Abstract
This study elucidated the role of CaN-NFAT signaling and neurotrophins on the transformation of myosin heavy chain isoforms in the rat soleus muscle fiber following aerobic exercise training. To do so, we examined the content and distribution of myosin heavy chain (MyHC) isoforms in the rat soleus muscle fiber, the activity of CaN and expression of NFATc1 in these fibers, and changes in the expression of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neutrophin-3 (NT-3) in the soleus and striatum following high-and medium-intensity aerobic treadmill training. Specific pathogen-free 2 month old male Sprague-Dawley (SD) rats were randomly divided into three groups: Control group (Con, n = 8), moderate-intensity aerobic exercise group (M-Ex, n = 8) and high-intensity aerobic exercise group (H-Ex, n = 8). We used ATPase staining to identify the muscle fiber type I and II, SDS-PAGE to separate and analyze the isoforms MyHCI, MyHCIIA, MyHCIIB and MyHCIIx, and performed western blots to determine the expression of NFATc1, NGF, BDNF and NT-3. CaN activity was measured using a colorimetric assay. In the soleus muscle, 8 weeks of moderate-intensity exercise can induce transformation of MyHC IIA and MyHC IIB to MyHC IIX and MyHC I (p < 0.01), while high-intensity treadmill exercise can induce transform MyHC IIx to MyHC IIB, MyHC IIA and MyHC I (p < 0.01). In comparison to the control group, CaN activity and NFATcl protein level were significantly increased in both the M-Ex and H-Ex groups (p < 0.05, p < 0.01), with a more pronounced upregulation in the M-Ex group (p < 0.05). Eight weeks of moderate- and high-intensity aerobic exercise induced the expression of NGF, BDNF and NT-3 in the soleus muscle and the striatum (p < 0.01), with the most significant increase in the H-Ex group (p < 0.01). In the rat soleus muscle, (1) CaN–NFATcl signaling contributes to the conversion of MyHC I isoform in response to moderate-intensity exercise; (2) Neurotrophins NGF, BDNF and NT-3 might play a role in the conversion of MyHC II isoform in response to high-intensity treadmill exercise.
Key points.
Eight weeks of moderate-intensity treadmill training induces the transformation MyHC IIA and MyHC IIB to MyHC IIX and MyHC I in the soleus muscles, while high-intensity exercise leads to transformation of MyHC IIX to MyHC IIA, MyHC IIB and MyHC I.
MyHC I conversion in response to moderate-intensity aerobic exercise is mediated by calcineurin-NFATcl signaling.
Eight weeks of moderate- and high-ntensity aerobic exercise induces the expression of NGF, BDNF and NT-3 in expression noted in rats subjected to high-intensity training. NGF and NT-3 expression in the striatum is lower than in the soleus muscle, while BDNF levels are similar. Neurotrophins may be involved in mediating MyHC II conversion in response to high-intensity aerobic exercise.
Key words: Aerobic treadmill training, the muscle fiber type, neurotrophins, Myosin heavy chain isoforms transformation, Calcineurin/Nuclear factor of activated T cells c1
Introduction
Contraction of the skeletal muscle requires the function of myosin heavy chain (MyHC). Isoforms of MyHC perform a variety of pleiotropic functions, depending on the isozyme type, the content, composition and distribution of skeletal muscle fiber, and the expression of neural, hormonal and mechanical factors. Skeletal muscles can develop into two types of fibers: fast and slow, that differ in the pattern of expression of MyHC isoforms. Several hypotheses have been proposed to explain the mechanism of action of skeletal muscle fibers. A variety of different signaling pathways and molecular mechanisms, including calcineurin (CaN)/nuclear factor of activated t-cells (NFAT) signaling, Ca2+/calmodulin-dependent kinase (CaMK) signaling, histone deacetylase(HDAC)/ myocyte enhancer factor 2 (MEF2) signaling, myogenic regulatory factor (MRFS) pathway, Ras/MAPK signaling, Myostatin and Wnt signaling, and PGC-lα/β, AMPK and PPARδ signaling have been shown to regulate gene expression and the choice of MyHC isoforms in the skeletal muscle (Carlsen et al., 2000). CaN-NFAT signaling is also involved in T cell differentiation and maturation, production of cytokines, vascular smooth muscle cell proliferation, synaptic transmission and myocardial hypertrophy. More importantly, some studies have demonstrated its role in the transformation of skeletal muscle fibers (Dupont-Versteegden et al., 2002; Serfling et al., 2006). Calcineurin is a cyclosporine- sensitive, calcium-regulated serine/threonine phosphatase, shown to be essential for skeletal muscle remodeling, where it facilitates the transduction of extracellular signals to the nucleus by targeting members of the NFAT family of transcription factors (Bassel-Duby et al., 2006). McCullagh et al. (2004) showed that expression of a constitutively active form of NFAT (NFATc1) stimulates expression of the MyHC-slow isoforms in regenerating muscles, and inhibits the fast MyHC IIB promoter in fast muscles of the adult. These results support the hypothesis that CaN-NFAT signaling acts as a sensor of nerve activity in skeletal muscles in vivo and consequently controls nerve activity- dependent switch in expression of MyHC isoforms. NFATcl, NFATc2 and NFATc3 have been detected in both the cytoplasm and nucleus of skeletal muscle cells. Despite the above findings on the role of NFAT signaling in the specification of skeletal muscle fiber type, the issue remains controversial (Schiaffino et al., 2002). In one study, Bigard et al. (2000) showed that CaN-mediated synergistic activation of NFAT (by phosphorylation) leads to the expression of slow muscle fiber-associated proteins in cooperation with the myocyte enhancer factor, and contributes to the specification of skeletal muscle fiber type. However, the involvement of CaN-NFAT signaling in exercise training-induced skeletal myofiber transformation is unclear.
Although NFAT is a key transcriptional regulator of neuronal development and function, it is poorly characterized as a possible downstream target of nerve growth factor (NGF) in neurons. Groth et al. (2007) showed that NGF can induce NFAT-dependent transcription of brain-derived neurotrophic factor (BDNF) and COX-2 in dorsal root ganglion cells. Nguyen et al. (2009) reported that NGF can repress the expression of GAP-43 (growth associated protein 43) in PC12 cells and in cultured cortical neurons through signaling mediated by NFAT3. Finally, Stefos et al. (2013) provided evidence supporting the hypothesis that CaN-NFAT signaling mediates gene regulatory effects of NGF in neurons.
An increasing quantity of experimental data supports the hypothesis that mature adult mammalian and human skeletal muscles maintain a high degree of plasticity, even after development is complete. The expression of skeletal muscle fiber type-specific proteins and changes in the composition of muscle fiber depend on developmental factors, neuromuscular activity, muscle load, hormonal levels and aging (Pette and Staron, 1997). Previous studies have reported that exercise can affect the composition and distribution of muscle fiber types. For example, increased neuromuscular activity results in a shift in MyHC isoform from fast to slow muscle fiber (Pette and Staron, 1997), while inactivity leads to a general shift in MyHC expression and associated metabolic properties along the following line of progression- from type I→IIA→IIX→IIB (Talmadge., 2000). It is well documented that exercise induces several physiological and biochemical changes in the brain. Different mechanisms, including CaN and Ras-ERK signaling, have been implicated in fiber type specification induced by nerve activity (Murgia et al., 2000; Sharma et al., 1991). Wang et al. (2002a; 2002b) found that regionalization during reinnervation of muscle fiber type is indirect evidence of the important role played by muscle fiber composition during normal innervation. In cases of reinnervation of the lower limb muscles in rats by the sciatic nerve, and reinnervation of the gastrocnemius muscle in rats by its own nerve, quantitative analysis of the distribution of muscle fiber type indicated that the normal muscle fiber type has a "mosaic" distribution, with a significantly similar fiber aggregation style, called fiber type grouping. The detection of similar fiber aggregation suggests that at least part of the shift in muscle fiber type would allow innervation by different types of motor neurons (Wang et al., 2002a; 2002b).
Neurons influence muscle fiber type-specific protein expression because changes in nerve activity can induce muscle fiber to release neurotrophic factors such as neuregulin even two species passing through the joint action. Mousavi et al. (2004) investigated the role of ciliary neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF) in the survival and maturation of a subset of motor neurons innervating the extensor digitorum longus (EDL) and tibialis anterior (TA) muscles. Their findings demonstrated the importance of muscle-derived BDNF in the survival and maturation of a subpopulation of motor neurons, and the significance of MyHC IIB muscle fibers during neonatal development of the neuromuscular junction. Carrasco et al. (2003) found that during normal postnatal development in rats, the expression of neurotrophin (NT)-4/5 in the slow-twitch soleus muscles is indispensable to the fast to slow conversion of MyHC isoforms. Simon et al. (2003) found that the expression of NT-4 in neurons following denervation or reinnervation can selectively promote the recovery of the slow motor units. In vitro, Rende et al. (2000) showed that another neurotrophic factor NGF regulates myoblast proliferation and differentiation by signaling through its specific receptor Tyrosine Kinase A (TrK A). Despite the above findings, the expression and distribution of different types of neurotrophic factors, and their respective roles in determining the type of muscle fiber needs to be explored further. NGF, BDNF and NT-3 are three important members of the family of neurotrophic factors, mainly expressed in the brain and peripheral tissues, and known to affect neuronal survival and differentiation. Interestingly, neurotrophic factors are expressed not only by neurons, but also by muscle cells. However, it is not known whether their expression is specific to the type of muscle fiber, or if specific stimulation conditions such as long-term exercise training can change their expression in the muscle.
In adult vertebrates, subtypes of skeletal myofibers differ markedly in their contractile physiology, metabolic capabilities, ultrastructural morphology, and susceptibility to fatigue. In vivo experiments need to be conducted in order to establish the contribution of the signaling pathways and molecular mechanisms discussed above to pathophysiological alterations in MyHC isoforms. In this study, we investigated the activity of CaN-NFAT signaling, and changes in the expression and content of NGF, BDNF and NT-3 in response to aerobic treadmill training. Our results suggest that NGF, BDNF and NT-3 regulate gene expression in myocytes of the soleus muscle and neurons of the striatum by regulating the activity of the calcineurin-NFAT signaling pathway.
Methods
Ethics statement
All animal procedures were approved by the local ethics committee (the Institutional Review Board of Hunan Normal University) and the Guidelines for Care and Use of Laboratory Animals (2011). Disposal of animals was done in accordance with “The guidance on the care of laboratory animals” (The provisions were issued in 2006 by the Ministry of Science and Technology of the People's Republic of China).
Experimental animals and treatments
Experimental animals
Specific pathogen-free 2-month-old male Sprague-Dawley (SD) rats (n=24, 220 ± 10 g) were supplied by the Animal Center of East Biotechnology Services Company (Changsha, Hunan, China; License number: Xiang scxk 2009 - 003). Four rats were housed in one standard cage with free access to food and water. All animals were kept in an air-conditioned room maintained at a constant temperature of 20°C to 25°C with a relative humidity of 45%–55%. Rats were subjected to a cycle of 12 h light and 12 h darkness. All animals were acclimated to laboratory conditions for 1 week, prior to the start of the experiment. A total of 24 rats were randomly assigned to three groups—sedentary control group (Con, n = 8), moderate-intensity aerobic exercise runner group (M-Ex, n = 8), and high-intensity aerobic exercise runner group (H-Ex, n = 8).
Exercise protocol
The aerobic exercise regime used in this study was proposed by Shuzhe et al. (2008), with reference to the exercise load standards of Bedford et al. (1979).All animals in the M-Ex and H-Ex groups were first subjected to a 5-day adaptation period on a rat treadmill (slope gradient 0%, ZH-PT-1 Treadmill, Li Tai Bio-Equipment Co., Ltd, Hangzhou, Zhejiang, China). Adapted training was carried out at a speed of 10 m/min and a gradient of 0°, for a gradually increasing duration of time—10 min on the first day, 20 min on the second day, 25 min on the third day. During this period they were placed on a belt facing away from the electrified grid (0.6 mA intensity) twice a day. For the actual experiment, the M-Ex group underwent daily training by running at 19.3 m/min (equivalent to approx. 76% peak oxygen uptake) (Bedford et al., 1979), on a slope of 5°, for a duration of 60 min. During the first 3 days at the start of the training, the exercise duration was increased gradually from 10 min to 25 min to 60 min. The H-Ex group underwent daily training by running at 26.8 m/min (equivalent to approx. 92.3% peak oxygen uptake) (Bedford et al., 1979), on a slope of 10°, for a duration of 40 min. The acceleration of the treadmill was set such that at about 3 min after the start of the training, the final speed of 26.8 m/min was achieved. To ensure the animals completed the exercise regime, we used sound stimulation and a small wooden stick to stimulate the animals’ tails, when necessary. We also used electrical stimulation to keep the rats at one-third distance on the treadmill runway. Animals were required to perform the training 5 days a week for a total of 8 weeks.
Tissue specimen collection and analysis
On the day following the last exercise schedule, the rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and decapitated. The striatum (Stereotaxic Atlas of the Rat Brain; George and Charles, 2005) and the right limb soleus muscle fibers were excised from eight rats. Tissue samples were stored at –808C until ready to be used for immunoblot assays. All surgical procedures were performed under anesthesia induced by chloral hydrate. All efforts were made to minimize suffering and distress in animals. The slow soleus muscle in adult rats is composed of approximately 80% slow-twitch (type I) fibers and 20% fast-twitch (type IIA) and a small number of undifferentiated (type IIC) fibers (Pullen et al., 1977; Tasic et al., 2003).
ATPase staining
Fibers in cross sections of rat soleus muscle were typed by using histochemical ATPase stains, and the results were compared with those of quantitative enzyme assays of fragments of the same fibers dissected from serial freeze-dried sections. The muscle samples were perpendicularly fixed in the metallic supports of the microtome where several series of 8 μm thick cross-sections were obtained and fixed in previously identified slides. In order to measure the area density and the number density of muscle fibers, five slices were sliced randomly in each group and five areas were sampled in each cut, resulting in approximately 400 fibers per animal under light microscope (×200) (Olympus BX51/BX51M/BX61, Japan).Two enzymes previously used to assess the metabolic type were measured in each case: lactate dehydrogenase and malate dehydrogenase. With rat soleus muscle there was essentially complete agreement between ATPase staining and the metabolic enzyme assays in distinguishing types I and II fibers. Calculator's image analysis system is the Simple PCI software version 6.0 (Compix Inc, Sewickly, PA).
SDS-PAGE
A simple, effective and very high resolution method to fractionate and analyze protein mixtures is the sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE). To determine the type of MyHC isoforms expressed in the soleus muscles, and the change in expression following exercise, we performed SDS-PAGE and specifically examined the levels and expression patterns of MyHC- IIA, IIB, IIX, and MyHC-I. MyHC isoform separation was carried out with some adaptations of the methodology by Francisco et al. (2011) and Mizunoya et al. (2008). The stained gel was then scanned and analyzed on a Tanon gel image shooting system scan (Shanghai Energy Technology Co., Ltd., China) in order to determine the gradation values of MyHC I, MyHC IIA, MyHC IIB and MyHC IIX. Finally, the percentage of MyHC I, MyHC IIA, MyHC IIB and MyHC IIX protein level was calculated using the Tanon gel image processing system (Shanghai Energy Technology Co., Ltd.,China).
Calcineurin phosphatase assay
In this study, calcineurin activity in skeletal muscle fiber was estimated using a colorimetric assay. Our experimental procedure was strictly in accordance with the instructions on the assay kit (Genmed Scientifics Inc., USA).
Western blot analysis of NFATc1, NGF, BDNF and NT-3
We used the BCA Protein Assay Kit (Wellbio, American Diagnostica Inc.) to determine total protein concentration, and performed the assay according to the manufacturer’s instructions. All antibodies used in this study were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Total cellular protein extracts were separated on 10% SDS-polyacrylamide gel and transferred to PVDF membranes. A T-Pro pre-stained protein ladder was used as a molecular marker to estimate the size of the proteins. The membranes were incubated with the primary antibodies, anti-NFATc1, anti-NGF, anti-BDNF and anti-NT-3, followed by an HRP-conjugated secondary antibody (Proteintech, 1:3000). The separated proteins were detected by developing the membranes with a ChemiLucent ECL Detection System (Millipore, Billerica, MA, USA). The exposed X-ray films were scanned using the Tanon Gel Image Shooting System, and data analysis was performed on the Tanon Gel Image Processing System.
Statistical analysis
Data from three independent experiments are presented as mean ± SEM. Statistical analysis was performed using predictive analytics software statistics 16.0 (SPSS Inc., Chicago, IL, USA). Comparisons across the experimental groups were performed using one-way analysis of variance (ANOVA). Statistical significance of the effects of the experimental treatment was determined by comparing the areas under the curve (p < 0.05; Student’s t-test).
Results
The dynamic changes of the body weight of rats
There was no significant difference between the groups of rats through adaptive stratified according to the body weight of rats. With the extension of the feeding, the body weight of rats in each group were increased greatly, the body weight of rats in H-Ex was significantly lighter than the control group (p < 0.05, Figure 1). But the body weight of rats in M-Ex was slightly lighter than the control group, and had no significant difference (p > 0.05, Figure 1).
Figure 1.
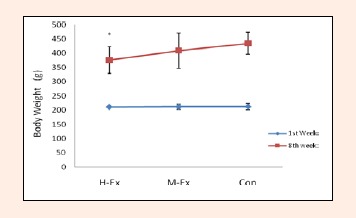
The dynamic changes of the body weight (g) of rats in each group. H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control group. * p < 0.05 vs. Con.
Treadmill training leads to increased the muscle fiber type I in the rat soleus by ATPase staining
We examined the muscle fiber type in the rat soleus by using ATPase staining. As shown in Figures 2, 3 and 4, after 8 weeks of treadmill training, the area density of the muscle fiber type I in the soleus muscle in the M-Ex and H-Ex groups was slightly higher (p >0.05, Figure 2 and 3) than the control group. But the number density of the muscle fiber type I in the M-Ex and H-Ex groups was significantly higher (p < 0.05, Figure 4) than the control group. Then the area density and the number density of the muscle fiber type II showed only a slight change in expression.
Figure 2.
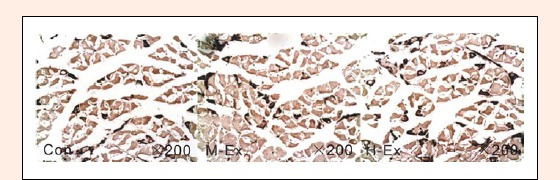
ATPase staining to idenfity the muscle fiber type I and II in the rat soleus. H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control.
Figure 3.
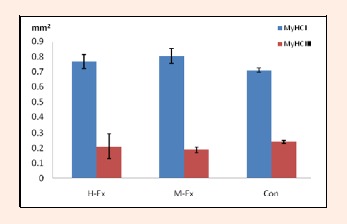
The area density of the muscle fiber type I and II in the rat soleus(mm2). H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control
Figure 4.
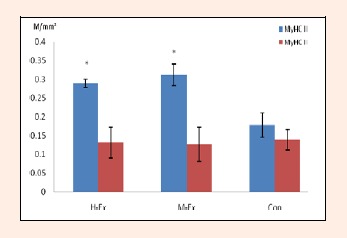
The number density of the muscle fiber type I and II in the rat soleus(M/mm2). H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control group. * p < 0.05 vs. Con.
Multiple myosin heavy chain isoforms are expressed in the rat soleus muscle
We first examined changes in the expression of the myosin heavy chain isoforms MyHC I, MyHC IIA, MyHC IIB and MyHC IIX, following 8 weeks of moderate or high intensity treadmill exercise, using SDS-PAGE. We found that in comparison to the control group, the percentage of MyHC I in the soleus muscle of the M-Ex group was significantly higher (p < 0.01, Table 1), while the percentage of MyHC IIA and MyHC IIB was significantly decreased (p < 0.01, Table 1), and the percentage of MyHC IIX was only slightly increased. In the H-Ex group, the percentage of MyHC I, MyHC IIA and MyHC IIB was increased significantly (p < 0.01, Table 1), while the percentage of MyHC IIX was decreased (p < 0.01, Table 1), in comparsion to the control group. Between the two exercise groups, the percentage of MyHCIIA and MyHCIIB in the soleus muscle was significantly higher in the H-Ex than the M-Ex group (p < 0.01, Table 1). In contrast, the percentage of MyHC IIX was significantly reduced (p < 0.01, Table 1), while MyHC I showed only a slight decrease in expression.
Table 1.
Changes in the percentage of MyHC isoforms in the soleus muscle. Data are means (±SD).
| Groups | MyHC I% | MyHC IIA% | MyHC IIX% | MyHC IIB% |
|---|---|---|---|---|
| Con | 62.29 (.47) | 14.2 (.11) | 13.5 (.17) | 10.01±0.48 |
| M-Ex | 69.23 (.55) ** | 8.43 (.55) ** | 14.46 (.34) | 7.88±0.27 ** |
| H-Ex | 68.54 (1.20) ** | 14.45 (1.10) ## | 6.33 (.40) **## | 10.68 (1.00)# |
MyHC: Myosin Heavy Chain; H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control group. Lane 2: soleus, H-Ex; lane 6: soleus, M-Ex; lane 8: soleus, Con.
** p < 0.01 vs. Con
# p < 0.05
## p < 0.01 vs. M-Ex.
Treadmill training leads to increased calcineurin-NFAT signaling in the rat soleus
We examined the level of activity of CaN signaling in the rat soleus using a colorimetric assay. As shown in Figure 5, after 8 weeks of treadmill training, CaN activity in the soleus muscle in the M-Ex and H-Ex groups was significantly higher (p < 0.05, Figure 5) than the control group. Interestingly, CaN activity in the M-Ex group was more pronounced than the H-Ex group. Next, we measured the expression of NFAT, known to be activated downstream of CaN signaling, by western blotting. Compared with the control group, NFATc1 protein level in the soleus muscle of both M-Ex and H-Ex groups was significantly upregulated (p < 0.01, Figure 6). Consistent with the CaN activity reported above, NFATc1 protein level in the M-Ex group was significantly higher than the H-Ex group (p < 0.05, Figure 6).
Figure 5.
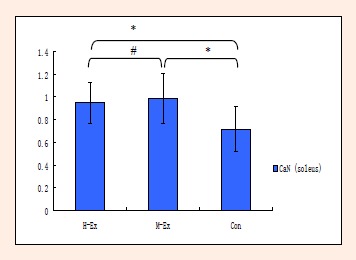
Colorimetric estimation of CaN activity in the rat soleus muscle (nmol·mg–1·min–1). CaN: Calcineurin; H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control group. ** p < 0.01 vs. Con; # p < 0.05, ## p < 0.01 vs. M-Ex.
Figure 6.
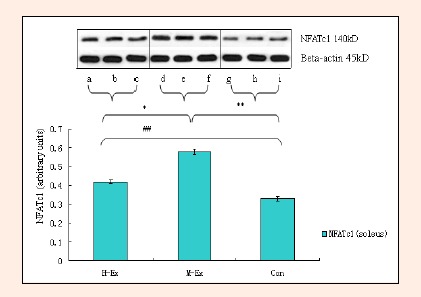
Western blot-based estimation of the quantity of NFATc1 in the soleus (means±SD, n = 3). NFATc1: Nuclear factor of activated T cells c1; H-Ex: High-intensity aerobic exercise group; M-Ex: Moderate-intensity aerobic exercise group; Con: Control group. * p < 0.05, ** p< 0.01 vs. Con; # p < 0.05, ## p < 0.01 vs. M-Ex.
Treadmill training leads to increased expression of NGF, BDNF and NT-3 in the rat soleus and striatum
Finally, we analyzed changes in the expression of neurotrophic factors NGF, BDNF and NT-3 in response to moderate and high intensity training, in both the soleus muscle as well as the striatum. The striatum is involved in mediating and regulating motor activity. As shown in Figures 7–9, western blot analysis revealed a significant upregulation in the expression of all three factors in the soleus and striatum in both M-Ex and H-Ex groups, in comparison to control rats (p < 0.01). The level of expression of NGF, BDNF and NT-3 relative to the internal control Beta-actin (ratios NGF/ Beta-actin, BDNF/Beta-actin and NT-3/Beta-actin) was also significantly enhanced (p < 0.01). Amongst the two exercise groups, the content of NGF, BDNF and NT-3 in the H-Ex group was significantly higher than the M-Ex group (p < 0.01). Similarly, the relative expression of all three factors (NGF/Beta-actin, BDNF/Beta-actin and NT-3/Beta-actin ratios) was also significantly higher in the H-Ex group (p < 0.01). Expression of NGF and NT-3 in the striatum was lower than in the soleus muscle. However, the expression of BDNF was similar in the soleus and the striatum and did not differ between the groups.
Figure 7.
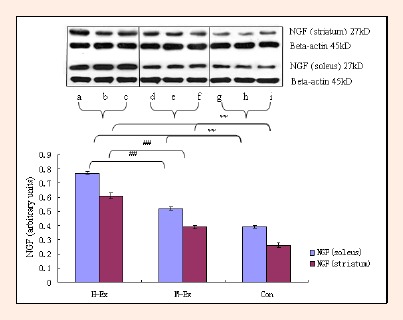
Western blot analysis of NGF in the soleus and striatum (means±SD, n = 3). NGF: Nerve growth factor; H-Ex: High-intensity aerobic exercise group, a, b and c; M-Ex: Moderate-intensity aerobic exercise group, d, e and f; Con: Control group, g, h and i. ** p < 0.01 vs. Con; ## p < 0.01 vs. M-Ex.
Figure 8.
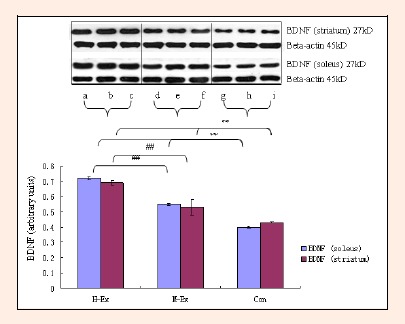
Western blot analysis of BDNF in the soleus and striatum (means±SD, n = 3). BDNF: brain-derived neurotrophic factor, H-Ex: High-intensity aerobic exercise group, a, b and c; M-Ex: Moderate-intensity aerobic exercise group, d, e and f; Con: Control group, g, h and i. ** p < 0.01 vs. Con; ## p < 0.01 vs. M-Ex.
Figure 9.
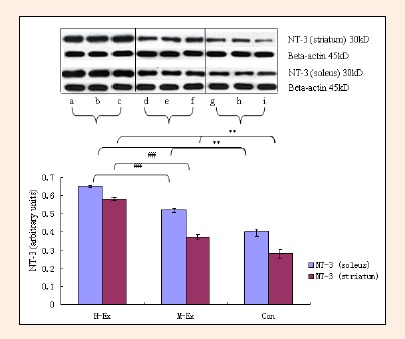
Western blot analysis of NT-3 in the soleus and striatum (means±SD, n = 3). NT-3: neutrophin-3; H-Ex: High-intensity aerobic exercise group, a, b and c; M-Ex: Moderate-intensity aerobic exercise group, d, e and f; Con: Control group, g, h and i. ** p < 0.01 vs. Con; ## p < 0.01 vs. M-Ex.
Discussion
Aerobic exercise influences the expression of MyHC isoforms in the skeletal soleus muscle
Skeletal muscles play an important role in mediating physical performance and adaptation to exercise (Pette et al., 1998). They can be functionally distinguished into two types of myofibers -slow (type I) and -fast (type II), that differ in the pattern of expression of the myosin heavy chain isoforms. Studies have shown that exercise can induce the transformation of skeletal muscle fibers from fast twitch to the slow type, accompanied by the interconversion of MyHC isoforms. In general, physical activity leads to transformation of MyHC IIB to MyHC IIX, followed by MyHC IIA, and finally MyHC I. Moreover, the effects of endurance training on MyHC expression may depend both on the amount and the type of training. Bigard et al. (2000) demonstrated that endurance training has a greater impact on the regeneration of the plantaris muscle with alterations in the pattern of expression of the fast fiber-type MyHC, but hardly affected the expression of MyHC I isoform. On the other hand, Yan-hong Su et al. (2007; 2008) reported that while 4 weeks of endurance training had no effect on the expression of MyHC proteins in the anterior tibialis muscle in rats, expression of MyHC IIA is reduced, while that of MyHC IIC and MyHC IIB is significantly improved. The quantity of MyHC IIA and MyHC IIB transcripts was found to be significantly increased under hypoxic training conditions. In addition, Bruton (2002) showed that endurance training can induce the conversion of MyHC IIX to MyHC IIA in skeletal muscle fibers, accompanied by an increase in the expression of MyHC I. Furthermore, Jinzhu and Ruiyuan (2005) demonstrated that exercise training can improve the level of expression level of p-actin in muscle fibers. They also found that the expression of slow myofiber-associated MyHC IIX and MyHC IIA isoforms in the quadriceps of rats increased after 2 weeks of endurance training, while the expression of the fast myofiber-associated MyHC IIB and slow myofiber-associated MyHC I isoforms remained unaltered. Further investigation by Walters et al. (2004) involving co-staining of endothelial cells and MyHCs isoforms indicated that there is a transient increase in capillary contact in MyHC IIB and MyHC IID/X fibers during the time (day 7) when a significant increase in total capillary density is observed. These findings suggest that endurance training induces angiogenesis in a subpopulation of MyHC IIA and MyHC IID/X myofibers before switching to MyHC IIA fibers. Cornachione et al. (2011) compared the response of the soleus muscles to endurance training versus eccentric exercise, and found a significant decrease in MyHC IIA and increase in MyHC IIB content following endurance training.
Aerobic exercise activates calcineurin-NFAT signaling in the skeletal soleus muscle
It is known that endurance training, especially moderate-intensity exercise, can induce the transformation of soleus muscle fibers from fast to slow type. Also, the signaling pathway mediated by calcineurin, a Ca2+/calmodulin-activated phosphatase, is involved in nerve activity-dependent fiber type specification of the skeletal muscle, specifically the expression of slow muscle fiber-specific proteins. In this study, we have shown that in comparison to the control group, the content of calcineurin phosphatase and the expression of NFATcl are significantly increased in the rat soleus muscle fibers in response to moderate-intensity exercise. These findings suggest that the increase in CaN activity induced by moderate physical exertion leads to upregulation, and possibly activation, of NFATc1.
Our results are consistent with the findings made by Bagen et al. (2008). In this study, rats subjected to no-slope endurance training showed an increase in the ratio of slow muscle fibers in the soleus. When treated with cyclosporin A (CSA), an inhibitor of calcineurin this effect was inhibited, and the cross-sectional area of the type I fiber was significantly reduced. This and other studies have suggested that CaN signaling might be involved in the regulation of skeletal muscle fiber type and size, and that this function might be muscle-specific in response to endurance exercise.
Researchers have studied the regulation of gene expression during muscle contraction and neuromuscular activity, and although the role of excitation–contraction coupling in this context has been elucidated, its involvement in specification and transformation of skeletal muscle fiber type remains unclear. Also, the contribution of the speed of neuronal activity to myofiber type conversion and the myogenic signaling mechanisms underlying this conversion process have not been clarified (Costelli et al., 2007). CaN, a calcium/calmodulin-dependent phosphatase, is an important regulator of muscle growth and development. It is involved in regulating maturation of myocytes and differentiation of muscle fibers in adults. However, the role of NFAT isoforms in muscle development and physiology has been rarely studied. Some researchers showed that nuclear translocation of different combinations of activated NFATs might lead to muscle fiber type-specific gene expression and subsequently conversion. There is experimental evidence that specific combinations of NFAT family members are involved in the interconversion of fast and slow MyHC isoforms. Specifically, while NFATcl is involved in the induction of MyHC IIB expression, four different NFATs have been shown to influence MyHC I expression. Chin et al. (1998) reported that fiber type-specific gene expression in skeletal muscles is regulated by signaling mechanisms mediated by calcineurin. CaN activation in skeletal myocytes selectively upregulates the activity of slow fiber-specific gene promoters, thus facilitating the conversion of fast to slow muscle fibers. Conversely, inhibition of calcineurin activity by administration of cyclosporin A to healthy animals promotes slow to fast fiber transformation. Activation of slow fiber-specific transcription appears to be mediated by a combinatorial mechanism involving members of the NFAT and MEF2 family of transcriptional regulators. Altogether, these findings have identified a molecular mechanism that could be responsible for translating different patterns of motor nerve activity into selective changes in gene expression to establish the specialized characteristics of slow and fast myofibers. The proposed hypothesis states that motor neurons are electrochemically linked to, and hence act as initiators of muscle fiber action potentials, causing a persistent increase in Ca2+ concentration in the muscle. These Ca2+ ions in turn bind to the calmodulin–calcineurin enzyme complex, and modulate the activity of NFAT proteins. Phosphorylation and subsequent translocation of NFATs from the cytosol to the nucleus is tightly regulated by calcineurin. Nuclear NFATs bind to corresponding nucleotide recognition sequences and induce transcription of the target genes (Rao et al., 1997). Several studies have elucidated the diversity of the NFAT family of transcription factors, and their functional association with other transcription factors to regulate the expression of inducible genes.
Our results show that NFATs play an important role in skeletal muscle physiology, by mediating a selective response to slow muscle-specific motor neuron activity. Just as receptors are essential in transducing the effects of neural activity, NFATs are required for mediating subsequent specification of myofiber type. Furthermore, changes in the level of activated NFATc1 further corroborate the significance of NFATs in differentiation of myofibers. Altogether, our findings support the physiological role of CaN-NFAT signaling in promoting the growth and repair of slow muscles and inhibiting gene expression associated with the fast-twitch MyHC IIX-IIB muscles. Moreover, it is known that the intracellular calcium level in slow muscle fibers is higher than fast- twitch muscles, and that long-term low-frequency stimulation can elevate the calcium levels in fast-twitch fibers. In vitro experiments demonstrated that fast to slow myosin conversion of skeletal muscle cells can be induced by a ten-fold increase in intracellular calcium concentration (Kubis et al., 1997). This finding further supports our own results on the activity of CaN in skeletal muscles.
Aerobic exercise induces upregulation of neurotrophins in the skeletal soleus muscle
NGF, BDNF, NT-3 and NT-4/5 are small molecule peptides/proteins that belong to a family of neurotrophic factors, and perform essential nutritional functions in the central and peripheral nervous system. In recent years, BDNF was shown to interact with the cellular cytoskeleton and affect its rapid reorganization, while establishing protrusions. Lanier et al. (2000) reported that the effect of BDNF on cytoskeletal architecture is likely mediated via Rho G-protein-coupled receptors and TrkB receptors, known to be activated by BDNF. TrkB-mediated activation of the Src family kinases, the subsequent interaction of Src with Abl tyrosine kinases, and regulation of TrkB activity by receptor endocytosis, could possibly explain the relationship between BDNF and cytoskeletal reorganization. Smart et al. and Gallo et al. provided additional evidence of the involvement of BDNF in regulating microtubule organization when they reported that changes in BDNF levels can regulate the content of F-actin in the active growth cone near the synapse, and influence their expansion (Gallo et al., 2004; Smart et al., 2003).
Funakoshi et al. first reported the expression of NT-4 in slow-twitch myofibers (Funakoshi et al., 1995). Recent studies, however, have shown that NT-4 expression in fast-twitch fibers is significantly higher than in slow muscles (Sakuma et al., 2001). In addition, Carrasco et al. (2003) showed that postnatal rat soleus muscles undergo a shift from fast-twitch to slow-twitch fibers, a process that can be accelerated upon intramuscular injection of NT-4/5. In addition, some researchers identified multipotent muscle-derived stem cells (MDSCs) and muscle satellite cells in the skeletal muscle that can undergo mesodermal differentiation and potentially contribute to muscle regeneration. These cells can differentiate into several mesenchymal lineages, including adipocytes, chondrocytes and osteoblasts, as well as endothelial cells and even neurons (Lee et al., 2000; Qu-Petersen et al., 2002).
In this study, we show that following 8 weeks of moderate- or high-intensity aerobic exercise, the absolute and relative expression of NGF, BDNF and NT-3 in the soleus muscle fibers is significantly enhanced, suggesting that aerobic exercise can trigger the expression of various skeletal muscle-associated neurotrophic factors. Others have performed similar studies and made similar findings. For example, Gomez-Pinilla et al. (2001) investigated the effect of movement on spinal cord neurons and skeletal muscles in rats. They found that while 1 day of exercise on the treadmill did not affect BDNF expression either in the spinal cord or the soleus muscle, after 5 days of training, at 2 h after the end of the exercise, there was a significant increase in BDNF mRNA levels. Next, the authors (Gómez-Pinilla et al., 2001; 2002) showed that following 3–7 days of voluntary running wheel exercise in rodents, the expression of BDNF and its receptor expression was significantly increased in convalescent lumbar spinal nerve and the soleus muscle. Interestingly, they also noted an increase in neurotransmitter function, the mRNA and protein levels of synaptophysin I, known to mediate synaptic plasticity, and expression of CREB (cAMP response element binding protein) mRNA and growth protein-43 in the joints.
In this study, we also show that the expression level of NGF, BDNF and NT-3 is significantly higher following 8 weeks of high-intensity aerobic training than moderate-intensity exercise. This is an interesting observation because the effect of intensity of physical training on nutrition of different muscle fibers is not clear. Our results are in contrast with other reports where BDNF expression during the development of the neuromuscular system in newborn animals was shown to play an important role in the survival and maturation of motor neurons and fast-twitch MyHC IIB muscle fibers (Mousavi et al., 2004). Furthermore, Nagano et al. (2003) showed that in the neonatal gastrocnemius muscle, which contains a higher proportion of MyHC IIB fibers than the soleus muscle, BDNF levels are higher. Altogether, these results demonstrate that there is a preferential expression of BDNF in those developing myofibers that mature into MyHC IIB fibers in adults. The pattern of expression of the different neurotrophic factors and their respective roles in determining the specification of muscle fiber type needs to be explored further.
During neural development, BDNF promotes the survival of neurons, including dopaminergic neurons in the striatum and cholinergic neurons in the basal forebrain. It is also essential for the maintenance of the cranial nerve, the optic nerve, and for the growth and survival of motor neurons (McTigue et al., 1998). BDNF shares 55%–60% sequence homology with other members of the same family of neurotrophic factors, NGF and NT-3 (Jun et al., 2004), and is mostly expressed in the cerebral cortex, hippocampus and striatum (Hofer et al., 1990; Rosenthal et al., 1990). In this study, we found that following 8 weeks of moderate- or high-intensity aerobic exercise, the absolute and relative expression of NGF, BDNF and NT-3 in the striatum is significantly upregulated, suggesting that aerobic exercise can trigger the expression of neurotrophic factors in the striatum. Our results are consistent with the findings of Bo et al. (2007) who used the Morris Water Maze to test the effects of swimming on learning and memory in rodents, and examined associated changes in BDNF expression in the hippocampus and striatum. They found that in comparison to the control group, 8 weeks of swimming training significantly enhanced the levels of BDNF mRNA in the hippocampus (38%) and striatum (14%), and was associated with improved learning and memory.
Here, we show that in the striatum, the absolute and relative expression of NGF, BDNF and NT-3 is significantly higher following 8 weeks of high-intensity aerobic training compared with moderate-intensity exercise. This indicates that high-intensity aerobic exercise contributes significantly to the expression of at least three nutritional factors in the striatum. Our results are consistent with the report by Decai and Lijuan (2007) where the authors noted an upregulation in the expression of BDNF and GFAP in the hippocampus and striatum in response to exercise-induced fatigue. They concluded that BDNF and GFAP contribute to the neurobiological regulation of sports-associated fatigue, and that BDNF and astrocytes might be necessary to avoid neuronal damage in response to exercise-induced fatigue, as they might mediate protective pro-survival and immunomodulatory effects. In addition, Pengpai (2011) showed that immediately after a single exhaustive exercise, the brain shows signs of early cortical ischemia-like changes, with significant upregulation of NGF and its cognate receptor TrkA. After 12 h, structural damage to the cerebral cortex was more apparent, but the expression of NGF and TrkA was significantly reduced. The authors further showed that at 24 h after exercise, cortical damage increased progressively accompanied by significant enhancement in NGF and TrkA expression. Recovery and remodeling of the damaged cortex was noted after 48 h, together with a decrease in NGF and TrkA expression, which continued to remain high. These results suggest that there is a dynamic association between structural damage and remodeling of the cerebral cortex and the expression of NGF and TrkA following a single exhaustive exercise.
Conclusion
Our experiments demonstrate that while the expression of NGF and NT-3 in the striatum is lower than in the soleus muscle, the levels of BDNF are similar and show no significant differences between the experimental groups. To our knowledge, there are no reports on the expression of neurotrophins in either the central striatum or the peripheral nerves innervating the skeletal muscle and. It was worthy of further study.
Acknowledgment
The authors contributed equal to this paper. The current project was supported by the National Natural Science Foundation of China (grant No. 31271257), The National 863 Grants of China (2008AA02Z411), and partially supported by the Hunan Provincial Natural Science Foundation of China (No. 11JJ6082 and 14JJ7035) and Scientific Research Fund of Hunan Provincial Education Department (No. 12B088).
Biographies
Wenfeng LIU
Employement
Lecturer, Hunan Provincial Key Laboratory of Physical Fitness and Sports Rehabilitation and The Key Laboratory of Protein Chemistry and Developmental Biology of Ministry of Education, Hunan Normal University
Degree
PhD
Research interests
Anti-aging medicine and Neurobiology; Movement science and Health management; Hypoxia physiology; Physical fitness.
E-mail: lwf1896@163.com
Gan CHEN
Employement
Lecturer, Hunan Provincial Key Laboratory of Physical Fitness and Sports Rehabilitation, Hunan Normal University
Degree
M.M.
Research interests
Sports medicine; Sports physiology; Muscle physiology; Physical fitness
E-mail: 283835603@qq.com
Fanling LI
Employement
Lecturer, Hunan Provincial People’s Hospital, the first affiliated hospital of Hunan Normal University
Degree
M.M.
Research interests
Sports medicine; Sports physiology; Muscle physiology; Physical fitness
E-mail: 286916836@qq.com
Changfa TANG
Employement
Professor, Hunan Provincial Key Laboratory of Physical Fitness and Sports Rehabilitation, Hunan Normal University
Degree
MD
Research interests
Sports medicine; Sports physiology; Muscle physiology; Physical fitness
E-mail: tangchangfa@sina.com
Dazhong YIN
Employement
Professor, The Key Laboratory of Protein Chemistry and Developmental Biology of Ministry of Education, Hunan Normal University, Qingyuan People's Hospital of Jinan University
Degree
PhD
Research interests
Aging biochemistry; Anti-aging medicine; Movement science and Physical fitness;
E-mail: dazhongyin002@126.com
References
- Bagen L., Yong X., Yaoming X. (2008) The Role of calcineurin in the changes in skeletal muscle fiber type and size of rats induced by endurance exercise. Chinese Journal of Sports Medicine 27, 551-556. [Google Scholar]
- Bassel-Duby R., Olson E.N. (2006) Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75, 19-37. [DOI] [PubMed] [Google Scholar]
- Bedford T.G., Tipton C.M., Wilson N.C., Oppliger R.A., Gisolfi C.V. (1979) Maximum oxygen consumption of rats and its changes with various experimental procedures. Journal of Applied Physiology 47, 1278-1283. [DOI] [PubMed] [Google Scholar]
- Bigard A.X., Mateo P.H., Sanchez H., Senuner B., Ventura-Clapier R. (2000) Lack of Coordinated changes in metabolic enzymnes and myosin heavy chain isoforms in regenerated muscles of trained rats. Joumal of Muscle Research and Cell Motility 21, 269-278. [DOI] [PubMed] [Google Scholar]
- Bo X., Yifei Y., Liu J., Tao H. (2007) Effects of Swimming Training on Rats’Learning and Memory and BDNFmRNA expression in Hippocampus and Striaturn. Chinese Journal of Beijing Sport University 30, 1352-1354. [Google Scholar]
- Bruton A. (2002) Muscle Plasticity: Response to training and detraining. Physiotherapy 88, 398-408. [Google Scholar]
- Carlsen H., Gundersen K. (2000) Helix loop helix transcription factors in electrically active and inactive skeletal muscles. Muscle Nerve 23, 1374-1380. [DOI] [PubMed] [Google Scholar]
- Carrasco D.I., English A.W. (2003) Neurotrophin 4/5 is required for the normal development of the slow muscle fiber phenotype in the rat soleus. The Journal of Experimental Biology 206, 2191-2200. [DOI] [PubMed] [Google Scholar]
- Chin E.R., Olson E.N., Richardson J.A., Yang Q., Humphries C., Shelton J.M., Wu H., Zhu W., Bassel-Duby R., Williams R.S. (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes & Development 12, 2499-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornachione A., Cação-Benedini L.O., Martinez E.Z., Neder L., Cláudia Mattiello-Sverzut A. (2011) Effects of eccentric and concentric training on capillarzation and myosin heavy chain contents in rat skeletal muscles after hindlimb suspension. Acta Histochemiea 113, 277-282. [DOI] [PubMed] [Google Scholar]
- Costelli P., Almendro V., Figueras M.T., Reffo P., Penna F., Aragno M., Mastrocola R., Boccuzzi G., Busquets S., Bonelli G., Lopez Soriano F.J., Argilés J.M., Baccino F.M. (2007) Modulations of the calcineurin/NF-AT Pathway in skeletal muscle atrophy. Biochimicaet Biophysiea Acta 1770, 1028-1036. [DOI] [PubMed] [Google Scholar]
- Decai Q., Lijuan H. (2007) Effects of Exercise-induced Fatigue on the Expression of BDNF and GFAP of Rats’Hippocampus and Striatum. Chinese Journal of Beijing Sport University 30, 781-783. [Google Scholar]
- Dupont-Versteegden E.E., Knox M., Gurley C.M., Houlé J.D., Peterson C.A. (2002) Maintenance of muscle mass is not dependent on the calcineurin NFAT pathway. American Journal of Physiology-cell Physiology 282, 1387-1395. [DOI] [PubMed] [Google Scholar]
- Francisco C.L., Jorge A.M., Dal-Pai-Silva M. (2011) Muscle fiber type characterization and myosin heavy chain (MyHC) isoform expression in Mediterranean buffaloes. Meat Science 88, 535-541. [DOI] [PubMed] [Google Scholar]
- Funakoshi H., Belluardo N., Arenas E., Yamamoto Y., Casabona A., Persson H., Ibáñez C.F. (1995) Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science 268, 1495-1499. [DOI] [PubMed] [Google Scholar]
- Gallo G., Letourneau P.C. (2004) Regulation of growth cone actin filaments by guidance cues. Journal of Neurobiology 58, 92-102. [DOI] [PubMed] [Google Scholar]
- George P., Charles W. (2005) The rat brain in stereotaxic coordinates. Boston, Elsevier Academic Press. [Google Scholar]
- Gómez-Pinilla F., Ying Z., Opazo P., Roy R.R., Edgerton V.R. (2001) Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. European Journal of Neuroscience 13, 1078-1084. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F., Ying Z., Roy R.R., Molteni R., Edgerton V.R. (2002) Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of Neurophysiology 88, 2187-2195. [DOI] [PubMed] [Google Scholar]
- Groth R.D., Coicou L.G., Mermelstein P.G., Seybold V.S. (2007) Neurotrophin activation of NFAT- dependent transcription contributes to the regulation of pronociceptive genes. Journal of Neurochemistry 102, 1162-1174. [DOI] [PubMed] [Google Scholar]
- Guidelines for Care and Use of Laboratory Animals. (2011) Committee for the Update of the Guide for the Care, Use of Laboratory Animals, National Research Council, Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington (DC). [Google Scholar]
- Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. (1990) Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO Journal 9, 2459-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinzhu P., Ruiyuan W. (2005) Effects of Treadmill Training on the Expression of α-actin and Myosin Heavy Chain Gene in Muscle of Rats. Chinese Journal of Sports Medicine 24, 415-415. [Google Scholar]
- Jun M., Ye T., Qi G. (2004) Influence of Excessive Exercise on the Morphology and Brain- derived Neurotrophic Factor Expression of Hippocampus Neurons. Chinese Journal of Sports Medicine 23, 510-514. [Google Scholar]
- Kubis H.P., Haller E.A., Wetzel P., Gros G. (1997) Adult fast myosin pattern and Ca2+-induced Slow myosin pattern in primary skeletal muse leculture. Proceedings of The National Academy of Sciences of The United States of America 94, 4205-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.M., Gertler F.B. (2000) From Abl to actin, Abl tyrosine kinase and associated proteins in growth cone motility. Current Opinion in Neurobiology 10, 80-87. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Qu-Petersen Z., Cao B., Kimura S., Jankowski R., Cummins J., Usas A., Gates C., Robbins P., Wernig A., Huard J. (2000) Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. Journal of Cell Biology 150 , 1085-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh K.J., Calabria E., Pallafacchina G., Ciciliot S., Serrano A.L., Argentini C., Kalhovde J.M., Lømo T., Schiaffino S.(2004) NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. C101, 10590-10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMcTigue D.M., Horner P.J., Stokes B.T., Gage F.H. (1998) Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. Journal of Neuroscience 18, 5354-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunoya W., Wakamatsu J., Tatsumi R., Ikeuchi Y. (2008) Protocol for highresolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Analytical Biochemistry 377, 111-113. [DOI] [PubMed] [Google Scholar]
- Mousavi K., Parry D.J., Jasmin B.J. (2004) BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. American Journal of Physiology-cell Physiology 287, 22-29. [DOI] [PubMed] [Google Scholar]
- Murgia M., Serrano A.L., Calabria E., Pallafacchina G., Lomo T., Schiaffino S. (2000) Ras is involved in nerve-activity-dependent regulation of muscle genes. Nature Cell Biology 2, 142-147. [DOI] [PubMed] [Google Scholar]
- Nagano M., Suzuki H. (2003) Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neuroscience Research 45, 391-399. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Lindner R., Tedeschi A., Forsberg K., Green A., Wuttke A., Gaub P., Di Giovanni S. (2009) NFAT-3 is a transcriptional repressor of the growth-associated protein 43 during neuronal maturation. Journal of Biological Chemistry 284, 18816-18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengpai Y. (2011) Structure and NGF, TrkA Dynamic Change in Rat Cerebral Cortex after Exhaustive Exercise. Chinese Journal of Chengdu Sport University 37, 60-64. [Google Scholar]
- Pette D., Staron RS. (1997) Mammalian skeletal muscle fiber type transition. International Review of Cytology 170, 143-223. [DOI] [PubMed] [Google Scholar]
- Pette D. (1998) Training effects on the contractile apparatus. Acta Physiologica Scandinavica 162, 367-376. [DOI] [PubMed] [Google Scholar]
- Pullen A.H. (1977) The distribution and relative sizes of fibre types in the extensor digitorum longus and soleus muscles of the adult rat. Journal of Anatomy 123, 467-486. [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z., Deasy B., Jankowski R., Ikezawa M., Cummins J., Pruchnic R., Mytinger J., Cao B., Gates C., Wernig A., Huard J. (2002) Identification of a novel population of muscle stem cells in mice, potential for muscle regeneration. Journal of Cell Biology 157, 851-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Luo C., Hogan P.G. (1997) Transcription factors of the NFAT family, regulation and function. Annual Review of Immunology 15, 707-747. [DOI] [PubMed] [Google Scholar]
- Rende M., Brizi E., Conner J., Treves S., Censier K., Provenzano C., Taglialatela G., Sanna P.P., Donato R. (2000) Nerve growth factor (NGF) influences differentiation and proliferation of myogenic cells in vitro via TrKA. International Journal of Developmental Neuroscience 18, 869-885. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Goeddel D.V., Nguyen T., Lewis M., Shih A., Laramee G.R., Nikolics K., Winslow J.W. (1990) Primary structure and biological activity of a novel human neurotrophic factor. Neuron 4, 767-473. [DOI] [PubMed] [Google Scholar]
- Sakuma K., Watanabe K., Sano M., Uramoto I., Nakano H., Li Y.J., Kaneda S., Sorimachi Y., Yoshimoto K., Yasuhara M., Totsuka T. (2001) A possible role for BDNF, NT-4 and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection and axotomy. Brain Research 907, 1-19. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Serrano A. (2002) Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends in Pharmacological Sciences 23, 569-575. [DOI] [PubMed] [Google Scholar]
- Serfling E., Klein-Hessling S., Palmetshofer A., Bopp T., Stassen M., Schmitt E. (2006) NFAT transcription factors in control of peripheral T cell tolerance. European Journal of Immunology 36, 2837-2843. [DOI] [PubMed] [Google Scholar]
- Sharma H.S., Cervós-Navarro J., Dey P.K. (1991) Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neuroscience Reserch 10, 211-221. [DOI] [PubMed] [Google Scholar]
- Shuzhe D., Caizhen C., Zhengtang Q. (2008) Effects of Exercise Intensity on p53 and COXI Expression of Skeletal Muscle in Rats. Chinese Journal of Sports Medicine 27, 454-457. [Google Scholar]
- Simon M., Porter R., Brown R., Coulton G.R., Terenghi G. (2003) Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres. European Journal of Neuroscience 18, 2460-2466. [DOI] [PubMed] [Google Scholar]
- Smart F.M., Edelman G.M., Vanderklish P.W. (2003) BDNF induces translocation of initiation factor 4E to mRNA granules, evidence for a role of synaptic microfilaments and integrins. Proceedings of The National Academy of Sciences of The United States of America 100, 14403-14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefos G.C., Soppa U., Dierssen M., Becker W. (2013) NGF Upregulates the Plasminogen Activation Inhibitor-1 in Neurons via the Calcineurin/NFAT Pathway and the Down Syndrome-Related Proteins DYRK1A and RCAN1 Attenuate this Effect. Plos One 8, e67470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge R.J. (2000) Myosin heavy chain isoform expression following reduced neuromuscular activity, potential regulatory mechanisms. Muscle Nerve 23, 661-679. [DOI] [PubMed] [Google Scholar]
- Tasic D., Dimov D., Gligorijevic J. (2003) Muscle Fiber Types and Fiber Morphometry in the Tibialis Posterior and Anterior of the Rat, A comparative Study. Medicine and Biology 10, 16-21. [Google Scholar]
- Walters R.E., Rotevatn S., Li P., Annex B.H., Yan Z. (2004) Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. American Journal of Physiology-cell Physiology 287, 1342-1348. [DOI] [PubMed] [Google Scholar]
- Wang L.C., Kernell D. (2002a) Recovery of type I fiber regionalization in gastrocnemius medialis of the rat after reinnervation along original and foreign paths, with and without muscle rotation. Neuroscience 114, 629-640. [DOI] [PubMed] [Google Scholar]
- Wang L., Copray S., Brouwer N., Meek M.F., Kernell D. (2002b) Regional distribution of slow-twitch muscle fibers after reinnervation in adult rat hindlimb muscles. Muscle Nerve 25, 805-15. [DOI] [PubMed] [Google Scholar]
- Yan-hong S., Rui-yuan W., Hua L. (2007) The effect of endurance training on heavy chain myosin as well as the regulating function of MyoD and Myogenin. Chinese Journal of Physical Education 14, 45-52. [Google Scholar]
- Yan-hong S., Rui-yuan W., Yue Z. (2008) Effects of Hypoxia and Hypoxic Training on the Expression of Rats’Myosin Heavy Chain. Chinese Journal of Beijing Sport University 31, 919-921. [Google Scholar]


