Abstract
The aim of this study was to investigate the effect of fatigue on electromyographic (EMG) parameters of healthy young adults during obstacle crossing of two different heights. Twelve untrained male adults (23 ± 5 years of age) were fatigued running on a treadmill with increasing speed and inclination and walked over an obstacle with a height set at 10% and 20% of each individual’s lower limb length. Maximal plantar flexor torque and EMG of the medial gastrocnemius, soleus, and tibialis anterior muscles of the trailing limb were assessed during obstacle crossing. Data were captured before, immediately after and 5 minutes after a fatigue session. Fatigue induced significant reduction on the plantar flexor torque output immediately after and 5 minutes after exhaustion. After fatigue gait speed was not affected, the minimum distance between the obstacle and the trailing or leading foot remained unchanged, and the trailing foot contacted the ground closer to the obstacle immediately after fatigue. Regarding the EMG, medial gastrocnemius became after fatigue more active during swing phase when increasing the obstacle height, whereas this was not the case before or 5 minutes after fatigue. No other significant difference was observed for any of the examined muscles. It is concluded that the assessed fatigue protocol induced only minimal changes in the EMG activity of the examined muscles during obstacle crossing. Therefore, it is suggested that the neuromuscular system of healthy young individuals is able to respond to the decreased force capacity after fatigue during obstacle crossing of heights up to the 20% of the limb length.
Key Points.
Exhaustion after running on a treadmill induces significant reduction in plantar flexion strength and changes in the positioning of the feet relative to the obstacle during obstacle crossing.
EMG activity of the calf muscles of the trailing limb does not change significantly after fatigue during the stance phase
During swing phase, medial gastrocnemius EMG activity of the trailing limb increases after fatigue when obstacle height increases.
These minor changes in EMG after fatigue, reveals that untrained, healthy, young subjects may compensate the deficit in muscular force due to fatigue when performing obstacle crossing.
Key words: Gait, obstacle, fatigue, electromyography
Introduction
Obstacle crossing during walking requires higher level of neuromuscular activation than level walking and is an important task for everyday life, especially for elderly people or people with balance impairments (Chou and Draganich, 1997; Overstall et al., 1977). One of the most serious causes which increase the risk of falls and injury, especially in elderly people, is an unsuccessful obstacle crossing (Galna et al., 2009). In addition to this, fatigue could be an aggravating factor in such circumstances (Barbieri et al., 2013; Parijat and Lockhart, 2008). However, the available literature in the field of obstacle crossing under fatigued state is very limited.
According to the current literature, fatigue affects the accuracy of movements during various tasks (Knicker et al., 2011), which might have implications in obstacle crossing. Inaccuracy in movement during obstacle crossing could result in tripping on the obstacle or slipping during foot strike, which increases the risk of falling and raises the probability for injury. Therefore, fatigue is an important element of everyday life that could influence the pattern of obstacle crossing. Nonetheless, this issue has not been investigated in healthy subjects yet.
Walking over obstacles has four objectives: 1. to maintain balance, 2. to prevent tripping during crossing, 3. to prevent slipping during landing, and 4. to progress the body forward. In order to cross an obstacle successfully, numerous muscles have to be activated in a certain sequence and intensity. Fatigue affects the coordination and the motor control accuracy (Sparto et al., 1997) and this could have consequences during obstacle crossing. For example, it is known that co-activation of agonist and antagonist muscles offers better joint stability (Kellis, 1998; Nagai et al., 2013) and could facilitate accuracy of the limb movement (Gribble et al., 2003). Many researchers have studied the complex relationship between fatigue and co-activation of antagonist muscles. It has been shown that fatigue has consequences on the accuracy of elbow flexion and extension movements and this could be explained by the reduced level of antagonist muscle co-activation (Missenard et al., 2008). Considering the above, it could be argued that during obstacle crossing such reduction in co-activation of the ankle joint muscles under fatigue conditions could impede accuracy and increase the risk of tripping. However, this issue remains to be verified.
Considering the above, the aim of this study was to investigate the effects of fatigue on the EMG characteristics during obstacle crossing of different heights. It is hypothesized that decrease in torque output of the plantar flexor muscles due to fatigue will induce changes in the kinematic and EMG response during obstacle crossing which could be linked to increased risk of tripping, especially at higher obstacle heights. This study will comprise the basis for future research in the field of obstacle crossing in order to understand how the neuromuscular system adapts under fatigue conditions. This could have applications to other populations which are more prone to fatigue and/or have increased risk of falling.
Methods
Participants
Twelve healthy men (age 18 to 30 years old) with no previous musculoskeletal injuries on the lower extremities or low back, participated voluntarily in this research (Table 1). Participants with estimated body fat above 30% were excluded, in order to avoid low signal to noise ratio in EMG signals (Lindström and Magnusson, 1977). All individuals were not systematically trained (less than 2 times a week physical activity) and gave their written consent before their participation, in accordance to the guidelines of the Local Ethics Committee.
Table 1.
Demographic and anthropometric characteristics of the participants (n = 12). Data are means (±SD).
| Age (years) | 22.8 (5.4) |
| Body mass (kg) | 83.8 (13.4) |
| Body fat (%) | 19.3 (1.9) |
| Body height (m) | 1.80 (.06) |
| Lower limb length (m) | .99 (.04) |
Measurement procedures
Subjects were instructed not to consume any alcohol or caffeine and not to exert any strenuous activity 24 hours prior to the examination. After the participants’ arrival to the laboratory the anthropometric characteristics were measured (postural height, limb length, skinfold thickness). Lower limb length was measured with scaled tape from the anterior superior iliac spine to the lateral malleolus. This measurement was used to calculate the obstacle height for each individual separately. Body fat, expressed as percentage of body mass, was estimated by measuring the skinfolds of the triceps brachialis, the abdominal, the suprailiac, and the thigh with an analog skinfold caliper (#1127A, Lafayette Instruments, Indiana, USA), as indicated by Jackson and Pollock (1985).
After the warm-up (low intensity running on a treadmill for 5 minutes and stretching for the lower limb muscle groups), the subject laid supine on the chair of the dynamometer (CYBEX Norm, HUMAC/NORM system, USA) in a comfortable position. The knee was fully extended and the foot was positioned at 90° relative to the shank. The foot was fixed with Velcro straps on the dynamometer, so that the projection of the ankle joint rotation axis crossed approximately the axis rotation of the dynamometer. The participants performed 2-3 submaximal plantar and dorsiflexions, before the Maximal Voluntary Contraction (MVC) test began. MVC test included 5 plantar flexions and 5 dorsiflexions with 3 minutes rest. The best trial in torque output was analyzed as baseline value.
After the MVC test, the participants were instructed to walk on their preferred speed along an 8-meter walkway with an obstacle placed on the midway, perpendicular to the direction of walking, and parallel to the ground. The obstacle consisted of a light-weight, plastic tube (1 cm w) that was placed on height-adjustable metal plates. The height of the obstacle was set at 10%, and 20% of the lower limb length of each participant. A series of trials with no obstacle were captured as well. These 3 conditions were assessed in random order. The self-selected pace was preserved for each participant during the whole experiment using a digital metronome (SAMWOO IMT – 020, Korea). Each individual started walking with the verbal cue “go”, starting from the same point marked on the ground. Five trials for each obstacle height with a brief rest period in between were captured as baseline measurements.
As soon as the baseline measurements were assessed, the participants performed the fatigue protocol (modified Bruce protocol). More particularly, it consisted of walking/running on a treadmill with the increments in speed and inclination every 3 minutes, as shown in Table 2. During the session, heart rate was monitored (Polar, Model A3, Polar Electro, Oy, Finland) and when exhausted, the participant defined on a scale from 1 to 20 the extent of fatigue that they experienced, according to the Borg scale. Immediately after the end of the fatigue protocol, two gait trials for each condition (no obstacle, 10%, 20% obstacle) were assessed randomly followed by an MVC isometric plantar flexion. These tests were repeated 5 minutes after the end of the fatigue protocol. At the end of the experiment, the participants performed stretching exercises for their lower limbs (5 minutes).
Table 2.
Properties of the treadmill fatigue protocol.
| Stage | Duration (mins) |
Speed (m·s-1) |
Inclination (%) |
Exhaustion level (n) |
|---|---|---|---|---|
| 1 | 3 | .76 | 10 | 0 |
| 2 | 3 | 1.12 | 12 | 0 |
| 3 | 3 | 1.52 | 14 | 5 |
| 4 | 3 | 1.88 | 16 | 4 |
| 5 | 3 | 2.23 | 18 | 3 |
| 6 | 3 | 2.46 | 20 | 0 |
| 7 | 3 | 2.68 | 22 | 0 |
Feet position tracking
In order to track the position of the feet relative to the obstacle three-dimensional trajectories were captured with 6 cameras (type M3) of a VICON 612 motion analysis system (Oxford Metrics, Ltd., Oxford, Oxforshire, UK). The sampling frequency was set at 120 Hz. Infrared reflective markers were fixed with adhesive tape, bilaterally, on the lateral malleolus, on the heel and between the 2nd and 3rd metatarsal distally. Two additional markers defined the position of the obstacle.
Data were processed offline with custom Matlab scripts (Matlab 7.0, Mathworks Inc.). The distance between the marker placed on the lateral malleolus and the horizontal line defined by the obstacle markers was calculated through the whole trajectory of the swing phase during crossing, and its minimum value was used to quantify how close the lower limb approached the obstacle. Furthermore, the heel and the obstacle markers were projected on the ground and the vertical distance between the heel marker and the line defined by the obstacle markers defined the distance of the feet from the obstacle. This distance was calculated for the leading limb during foot strike, after the obstacle clearance, and for the trailing limb during stance phase, before the obstacle clearance.
Electromyography
The EMG of the trailing limb was captured using a BTS TELEMG remote system (BTS Bioengineering, Milano, Italy). The pre-amplifiers had common-mode rejection ratio greater than 110 dB at 50/60 Hz and the gain was 1000 at a bandwidth from 10 to 500 Hz. Sampling frequency was set at 1 kHz. The signal was band-pass filtered (Butterworth zero-lag, 4th order, 10 to 500 Hz) and the root mean square (RMS) was calculated over a window of 20 ms. Outcome EMG variables for each muscle was the mean RMS during the stance and swing phase normalized to the maximum EMG value during the isometric MVC test, when the specific muscle was acting as an agonist (plantar flexion for the medial gastrocnemius and soleus, and dorsiflexion for the tibialis anterior).
Bipolar disc surface electrodes (Ag/AgCl, 1 cm diameter) were used to record EMG activity of the medial gastrocnemius, soleus, and tibialis anterior muscles. The center-to-center inter-electrode distance was set at 2 cm. The electrode placement was according to the SENIAM guidelines (Hermens et al., 1999). More specifically, for the medial gastrocnemius muscle the electrodes were placed on the most prominent part of the muscle, and for the soleus muscle at the 2/3 of the distance between the medial femoral condyle and the medial malleolus when knee is flexed. The recording electrodes for the tibialis anterior muscle were placed at the 1/3 of the distance between the tip of the fibula head and the medial malleolus. The ground electrode was set over the medial malleolus of the contralateral foot. Manual tests consisted of short isometric contractions activating selectively the preferred muscle, with simultaneous EMG feedback, ensured the appropriate electrode placement and minimized the effect of crosstalk (Winter et al., 1994). Prior electrode placement, the skin was carefully dry-shaved, rubbed with sandpaper, and cleaned with an alcohol solution. This preparation resulted skin impendence below 5kΩ.
Statistical analysis
Descriptive statistics (mean values and standard deviation) were calculated for all dependent variables. Two-way ANOVA with repeated measurements was assessed for all dependent variables. The first factor was the presence of the obstacle or obstacle height, and the second the evaluation before, immediately after and 5 minutes after the fatigue protocol. To determine the effect of fatigue on the isometric plantar flexion torque, one-way ANOVA with repeated measurements was used. The level of significance was set at an alpha level of 0.05. All statistical analyses were undertaken using SPSS 17 statistical software (SPSS Inc., Chicago, IL, USA).
Results
All participants were able to complete the experiment. Five subjects quit at stage 3 of the fatigue protocol, 6 at stage 4, and 3 at stage 5 (Table 2). They evaluated their exhaustion with 19 (n = 9) or 20 (n = 5) according to Borg’s scale. The heart rate at the end of the fatigue protocol was 190 ± 10 beats per minute.
Before the fatigue protocol the torque of the plantar flexors was 179.4 ± 22.7 Nm (Figure 1). This torque decreased significantly by 29.4 ± 8.7% after exhaustion (F = 196, p < 0.001). Five minutes after the end of the fatigue protocol the torque remained statistically significantly reduced by 10.8 ± 4.7% relative to the pre-fatigue values (p < 0.05).
Figure 1.
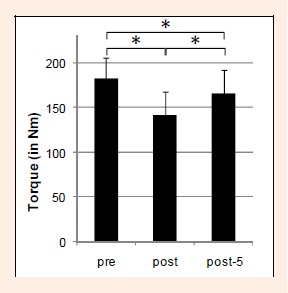
Mean values of the plantar flexion moment during an MVC before (pre), after fatigue (post) and 5 minutes after fatigue (post-5). Vertical lines indicate one standard deviation, and asterisks designate significant difference between measurements (p < 0.05).
Gait speed was not affected by the fatigue protocol when walking without obstacle (before: 1.08 ± 0.09 m·s-1, after: 1.13 ± 0.11 m·s-1, after 5 minutes: 1.09 ± 0.12 m·s-1, p > 0.05), or by the obstacle of height at 10% (before: 0.98 ± 0.07 m·s-1, after: 1.01 ± 0.10 m·s-1, after 5 minutes: 1.04 ± 0.21 m·s-1, p>0.05) and 20% (before: 0.93 ± 0.07 m·s-1, after: 1.02 ± 0.12 m·s-1, after 5 minutes: 0.97 ± 0.12 m·s-1, p > 0.05) of the limb length. As shown in Table 3, the minimum distance from the obstacle during clearance decreased significantly for both limbs when increasing the obstacle height (p < 0.05). However, no significant main effect of fatigue or interaction was observed (p > 0.05). The distance of the leading limb from the obstacle during stance after obstacle clearance was not affected by obstacle height, fatigue or their interaction. However, the distance of the trailing foot before crossing decreased significantly immediately after fatigue for the 10% obstacle height, and returned to pre-fatigue levels 5 minutes after the fatigue protocol.
Table 3.
Means (±standard deviation) of the minimum distance of the lateral malleolus marker from obstacle for the leading and trailing limb and the distance of the heel marker from the obstacle during heel stance phase after and before the obstacle for the leading and trailing limb, respectively.
| 10% | 20% | p-value | |||
|---|---|---|---|---|---|
| Leading limb | Minimum distance (cm) | Before fatigue | 17.0 (2.9) | 15.8 (2.8) | pf = .950 |
| After fatigue | 17.4 (3.7) | 15.9 (4.7) | ph = .004 | ||
| 5 minutes after fatigue | 17.0 (4.8) | 16.0 (5.1) | pf×h = .927 | ||
| Distance after obstacle (cm) | Before fatigue | 28.0 (6.7) | 27.1 (7.1) | pf = .216 | |
| After fatigue | 31.0 (7.7) | 28.2 (6.6) | ph = .055 | ||
| 5 minutes after fatigue | 28.9 (9.8) | 26.4 (7.5) | pf×h = .322 | ||
| Trailing limb | Minimum distance (cm) | Before fatigue | 10.7 (2.7) | 8.3 (2.9) | pf = .480 |
| After fatigue | 9.0 (5.7) | 7.9 (3.6) | ph = .027 | ||
| 5 minutes after fatigue | 9.4 (3.1) | 8.1 (3.9) | pf×h = .644 | ||
| Distance before obstacle (cm) | Before fatigue | 45. 8 (4.7) | 44.7 (4.7) | pf = .020 | |
| After fatigue | 40.5 (8.3) | 45.0 (4.5) | ph = .146 | ||
| 5 minutes after fatigue | 45.7 (4.5) | 46.3 (5.8) | pf×h = .010 | ||
pf, ph, and pf×h: significance level for the factors concerning fatigue (before, after and 5 minutes after), the obstacle height (10% and 20% of lower limb length), and their interaction, respectively.
During stance phase the EMG for the three examined muscles of the trailing foot was not affected by the obstacle height (p > 0.05) and no changes were observed before and after fatigue (p > 0.05; Table 4). Furthermore, no interaction between the two factors was evident in any of the examined muscles (p>0.05). During swing phase a significant interaction was observed for the medial gastrocnemius muscle (p < 0.05), indicating that after fatigue, EMG activity increased with obstacle height but this was not the case before and 5 minutes after fatigue. No further significant main effects or interactions were observed for any of the examined muscles during swing phase (p > 0.05).
Table 4.
Means (± standard deviation) of the electromyographic data (percentage of the maximum during MVC test) of the trailing limb during stance and swing phase.
| no obstacle | 10% | 20% | p-values | |||
|---|---|---|---|---|---|---|
| Stance Phase | Medial gastrocnemius | Before fatigue | 17.0 (5.3) | 17.8 (6.0) | 17.8 (6.7) | pf = .143 |
| After fatigue | 21.1 (12.9) | 20.5 (10.4) | 20.5 (9.5) | ph =.806 | ||
| 5 minutes after fatigue | 23.1 (12.7) | 22.3 (11.8) | 20.8 (11.7) | pf×h = .739 | ||
| Soleus | Before fatigue | 20.8 (8.8) | 20.9 (9.0) | 20.9 (9.3) | pf = .336 | |
| After fatigue | 21.2 (11.7) | 21.8 (10.0) | 22.6 (13.9) | ph = .992 | ||
| 5 minutes after fatigue | 25.6 (16.6) | 24.6 (13.3) | 24.3 (13.7) | pf×h = .904 | ||
| Tibialis anterior | Before fatigue | 20.2 (9.3) | 19.2 (8.1) | 19.0 (8.9) | pf = .306 | |
| After fatigue | 19.8 (10.7) | 20.4 (9.8) | 19.6 (10.9) | ph = .939 | ||
| 5 minutes after fatigue | 22.1 (12.5) | 23.5 (14.2) | 23.6 (11.8) | pf×h = .846 | ||
| Swing Phase | Medial gastrocnemius | Before fatigue | 12.3 (4.6) | 14.7 (4.6) | 15.3 (6.1) | pf = .248 |
| After fatigue | 15.5 (9.3) | 18.8 (11.2) | 20.0 (7.7) | ph = .531 | ||
| 5 minutes after fatigue | 19.5 (13.0) | 19.0 (10.7) | 16.5 (9.3) | pf×h = .026 | ||
| Soleus | Before fatigue | 17.4 (5.6) | 19.3 (7.4) | 19.5 (7.5) | pf = .616 | |
| After fatigue | 18.1 (9.4) | 20.3 (7.7) | 21.4 (10.5) | ph = .597 | ||
| 5 minutes after fatigue | 21.8 (14.7) | 22.2 (11.0) | 19.6 (9.8) | pf×h = .419 | ||
| Tibialis anterior | Before fatigue | 15.9 (5.7) | 17.6 (7.0) | 17.7 (8.0) | pf = .782 | |
| After fatigue | 16.1 (10.2) | 18.1 (6.7) | 17.7 (5.1) | ph = .483 | ||
| 5 minutes after fatigue | 17.7 (10.2) | 19.8 (10.1) | 17.9 (8.4) | pf×h = .955 | ||
pf, ph, and pf×h: significance level for the factors concerning fatigue (before, after and 5 minutes after), the presence of the obstacle or obstacle height (10% and 20% of lower limb length), and their interaction, respectively.
Discussion
This study shows that exhaustion after sustained running resulted in a significant decrease in the plantar flexor MVC torque, with only minor fatigue effects in the measured distances of the feet from the obstacle during crossing at the two different heights. The EMG amplitude of the calf muscles of the trailing foot did not change during stance phase before the obstacle clearance, whereas during swing phase, medial gastrocnemius showed increased EMG activity immediately after fatigue when increasing the obstacle height.
Fatigue as a result of a hard workout or training bouts could affect the contractile properties of the muscle and the neural output which could have consequences in the muscle force generation (Häkkinen, 1994). More specifically, exhaustive running may induce failure in the neuromuscular propagation (Girard et al., 2012), which could explain the reduced torque output of the plantar flexors in the present study after the fatigue protocol. It is worth noting that apart from the increased values in Borg scale, and the high heart rate recorded during exhaustion, and the high inclination achieved at the end of the fatigue protocol (14-18%), this reduction in plantar flexor torque was significant even 5 minutes after the end of the fatigue session. This implies the significant fatigue effect that occurred at least in terms of muscle torque output. This deficit could have implications in the neuromuscular properties during obstacle crossing.
After fatigue, only minimal changes were observed in the distance of the foot relative to the obstacle. Previous studies have shown that fatigue affects the accuracy of the ankle plantar flexors (Forestier et al., 2002) and the accuracy of the knee joint at low activation intensity (Skinner et al., 1986). In the present experiment the selected task of obstacle crossing requires relatively low level of activation and therefore it could be expected that the minimum distance of the foot from the obstacle might change after fatigue. However, our observations did not support this hypothesis, i.e. fatigue had no significant effect on the minimum distance from the obstacle for both feet. On the other hand, after fatigue, the distance of the trailing foot from the obstacle before crossing became shorter at the obstacle height of 10%, which supports the argument that accuracy is more affected at low level of activation (Jaric et al., 1999; Skinner et al., 1986). Stepping closer to the obstacle with the trailing limb, in the absence of visual feedback, could increase the risk of unsuccessful clearance and this should be taken into consideration, especially in pathological conditions under which this distance is already shorter (Law and Webb, 2005). On the other hand, the distance of the leading foot from the obstacle that stepping on the ground after obstacle crossing, does not change after fatigue. This is supported by previous findings, claiming that this distance is less variable (Niang and McFadyen, 2005, Patla et al., 1996) and probably less exposed to fatigue effects.
According to our results regarding the EMG measurements, higher obstacles induced minimal, non-significant increase in the EMG amplitude of the calf muscles during stance or swing phase. In a previous study, comparing the EMG of elderly and young people during obstacle crossing it was observed that increased obstacle height induces higher muscle activation (Hahn et al., 2005). Nonetheless, for the young population, this increase was marginal and probably not significant, which is in accordance with our findings. However, it cannot be excluded, that higher obstacle heights could have a significant effect on EMG amplitude of the examined muscles, but still control of gait speed should be required.
As far as fatigue concerns, due to the lack of literature regarding obstacle crossing at different obstacle heights, it is not possible to make direct comparisons with previous studies. Nevertheless, there are some indications from recent studies using a similar experimental setup. These studies showed that EMG during gait perturbations is not affected after fatigue (Granacher et al., 2010), but during sidecutting maneuver in handball players the antagonist co-activation of the hamstrings was reduced after fatigue (Zebis et al., 2011). The later behavior could also be expected during obstacle crossing as well. However, this was not the case during stance phase, probably because the selected task in the present study was less intense. Although minimum distance from the obstacle decreased for both limbs when increasing obstacle height from 10% to 20% of the limb length, which indicates that the task was more challenging, higher obstacle heights might give a clearer picture of the fatigue effects. This issue has to be considered in future studies investigating EMG during obstacle crossing in young healthy subjects.
Regarding the swing phase, medial gastrocnemius of the trailing limb showed increased activity after fatigue, when increasing the obstacle height. Considering that during this phase, medial gastrocnemius acts as antagonist, this is an indication of increased antagonist co-activation after fatigue. This behavior might have implications for the enhancement of the limb movement accuracy (Gribble et al., 2003), which is crucial for the trailing limb during obstacle crossing, especially in the absence of visual feedback.
A factor that could have an impact on our results is the condition of the participants examined in the present study, which were young, untrained, and non-sedentary people. Keeping in mind that skillful athletes are less prone to be affected by fatigue (Simoneau et al., 2006), it could be argued that subjects which are more susceptible to fatigue could have more evident signs of neuromuscular alterations during obstacle crossing after fatigue. Such subjects are not only elderly or sedentary healthy people, but also patients who suffer from chronic fatigue or patients that are more prone to fatigue such as multiple sclerosis, cerebrovascular disorders and muscular dystrophies (Angelini and Tasca, 2012; Zwarts et al., 2008). Additionally, regular exercise, as a component of physical fitness that might be beneficial especially for the above mentioned pathological populations, includes, at least in some extent, fatigue (Brogårdh and Lexell, 2012; Latimer-Cheung et al., 2013; Sveen et al., 2008; White et al., 2004). Therefore, future studies should examine the effect of fatigue on obstacle crossing in such populations. Besides, if the fatigue protocol would focus on a specific muscle or muscle group, the obstacle crossing EMG activation could be probably more affected. This approach followed in previous studies examining gait (Pohl et al., 2010) or running (Christina et al., 2001) should also be clarified in the future.
Conclusion
According to our findings, the function of the neuromuscular system was not substantially influenced during obstacle crossing after fatigue induced by exhaustive running. This implies that untrained, healthy, young subjects may compensate the deficit in muscular force due to fatigue and might not have higher risk of falls after exhaustion when stepping over an obstacle of height up to 20% of their lower limb length. However, there are indications of higher level of antagonist co-activation after fatigue for the medial gastrocnemius during swing phase. Therefore, these findings do not exclude possible differentiation in terms of EMG and kinematics for more intense and selective (targeted in a specific muscle or muscle group) fatigue protocols, higher obstacle heights or even for patients with deficits in neuromuscular adaptation during movement.
Biographies

Christos ANTONOPOULOS
Employment
Department of Physical Education and sport Science of the Aristotle University of Thessaloniki
Degree
BSc
Research interests
movement analysis, electromyography, fatigue, developmental ages
E-mail: chriant@phed.auth.gr

Dimitrios PATIKAS
Employment
Lecturer at the Department of Physical Education and Sport Science, at Serres.
Degree
PhD
Research interests
The biomechanical and neuromuscular function (fatigue, neuromuscular diseases, sport injuries, gender, age, training and footwear) and their underlying mechanisms.
E-mail: dpatikas@auth.gr

Nikolaos KOUTLIANOS
Employment
Assistant Professor of Athletes’ Physical Health Evaluation, Sports Medicine Laboratory, School of Physical Education & Sport Science, Aristotle University of Thessaloniki
Degree
PhD
Research interests
Athletes’ cardiovascular screening, clinical ergophysiology, doping
E-mail: koutlian@phed.auth.gr

Sofia D. PAPADOPOULOU
Employment
Assistant Professor at the Department of Physical Education and Sports Science, at the Aristotle University of Thessaloniki (A.U.TH.), Greece.
Degree
PhD
Research interests
Team sports, coaching science, sports medicine and performance, kinanthropometry, sports nutrition and sport injuries.
E-mail: sophpapa@phed.auth.gr

Dimitrios CHATZOPOULOS
Employment
Lecturer at Aristotle University of Thessaloniki, Department of Physical Education and Sport Science
Degree
PhD
Research interests
Stretching, warm-ups, fitness training.
E-mail: chatzop@phed.auth.gr

Konstantinos HATZIKOTOULAS
Employment
Postdoctoral fellow in Analytical Genomics of Complex Traits group of Wellcome Trust Sanger Institute
Degree
PhD
Research interests
Underlying mechanisms of neuromuscular fatigue in children and adults in parallel with all aspects of statistical genetics, and in particular in the genetic etiology of common disease.
E-mail: kh7@sanger.ac.uk

Eleni BASSA
Employment
Research fellow in the Department of Physical Education and Sport, Aristotle University of Thessaloniki, Greece.
Degree
PhD
Research interests
Motor development and neuromuscular control of children and adults (fatigue, strength, power, electromyography and electrostimulation) and their underlying mechanisms.
E-mail: lbassa@phed.auth.gr

Christos KOTZAMANIDIS
Employment
Assoc. Professor in the Department of Physical Education and Sport Science of Aristotle University Thessaloniki.
Degree
PhD
Research interests
Motor development and neuromuscular control of children and adults (fatigue, strength, power, electromyography and electrostimulation) and their underlying mechanisms.
E-mail: kotzaman@phed.auth.gr
References
- Angelini C., Tasca E. (2012) Fatigue in muscular dystrophies. Neuromuscular Disorders 22, S214-S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri F.A., Rocha dos Santos P.C., Vitorio R., van Dieen J.H., Gobbi L.T.B. (2013) Effect of muscle fatigue and physical activity level in motor control of the gait of young adults. Gait & Posture 38, 702-707. [DOI] [PubMed] [Google Scholar]
- Brogårdh C., Lexell J. (2012) Effects of cardiorespiratory fitness and muscle-resistance training after stroke. Physical Medicine and Rehabilitation 4(11), 901-907. [DOI] [PubMed] [Google Scholar]
- Chou L.S., Draganich L.F. (1997) Stepping over an obstacle increases the motions and moments of the joints of the trailing limb in young adults. Journal of Biomechanics 30(4), 331-337. [DOI] [PubMed] [Google Scholar]
- Christina K.A., White S.C., Gilchrist L.A. (2001) Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Human Movement Science 20, 257-276. [DOI] [PubMed] [Google Scholar]
- Forestier N., Teasdale N., Nougier V. (2002) Alteration of the position sense at the ankle induced by muscular fatigue in humans. Medicime & Sciense in Sports and Exercise 34(1), 117-122. [DOI] [PubMed] [Google Scholar]
- Galna B., Peters A., Murphy A.T., Morris M.E. (2009) Obstacle crossing deficits in older adults: A systematic review. Gait & Posture 30, 270-275. [DOI] [PubMed] [Google Scholar]
- Girard O., Millet G.P., Micallef J.P., Racinais S. (2012) Alteration in neuromuscular function after a 5 km running time trial. European Journal Applied Physiology 112(6), 2323-2330. [DOI] [PubMed] [Google Scholar]
- Granacher U., Gruber M., Forderer D., Strass D., Gollhofer A. (2010) Effects of ankle fatigue on functional reflex activity during gait perturbations in young and elderly men. Gait & Posture 32, 107-112. [DOI] [PubMed] [Google Scholar]
- Gribble P.L., Mullin L.I., Cothros N., Mattar A. (2003) Role of Cocontraction in Arm Movement Accuracy. Journal of Neurophysiology 89, 2396-2405. [DOI] [PubMed] [Google Scholar]
- Hahn M.E., Lee H.-J., Chou L.-S. (2005) Increased muscular challenge in older adults during obstructed gait. Gait & Posture 22, 356-361. [DOI] [PubMed] [Google Scholar]
- Häkkinen K. (1994) Neuromuscular fatigue in males and females during strenuous heavy resistance loading. Electromyography and Clinical Neurophysiology 34(4), 205-214. [PubMed] [Google Scholar]
- Hermens H.J., Freriks B., Merletti R., Stegeman D.F., Blok J.H., Raw G., Klug C.D., Hägg G.M. (1999) European recommendations for surface electromyography. Enshede, The Netherlands: Roessingh Research and Development; 1-122. [Google Scholar]
- Jackson A.S., Pollock M.L. (1985) Practical assessment of body composition. The Physician and Sportsmedicine 13, 76-90. [DOI] [PubMed] [Google Scholar]
- Jaric S., Blesic S., Milanovic S., Radovanovic S., Ljubisavljevic M., Anastasijevic R. (1999) Changes in movement final position associated with agonist and antagonist muscle fatigue. European Journal Applied Physiology 80, 467-471. [DOI] [PubMed] [Google Scholar]
- Kellis E. (1998) Quantification of quadriceps and hamstring antagonist activity. Sports Medicine 25(1), 37-62. [DOI] [PubMed] [Google Scholar]
- Knicker A.J., Renshaw I., Oldham A.R.H., Cairns S.P. (2011) Interactive Processes Link the Multiple Symptoms of Fatigue in Sport Competition. Sports Medicine 41(4), 307-328. [DOI] [PubMed] [Google Scholar]
- Latimer-Cheung A.E., Ginis K.A.M., Hicks A.L., Motl R., Pilutti R., Duggan M., Wheeler G., Persad R., Smith K. (2013) Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Archives of Physical Medicine and Rehabilitation 94, 1829-1836. [DOI] [PubMed] [Google Scholar]
- Law S.H.L., Webb Y.C. (2005) Gait adaptation of children with cerebral palsy compared with control children when stepping over an obstacle. Medicine & Child Neurology 47, 321-328. [DOI] [PubMed] [Google Scholar]
- Lindström L.H., Magnusson R.I. (1977) Interpretation of myoelectric power spectra: a model and its applications. Proceedings of the IEEE 65, 653-662. [Google Scholar]
- Missenard O., Mottet D., Perrey S. (2008) The role of cocontraction in the impairment of movement accuracy with fatigue. Experimental Brain Research 185, 151-156. [DOI] [PubMed] [Google Scholar]
- Nagai K., Yamada M., Mori S., Tanaka B., Uemura K., Aoyama T., Ichihashi N., Tsuboyama T. (2013) Effect of the muscle coactivation during quiet standing on dynamic postural control in older adults. Gerontology and Geriatrics 56, 129-133. [DOI] [PubMed] [Google Scholar]
- Niang E.S.A., McFadyen B.J. (2005) Effects of physical activity level on unobstructed and obstructed walking in young male adults. Gait & Posture 22, 75-81. [DOI] [PubMed] [Google Scholar]
- Overstall P.W., Exton-Smith A.N., Imms F.J., L. J.A. (1977) Falls in the elderly related to postural imbalance. British Medical Journal 1, 261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parijat P., Lockhart T.E. (2008) Effects of lower extremity muscle fatigue on the outcomes of slip-induced falls. Ergonomics 51, 1873-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla A.E., Rietdyk S., Martin C., Prentice S. (1996) Locomotor patterns of the leading and trailing limb while going over solid and fragile obstacles: Some insight into the role of vision during locomotion. Journal of Motor Behavior 28(1), 35-47. [DOI] [PubMed] [Google Scholar]
- Pohl M.B., Rabbito M., Ferber R. (2010) The role of tibialis posterior fatigue on foot kinematics during walking. Journal of Foot and Ankle Research 3(6), 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau M., Bégin F., Teasdale N. (2006) The effects of moderate fatigue on dynamic balance control and attentional demands. Journal of NeuroEngineering and Rehabilitation 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H.B., Wyatt M.P., Hodgdon J.A., Conard D.W., Barrack R.L. (1986) Effect of fatigue on joint positioning of the knee. Journal of Orthopaedic Research 4, 112-118. [DOI] [PubMed] [Google Scholar]
- Sparto P.J., Parnianpour M., Reinsel T.E., Simon S. (1997) The Effect of fatigue on multijoint kinematics, coordination, and postural stability during a repetitive lifting test. Journal of Orthopaedic & Sports Physical Therapy 25(1), 3-12. [DOI] [PubMed] [Google Scholar]
- Sveen M.L., Jeppesen T.D., Hauerslev S., Kober L., Krag T.O., Vissing J. (2008) Endurance training improves fitness and strength in patients with Becker muscular dystrophy. Brain 131, 2824-2831. [DOI] [PubMed] [Google Scholar]
- White L.J., McCoy S.C., Castellano V., Gutierrez G., Stevens J.E., Walter G.A., Vandenborne K. (2004) Resistance training improves strength and functional capacity in persons with multiple sclerosis. Multiple Sclerosis 10(6), 668-674. [DOI] [PubMed] [Google Scholar]
- Winter D.A., Fuglevand A.J., Archer S. (1994) Crosstalk in surface electromyography: theoretical and practical estimates. Journal of Electromyography and Kinesiology 4, 15-26. [DOI] [PubMed] [Google Scholar]
- Zebis M.K., Bencke J., Andersen L.L., Alkjær T., Suetta C., Mortensen P., Kjær M., Aagaard P. (2011) Acute fatigue impairs neuromuscular activity of anterior cruciate ligament-agonist muscles in female team handball players. Scandinavian Journal of Medicine & Science in Sports 21, 833-840. [DOI] [PubMed] [Google Scholar]
- Zwarts M.J., Bleijenberg G., van Engelen B.G.M. (2008) Clinical neurophysiology of fatigue. Clinical Neurophysiology 119, 2-10. [DOI] [PubMed] [Google Scholar]


