | <0.001 | <0.001 | <0.001 | 2.2 (1.2-3.2) | 2.4 (1.4-3.5) | 0.6 (0.0-1.2) | 0.6 (0.0-1.2) | 0.4 (−0.1-0.9) | <0.001 | <0.001 | <0.001 |
| Alcohol user | 45.6 (38.9-52.3) | 38.8 (32.6-45.0) | 38.4 (32.1-44.7) | 35.1 (29.1-41.2) | 35.4 (29.4-41.3) | <0.001 | 0.016 | 0.003 | 31.8 (26.6-37.0) | 30.7 (25.6-35.8) | 31.5 (26.2-36.8) | 30.7 (25.5-35.9) | 30.9 (25.7-36.1) | 0.034 | 0.918 | 0.783 |
| Weekly drinker | 23.9 (19.2-28.6) | 19.1 (14.9-23.3) | 18.1 (14.0-22.3) | 13.8 (10.2-17.4) | 16.8 (12.9-20.8) | <0.001 | 0.015 | 0.001 | 6.0 (4.0-8.0) | 6.5 (4.3-8.6) | 5.5 (3.5-7.5) | 4.2 (2.6-5.8) | 5.2 (3.3-7.1) | 0.058 | 0.540 | 0.158 |
| Low physical activity | 19.8 (16.4-23.3) | 18.6 (15.1-22.1) | 20.5 (16.7-24.3) | 19.8 (15.9-23.7) | 22.8 (18.7-26.9) | 0.981 | 0.198 | 0.230 | 37.3 (32.9-41.7) | 35.9 (31.6-40.1) | 37.9 (33.4-42.4) | 39.5 (34.9-44.0) | 38.5 (34.0-43.1) | 0.719 | 0.385 | 0.397 |
| Low fruit and vegetable consumption | 72.7 (65.5-79.8) | 75.2 (67.7-82.7) | 73.5 (66.1-81.0) | 72.9 (65.4-80.5) | 72.9 (69.7-85.2) | 0.381 | 0.298 | 0.499 | 74.8 (68.3-81.4) | 75.9 (69.5-82.3) | 79.2 (72.6-85.7) | 77.9 (71.5-84.3) | 79.3 (72.7-85.8) | 0.260 | 0.248 | 0.299 |
| High staple consumption1 | 18.9 (11.4-26.4) | 15.8 (9.4-22.2) | 20.5 (12.5-28.6) | 19.9 (12.1-27.8) | 19.4 (11.7-27.0) | 0.718 | 0.680 | 0.365 | 15.7 (9.5-21.9) | 15.8 (9.6-22.1) | 16.8 (10.2-23.3) | 18.4 (11.2-25.5) | 18.4 (11.2-25.5) | 0.404 | 0.384 | 0.088 |
| Physical risk factors, % (95% CI) | ||||||||||||||||
| Overweight | 2.1 (1.0-3.2) | 3.4 (1.9-4.9) | 4.2 (2.5-5.9) | 5.4 (3.4-7.5) | 10.1 (7.1-13.1) | <0.001 | <0.001 | <0.001 | 12.0 (9.4-14.7) | 12.7 (10.0-15.3) | 16.5 (13.4-19.7) | 23.3 (19.5-27.2) | 23.4 (19.6-27.3) | <0.001 | <0.001 | <0.001 |
| Abdominal obesity | 0.4 (−0.1-0.8) | 0.6 (−0.0-1.1) | 0.8 (0.1-1.6) | 1.1 (0.2-2.0) | 4.6 (2.4-6.8) | <0.001 | <0.001 | <0.001 | 25.3 (21.5-29.0) | 26.6 (22.7-30.4) | 30.3 (26.1-34.6) | 35.2 (30.6-39.8) | 37.5 (32.7-42.4) | <0.001 | <0.001 | <0.001 |
| Raised blood pressure | 13.7 (11.1-16.4) | 15.2 (12.2-18.3) | 15.7 (12.5-18.9) | 18.8 (15.1-22.6) | 18.0 (14.5-21.6) | 0.561 | 0.017 | 0.018 | 15.9 (13.2-18.6) | 14.7 (12.0-17.3) | 16.5 (13.4-19.5) | 19.0 (15.6-22.3) | 18.5 (15.2-21.8) | 0.206 | 0.047 | 0.051 |
| Cardiometabolic risk factors , % (95% CI) | ||||||||||||||||
| Raised total cholesterol | 1.4 (0.5-2.3) | 2.5 (1.3-3.8) | 2.1 (0.9-3.4) | 4.1 (2.2-6.0) | 5.4 (3.3-7.6) | <0.001 | <0.001 | <0.001 | 5.0 (3.3-6.6) | 5.9 (4.0-7.8) | 5.2 (3.4-7.0) | 9.8 (7.0-12.6) | 9.8 (7.1-12.6) | 0.003 | <0.001 | <0.001 |
| Low HDL-cholesterol | 65.4 (58.8-72.0) | 63.3 (56.8-70.0) | 62.3 (55.8-68.7) | 61.8 (55.2-68.4) | 53.5 (47.5-59.5) | 0.040 | 0.007 | 0.013 | 86.5 (79.6-93.3) | 80.9 (74.5-87.3) | 79.6 (73.2-85.9) | 81.3 (75.0-87.5) | 73.6 (67.6-79.7) | 0.047 | 0.017 | 0.017 |
| Raised triglycerides | 12.1 (9.4-14.8) | 12.3 (9.4-15.2) | 11.5 (8.7-14.3) | 11.5 (8.5-14.4) | 12.7 (9.7-15.8) | 0.949 | 0.731 | 0.914 | 14.4 (11.7-17.1) | 14.3 (11.6-16.9) | 13.0 (10.4-15.7) | 14.7 (12.0-17.5) | 14.5 (11.8-17.3) | 0.491 | 0.866 | 0.866 |
| Raised HbA1c | 0.2 (−0.1-0.6) | 0.6 (−0.0-1.2) | 1.3 (0.3-2.3) | 1.0 (0.1-1.9) | 0.9 (0.1-1.8) | 0.245 | 0.073 | 0.074 | 0.2 (−0.1-0.5) | 0.5 (0.3-1.0) | 0.9 (0.1-1.7) | 1.0 (0.1-1.8) | 1.6 (0.4-2.8) | 0.005 | <0.001 | <0.001 |
| Health screening, % (95% CI) | ||||||||||||||||
| Blood pressure screen | 39.5 (34.6-44.5) | 39.7 (34.5-44.9) | 40.4 (35.1-45.8) | 45.0 (39.0-50.9) | 47.0 (41.1-53.0) | 0.506 | 0.030 | 0.018 | 48.7 (43.4-53.9) | 53.2 (47.7-58.7) | 55.4 (49.6-61.2) | 60.1 (54.1-66.0) | 59.6 (53.6-65.6) | 0.163 | 0.001 | 0.001 |
| Diabetes screen | 17.6 (13.8-21.4) | 18.7 (14.6-22.9) | 21.6 (16.9-26.2) | 21.9 (17.0-26.7) | 26.2 (20.7-31.6) | 0.136 | 0.009 | 0.001 | 20.9 (16.5-25.4) | 23.7 (18.8-28.6) | 25.2 (20.0-30.5) | 26.9 (21.4-32.4) | 28.8 (22.9-34.6) | 0.266 | 0.008 | 0.001 |
| Cholesterol screen | 0.7 (0.1-1.4) | 1.5 (0.5-2.4) | 1.0 (0.2-1.8) | 0.9 (0.1-1.6) | 1.7 (0.6-2.7) | 0.374 | 0.335 | 0.335 | 1.1 (0.3-1.9) | 0.7 (0.1-1.3) | 1.0 (0.3-1.7) | 1.3 (0.4-2.1) | 1.1 (0.3-1.9) | 0.702 | 0.621 | 0.700 |
| Diagnosis, % (95% CI) | ||||||||||||||||
| Diagnosed with raised blood pressure | 2.7 (1.5-3.8) | 3.6 (2.1-5.0) | 4.2 (2.5-5.9) | 3.9 (2.1-5.6) | 5.7 (3.6-7.7) | 0.298 | 0.010 | 0.010 | 5.5 (3.9-7.1) | 6.4 (4.6-8.2) | 7.6 (5.4-9.8) | 10.1 (7.5-12.7) | 10.5 (7.8-13.2) | 0.046 | <0.001 | <0.001 |
| Diagnosed with diabetes | 0.9 (0.2-1.6) | 1.2 (0.4-2.1) | 1.6 (0.5-2.6) | 1.1 (0.2-2.1) | 1.2 (0.3-2.2) | 0.963 | 0.637 | 0.637 | 0.6 (−0.0-1.1) | 0.7 (0.0-1.3) | 1.0 (0.1-1.8) | 0.8 (0.0-1.5) | 1.0 (0.1-2.0) | 0.364 | 0.146 | 0.174 |
| Diagnosed with raised cholesterol | 0.6 (−0.0-1.2) | 0.6 (−0.0-1.2) | 0.1 (−0.1-0.4) | 0.5 (−0.1-1.0) | 0.6 (−0.1-1.2) | 0.957 | 0.939 | 0.936 | 0.7 (0.0-1.4) | 0.3 (−0.1-0.8) | 0.2 (−0.1-0.6) | 0.5 (−0.0-1.0) | 0.4 (−0.1-0.8) | 0.349 | 0.427 | 0.444 |
| Treatment among those diagnoses, % (95% CI) | ||||||||||||||||
| On treatment for raised blood pressure | 45.0 (17.1-73.0) | 24.9 (5.0-44.9) | 43.0 (16.4-69.7) | 52.2 (19.8-84.6) | 47.8 (22.7-72.8) | 0.476 | 0.434 | 0.434 | 48.3 (29.7-66.8) | 42.6 (25.6-59.7) | 52.0 (33.0-70.9) | 49.8 (33.3-66.4) | 53.6 (36.8-70.4) | 0.678 | 0.518 | 0.518 |
| On treatment for diabetes | 29.7 (−11.5-71.0) | 27.2 (−10.9-65.3) | 21.1 (−8.2-50.5) | 49.8 (−6.6-106.2) | 54.0 (0.6-107.3) | 0.255 | 0.319 | 0.319 | 0.0 (0.0-0.0) | 53.4 (6.5-100.2) | 16.4 (−6.3-39.2) | 41.3 (0.8-81.7) | 17.6 (−7.2-42.4) | 0.960 | 0.716 | 0.716 |
| On treatment for raised cholesterol | 25.2 (−24.0-74.1) | 23.7 (−22.8-70.2) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 36.2 (−48.3120.8) | 0.765 | 0.777 | 0.777 | 0.0 (0.0-0.0) | 69.1 (−28.0166.2) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 94.8 (−17.5207.0) | 0.139 | 0.153 | 0.153 |
Prevalences are adjusted for multi-level mixed-effects, with random effects to account for clustering at household and village levels, apart from treatment for diabetes among women and treatment for raised cholesterol as the model could not complete due to values equalling zero.
P values were determined by fitting Poisson regression models with categories of SES as the independent variable and adjusted for age as a continuous variable.
Adjusted p-values were determined by fitting multi-level mixed-effects Poisson regression models adjusted for age and with random effects to adjust for clustering at household and village levels.
n=6057 for total population, n=2701 for men, and n=3356 for women
Women and those of higher SES were more likely to report having previously been screened for raised blood pressure and diabetes than men and those of lower SES, respectively (table 3 and 5). No marked difference between men and women or SES groups was found with regard to screening for cholesterol. Furthermore, women and those of higher SES were also more likely than men and those of lower SES, respectively, to report having been diagnosed with raised blood pressure. However, no substantial differences were seen by sex or SES for treatment of raised blood pressure.
Figure 1 illustrates the interrelation between sociodemographic factors and NCD risk factors. These analyses suggest that the associations among sociodemographic factors and NCD risk factors may be complex. Exploratory analyses showed that the association between SES and low physical activity (p-value 0.0063 for likelihood ratio test), overweight (p-value 0.0004), abdominal obesity (p-value 0.0031), raised total cholesterol (p-value 0.0082), and raised triglycerides (p-value 0.0001), were modified by age and the association between SES and abdominal obesity (p-value <0.0001) was modified by sex (supplemental table S4). Furthermore, modification by sex was also found for the association between age and low physical activity (p-value <0.0001), abdominal obesity (p-value 0.0015), raised blood pressure (p-value 0.0002), and low HDL-cholesterol (p-value <0.0001). These analyses suggest that the strength of association between SES and some NCD risk factors may not be the same for all age groups and for both men and women. Additionally, the strength of association between age and some NCD risk factors may also differ between men and women.
Figure 1. Selection of patterns of association of NCD risk factors with age and socioeconomic status by sex.
Discussion
In this rural population in south-western Uganda we found a clear gradient in SES status. SES was found to be associated with several NCD risk factors, yet these associations were inconsistent, with some NCD risk factors being positively associated and some inversely associated. Furthermore, these associations might be modified by other sociodemographic factors. By contrast to urban-rural assessments, our findings suggest that social inequalities may exist within rural populations. These data may help us towards identifying the principal causes and social determinants of NCDs and their risk factors in rural African populations. Effective actions are needed to address NCDs in disadvantaged groups in order to achieve a substantial reduction in the total NCD burden, therefore making health inequalities an essential priority.10 20
The overall increase in NCD risk factors in developing countries is often considered to be largely due to three key transitions: urbanisation, nutritional transition and demographic transition.5-7 At the early stages of these transitions, risk factors tend to be concentrated among urban dwellers and older people.6,21 Uganda may be undergoing epidemiological transition at the early stages, rapid urbanisation, and slow changes to demography, though the fertility rate remains high. However, it should not be presumed that the accompanying increase in burden of NCDs is restricted to older people and those in urban settings. In this study we found that NCD risk factors were present among rural dwellers, not all factors were highest among those of higher SES, and although risk factors were more common among older people there was still a sizable burden in this relatively young population. Furthermore, the reported burden of low fruit and vegetable consumption and low levels of physical activity were especially high even though the majority of the population were farmers. For example, the prevalence of low physical activity in this population was comparable to the global estimate of 31% worldwide.22 Other studies of young rural African populations have also found a substantial burden of NCD and their risk factors (table 6). Caution should therefore be exercised in making presumptions about NCD risk for individual populations based on transition theories which are designed to describe large-scale changes over time. Rural populations, and the heterogeneity within, should therefore be considered in addition to urban areas when designing initiatives aimed at reducing NCD risk factors.The underlying causes of these factors should also be further investigated within rural settings. This is particularly important given that the majority of Africans still reside in rural areas.11 23
Table 6. Comparison of surveys of risk factors for NCDs in General Population Cohort study with similar case definitions.
| Study | GPC Uganda | Cameroon39* | South Africa40** | Nigeria41 *** |
|---|---|---|---|---|
| Methods | ||||
| Survey year | 2011 | 1998 | 1996 | 2002-2005 |
| Region | SW rural Uganda | Rural dwellers from Bafut | Rural black community in KwaZulu-Natal province | Egbeda local government area, rural SW Nigeria |
| Sample frame | General population 25 villages | Random sampling of households | Random-cluster sampling | Random-sample survey |
| Ages (years) | ≥13 | ≥15 | >15 | 18-64 |
| Sample size | 6867 | 1282 | 947 | 2000 |
| Results (percentages for men, women) | ||||
| Current smoker | 14, 1 | 32, 14 | NA | 4, 0 |
| Overweight (BMI 25-30) | 5, 13 | 1, 6 | 13, 25 | 2, 2 |
| Obesity (BMI ≥30) | 1, 4 | 0.5, 3 | 9, 23 | 2, 2 |
| Hypertension | 17, 16 | 16, 12 | 31, 25 | 42, 37 |
| Hypercholesterolaemia | 3, 7 | NA | NA | 3, 3 |
| Diabetes | 1, 1 | 5, 3 | 14, 11 | 4, 6 |
Diabetes defined as FG≥6.1mmol/l
Diabetes defined as FG≥5.6 mmol/l and hypertension ≥130/85
Cholesterol ≥200 mg/dl and diabetes FBG≥110 mg/dl
As differences in SES and demography evolve across and within populations with the epidemiological transition, it is important to understand the lifestyle implications which may affect health. The explanations for the differences in NCD risk factors between sociodemographic groups found in this study remain unclear. For example, overweight has frequently been reported as being associated with higher SES within LMICs, by contrast to its association with lower SES in high-income countries.24-28 Explanations for these observations often centre on an increased availability of food and a decrease in physical labour and activity, coupled with an environment of food insecurity and cultural factors among those of higher SES in LMICs.21 27 However, although we found that overweight and abdominal obesity were more common in higher SES groups, we did not find any association between SES and low physical activity, or consumption of fruit, vegetables, or staples. It is therefore unclear what the causes of overweight and abdominal obesity are in this population and how they differ for women and those of higher SES leading to a higher prevalence in these sociodemographic groups. More defined studies of lifestyle factors, including closer examination of diet components such as fat and sugar consumption, may be needed to better understand these causes. We also found that smoking and alcohol consumption were more common among those of lower SES, which is in keeping with findings from both LMICs and high-income countries.28-32 The reasons for this also remain unclear; although it may reflect a better understanding and knowledge of the adverse effects of smoking among those of higher SES. Given that smoking and weekly alcohol consumption are substantially more common among men than women, there may also be cultural factors at play. For example, smokeless tobacco has been showed to be more common among women than men in many African populations, as smoking tobacco was reportedly considered culturally unacceptable for women.33
The complex nature of associations between sociodemographic factors and NCD risk factors is further illustrated by the apparent interdependence between the sociodemographic factors in their association with NCD risk factors, as shown through interaction analyses. The difference between men and women in the strength of association between age and lifestyle risk factors may be due to cultural reasons and gender roles across age groups. However, there may also be underlying biological differences between men and women leading to differences in the relationship between age and risk factors, observed here for blood pressure and HDL-cholesterol.34 The relationship between SES and NCD risk factors may be modified by both age and sex in some cases. This may reflect a difference in how the lives of men and women and of those of different age groups are affected by their household’s SES.
Our study has a number of strengths and possible limitations which should be considered. The study was cross-sectional in design and we are therefore unable to comment on a temporal relationship between sociodemographic factors and NCD risk factors. The internal validity of this study is likely to be good given the highly rigorous quality control. The internationally standardised WHO STEPs questionnaire was used, equipment were regularly recalibrated, and staff followed detailed standard operating procedures to ensure accuracy and precision of measurements. Data checks were performed on a weekly and monthly basis to ensure consistency in data quality. However, some data were missing, requiring us to perform full-case analysis on 85% of those surveyed. There were some systematic differences between those with and those without data, suggesting that data may not be missing at random (supplemental table S2). This could therefore reduce the generalizability of our findings. NCD lifestyle risk factors and most socioeconomic variables were self-reported by participants, which may have introduced information bias. However, given the low levels of previous screening for NCD risk factors in this population and the rigorous training of staff, this potential for information bias is likely to be low. Physical activity and diet were self-reported. This may have caused misclassification in exposure measurement, which may in part explain the apparently discrepant results between lifestyle factors and overweight. SES was constructed on a household level which may have hidden differences in SES between those within a household, particularly potential differences between men and women.35 The wide age range of participants (13-97 years) is both a strength and a weakness of this study. Few datasets have examined NCD risk in early adulthood; our dataset has allowed us to do so. However, only 20.5% of the population are aged 50 years and above and therefore a small proportion of the sample size are older people who have the greatest NCD risk. Even still, we find a substantial burden of NCD risk factors in this relatively young population.
These analyses provide a comprehensive overview of the sociodemographic distribution of NCD risk factors. There are many ways in which future studies could explore this topic in more depth. Objective and better-refined tools for capturing diet and physical activity data, such as food diaries, sodium urine testing, and accelerometers, could provide better assessment of exposure. In the current study, data on treatment of NCDs are underpowered. Future work is needed to better examine how sociodemographic factors relate to the treatment of NCDs and access to healthcare facilities. Importantly, there is a need to disentangle the underlying determinants of the observed sociodemographic distributions of NCD risk factors.8 9 Longitudinal data on sociodemographic factors and NCDs could greatly enhance our understanding of their temporal relationship and the determinants of sociodemographic distribution in NCDs and their risk factors. A multi-disciplinary approach may be needed to achieve a holistic understanding of these determinants.
A clearer picture of the sociodemographic distribution of risk factors may provide insight into potential primary targets for intervention and policy. A better understanding of the underlying ‘causes of the causes’ of NCDs may also provide information about the aetiology of NCD and shed light on the observed differences between those of African descent and those of European descent with regard to NCDs and NCD risk factors.36-38 With the growing focus on NCDs in SSA, it is important to ensure that policies put in place in order to reduce the burden of NCD risk factors consider rural populations in addition to urban populations. The majority of Africans still reside in rural areas and thus such areas should be incorporated into the design and implementation of policy and healthcare programmes.
Supplementary Material
Key messages.
Rural African populations are not homogenous; here we identify a socioeconomic gradient in a relatively poor rural population in Uganda.
Within this relatively young population NCD risk factors were common and they varied across the socioeconomic gradient, with some risk factors being positively associated and some inversely associated with socioeconomic status.
A better understanding of the determinants of the sociodemographic distribution of NCDs and their risk factors in rural African populations will help identify populations at most risk of developing NCDs and help plan interventions to reduce their burden.
Acknowledgements
We would like to thank the GPC team and all other MRC staff who contributed to this study. We are also grateful to all study participants for their involvement in the study.
Funding: This work was sponsored by Medical Research Council (MRC), UK [grant numbers G0801566 and G0901213-92157] awarded to MSS and also MRC/Uganda Virus Research Institute core funding. GAVM was supported by the Gates Cambridge Scholarship.
Footnotes
Conflict of interest: None
References: I, Georgina A.V. Murphy, have checked the references for accuracy and completeness.
Guarantor: Manjinder S Sandhu will act as the guarantor for this paper.
References
- 1.World Health Organization . World health statistics 2012. Geneva: 2012. [Google Scholar]
- 2.World Health Organization 2009 http://www.who.int/healthinfo/global_burden_disease/DTHMDG%202004.xls.
- 3.World Health Organization . The global burden of disease: 2004 update. World Health Organization; Geneva: 2004. [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998;1(1):5–21. doi: 10.1079/phn19980004. [DOI] [PubMed] [Google Scholar]
- 6.Sobngwi E, Mbanya JC, Unwin NC, Porcher R, Kengne AP, Fezeu L, et al. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban Cameroon. Int J Epidemiol. 2004;33(4):769–76. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- 7.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. The Milbank Memorial Fund quarterly. 1971;49(4):509–38. [PubMed] [Google Scholar]
- 8.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 9.Marmot M, Friel S, Bell R, Houweling TA, Taylor S. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–9. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 10.Di Cesare M, Khang YH, Asaria P, Blakely T, Cowan MJ, Farzadfar F, et al. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381(9866):585–97. doi: 10.1016/S0140-6736(12)61851-0. [DOI] [PubMed] [Google Scholar]
- 11.United Nations Human Settlements Programme (UN-HABITAT) The State of African Cities 2012: Goverance, Inequality and Urban Land Markets. Nairobi: 2010. [Google Scholar]
- 12.Asiki G, Murphy G, Nakiyingi-Miiro J, Seeley J, Nsubuga RN, Karabarinde A, et al. The general population cohort in rural south-western Uganda: a platform for communicable and non-communicable disease studies. Int J Epidemiol. 2013;42(1):129–41. doi: 10.1093/ije/dys234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakibinge S, Maher D, Katende J, Kamali A, Grosskurth H, Seeley J. Community engagement in health research: two decades of experience from a research project on HIV in rural Uganda. Trop Med Int Health. 2009;14(2):190–5. doi: 10.1111/j.1365-3156.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization STEPS Surveillance Manual. http://www.who.int/chp/steps/manual/en/index.html.
- 15.Howe LD, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, et al. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41(3):871–86. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Global Strategy on Diet, Physical Activity and Health: Department of Chronic Diseases and Health Promotion. 2008. [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 18.American Diabetes Association What your cholesterol levels mean. http://www.heart.org/HEARTORG/Conditions/Cholesterol/AboutCholesterol/What-Your-Cholesterol-Levels-Mean_UCM_305562_Article.jsp.
- 19.World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of type 2 diabetes. World Health Organisation; Geneva: 2011. [Google Scholar]
- 20.Stuckler D, Basu S, McKee M. Commentary: UN high level meeting on non-communicable diseases: an opportunity for whom? BMJ. 2011;343:d5336. doi: 10.1136/bmj.d5336. [DOI] [PubMed] [Google Scholar]
- 21.BeLue R, Okoror TA, Iwelunmor J, Taylor KD, Degboe AN, Agyemang C, et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health. 2009;5:10. doi: 10.1186/1744-8603-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . Global status report on noncommunicable diseases 2010. World Health Organization; Geneva: 2011. [Google Scholar]
- 23.Uganda Bureau of Statistics . Uganda Demographic and Health Survey, Preliminary Report. 2011. [Google Scholar]
- 24.Ziraba AK, Fotso JC, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health. 2009;9:465. doi: 10.1186/1471-2458-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaren L. Socioeconomic status and obesity. Epidemiologic reviews. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian SV, Perkins JM, Ozaltin E, Davey Smith G. Weight of nations: a socioeconomic analysis of women in low- to middle-income countries. Am J Clin Nutr. 2011;93(2):413–21. doi: 10.3945/ajcn.110.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 28.Bovet P, Ross AG, Gervasoni JP, Mkamba M, Mtasiwa DM, Lengeler C, et al. Distribution of blood pressure, body mass index and smoking habits in the urban population of Dar es Salaam, Tanzania, and associations with socioeconomic status. Int J Epidemiol. 2002;31(1):240–7. doi: 10.1093/ije/31.1.240. [DOI] [PubMed] [Google Scholar]
- 29.Pampel F. Tobacco use in sub-Sahara Africa: estimates from the demographic health surveys. Soc Sci Med. 2008;66(8):1772–83. doi: 10.1016/j.socscimed.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–23. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- 31.Laaksonen M, Rahkonen O, Karvonen S, Lahelma E. Socioeconomic status and smoking: analysing inequalities with multiple indicators. European journal of public health. 2005;15(3):262–9. doi: 10.1093/eurpub/cki115. [DOI] [PubMed] [Google Scholar]
- 32.Doku D, Koivusilta L, Raisamo S, Rimpela A. Do socioeconomic differences in tobacco use exist also in developing countries? A study of Ghanaian adolescents. BMC Public Health. 2010;10:758. doi: 10.1186/1471-2458-10-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend L, Flisher AJ, Gilreath T, King G. A systematic literature review of tobacco use among adults 15 years and older in sub-Saharan Africa. Drug Alcohol Depend. 2006;84(1):14–27. doi: 10.1016/j.drugalcdep.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Cameron N, Getz B. Sex differences in the prevalence of obesity in rural African adolescents. Int J Obes Relat Metab Disord. 1997;21(9):775–82. doi: 10.1038/sj.ijo.0800472. [DOI] [PubMed] [Google Scholar]
- 35.Deere CD, Doss CR. Gender and the Distribution of Wealth in Developing Countries: World Institute for Development Economics Research. United Nations University: 2006. [Google Scholar]
- 36.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart. 1997;78(6):555–63. doi: 10.1136/hrt.78.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khattar RS, Swales JD, Senior R, Lahiri A. Racial variation in cardiovascular morbidity and mortality in essential hypertension. Heart. 2000;83(3):267–71. doi: 10.1136/heart.83.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godsland IF, Johnston DG, Chaturvedi N. Mechanisms of disease: lessons from ethnicity in the role of triglyceride metabolism in ischemic heart disease. Nat Clin Pract Endocrinol Metab. 2007;3(7):530–8. doi: 10.1038/ncpendmet0530. [DOI] [PubMed] [Google Scholar]
- 39.Sobngwi E, Mbanya JC, Unwin NC, Kengne AP, Fezeu L, Minkoulou EM, et al. Physical activity and its relationship with obesity, hypertension and diabetes in urban and rural Cameroon. Int J Obes Relat Metab Disord. 2002;26(7):1009–16. doi: 10.1038/sj.ijo.0802008. [DOI] [PubMed] [Google Scholar]
- 40.Motala AA, Esterhuizen T, Pirie FJ, Omar MA. The prevalence of metabolic syndrome and determination of the optimal waist circumference cutoff points in a rural South african community. Diabetes Care. 2011;34(4):1032–7. doi: 10.2337/dc10-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: a population-based survey. Cardiovasc J Afr. 2010;21(1):26–31. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.