Abstract
The purpose of the present study was to evaluate the acute effects of an upper limb static-stretching (SS) protocol on the maximal concentric jump performance. We recruited 25 young healthy, male, resistance trained individuals (stretched group, n = 15 and control group, n = 10) in this study. The randomized between group experimental protocol consisted of a three trials of maximal concentric jump task, before and after a SS of the upper limb. Vertical ground reaction forces (vGRF) and surface electromyography (sEMG) of both gastrocnemius lateralis (GL) and vastus lateralis (VL) were acquired. An extensive SS was employed consisting of ten stretches of 30 seconds, with 15 seconds of rest, and 70-90% of the point of discomfort (POD). ANOVA (2x2) (group x condition) was used for shoulder joint range of motion (ROM), vGRF and sEMG. A significant interaction for passive ROM of the shoulder joint revealed significant increases between pre- and post-SS protocol (p < 0.001). A significant interaction demonstrated decreased peak force and an increased peak propulsion duration between pre- and post-stretching only for stretch group (p = 0.021, and p = 0.024, respectively. There was a significant main effect between groups (stretch and control) for peak force for control group (p = 0.045). Regarding sEMG variables, there were no significant differences between groups (control versus stretched) or condition (pre-stretching versus post-stretching) for the peak amplitude of RMS and IEMG for both muscles (VL and GL). In conclusion, an acute extensive SS can increase the shoulder ROM, and negatively affect both the propulsion duration and peak force of the maximal concentric jump, without providing significant changes in muscle activation.
Key points.
The jump performance can be affected negatively by an intense extensive static-stretching protocol.
An intense acute extensive SS protocol can affect positively the shoulder ROM.
The intense acute extensive SS protocol does not change the level of muscle activation for vastus lateralis and gastrocnemius lateralis.
Key words: Kinesiology, neuroscience, physiology, performance
Introduction
The effect of static stretching (SS) on the neuromuscular system has been attributed to both central and peripheral mechanisms. These performance (force and power) reductions can originate from neurophysiological (i.e. mechanoreceptors of the skin, muscle and joint proprioception), hormonal, cellular (structural changes such as titin), or mechanical (i.e. stiffness, torque-length characteristics) factors (Avela et al., 2004; Behm et al., 2004; Behm and Chaouachi, 2011; Pacheco et al., 2011; Rubini et al., 2007; Serpa et al., 2014; Wallmann et al., 2005), and in some studies, it might persist for over several hours post-stretch (Brandenburg et al., 2007; Fowles et al., 2000; Haddad et al., 2014; Power et al., 2004).
The majority of these studies focus on how a specific stretching protocol affects the exercised muscle(s). On the other hand, there is evidence that the contralateral untrained limb might be affected by the trained limb, known as cross-over effect (Farthing, 2009; Zhou, 2000). Several articles have reported cross-over effects for different exercise conditions such as fatigue (Doix et al., 2013; Marchetti and Uchida, 2011; Rattey et al., 2006; Regueme et al., 2007; Strang et al., 2009; Todd et al., 2003), and force/power (Carroll et al., 2006; Farthing et al., 2005; Lee and Carroll, 2007; Sariyildiz et al., 2011; Shima et al., 2002), however, few articles have examined the cross-over effect after SS routines (Lima et al., 2014; Nelson et al., 2012). Nelson, et al. (2012) analyzed 10-week stretching program (4 times for 30s, with 30s rest, 3 d·wk–1). The results indicated an increase in strength (1RM) and ROM for both legs (stretched and non-streched limb), where the strength gain of the non-stretched leg was 56% of the stretched leg. Lima et al. (2014) investigated the acute effects of unilateral ankle plantar flexors SS (6 sets of 45s/15s, 70-90% point of discomfort (POD)) on surface electromyography (sEMG) and the center of pressure (COP) during a single-leg balance task in both lower limbs. The results indicated no differences on non-stretched limb for all dependent variables when compared to stretched limb.
The investigation of cross-over effect is mainly limited to assessing how the stretching protocols of an ipsilateral muscle affects the performance of the contralateral homologous muscle. There is less research investigating possible non-localized effects of upper-body on lower-body performance or vice versa. This type of question addresses whether fatigue is specific to working muscles or is it more of a systemic response. Previous studies have shown evidence for the existence of neural coupling between upper and lower-limb neural networks which may modulate the muscle activation, reflexes and coordination of muscles (Huang and Ferris, 2004; 2009). Considering the neural coupling between upper and lower-limb, some articles have reported this effect for fatigue conditions (Kennedy et al., 2013; Takahashi et al., 2011). Halperin et al. (2014) in two separate papers reported that fatigued elbow flexors impaired force and activation of contralateral knee extensors while in the second paper, knee extension fatigue caused decrements in the ability to perform repeated maximal voluntary contractions of the elbow flexors. Neural coupling could be facilitated by group III and IV muscle afferents (Martin et al., 2008). This feedback loop from muscle afferents can provide an inhibitory effect to the central nervous system leading to systemic decrements in the central drive to exercised and non-exercised muscles (Martin et al., 2008). Gandevia (2001) provides extensive evidence of supraspinal inhibition concluding that muscle feedback concerning biochemical and force-generating status can impair cortical sites. Long loop reflexes from muscle spindle afferents (Marsden et al., 1983) as well as skin and subcutaneous afferents (Corden et al., 2000) can influence cortical activation of the exercised and non-exercised remote muscles (Kagamihara et al., 2003). Hence, it would be important to ascertain the non-local muscle effects of stretching the upper body on lower body performance.
However, there is no scientific evidence for acute stretching-induced crosover or non-local muscle fatigue effects. Therefore, the purpose of the present study was to evaluate the acute effects of an upper limb SS protocol on the maximal concentric jump performance. It was hypothesized that extensive upper body SS would impair lower body jump performance.
Methods
Subjects
Based on a statistical power analysis derived from the maximum jump height data from a pilot study, fifteen subjects would be necessary to achieve an alpha level of 0.05 and a power (1-β) of 0.80 (Eng, 2003). Therefore, we recruited 25 young healthy, resistance trained individuals (stretched group: 15 males, age: 23 ± 5 years, height: 1.78 ± 0.08 m, and weight: 74.4 ± 13kg and control group: 10 males, age: 22 ± 6 years, height: 1.79 ± 0.07 m, and weight: 74 ± 15kg) to participate in this study. Individuals who participated in this study had no previous surgery on both upper and lower extremities, no history of injury with residual symptoms (pain, “giving-away” sensations). All the participants signed an informed consent form after receiving instruction on the experimental protocol of the research. This study was approved by the University research ethics committee (#35/2014).
Procedures
Prior to the data collection, the subjects were asked to identify the preferred leg for kicking a ball, which was then considered the dominant leg (Maulder and Cronin, 2005), and all subjects were right-arm dominant based on their preferred arm to write. All subjects were right leg and arm dominant. The experimental protocol consisted of: (1) a brief sub-maximal jumping warm-up for 5 minutes (20 jumps, 4 jumps with a rest interval of 45 seconds); (2) concentric jump task familiarization; (3) a pre-stretching evaluation of the upper limb (passive ROM); (4) three trials of maximal concentric jump task; (3) shoulder joint SS protocol; (4) immediately post-stretching evaluation of the upper limb (passive ROM); and (5) three trials of maximal concentric jump task. The control group followed the same procedures as the stretched group, except for the shoulder joint SS protocol. The control group remained seated during the same time interval that the stretched group performed the stretching protocol. All measures were performed by the same researcher and at the same hour of the day, between 9 and 12 AM.
Concentric jumping task (CJ)
The subjects initially stood on the force plate (Biomec410, EMG System do Brasil, São José dos Campos, Brazil) with knee flexed to 90°, with the hands placed on the hips. The participant then held the position for a 2-s period at which time they were instructed to jump as high and fast as possible. The subjects left the force plate with the knee and ankle fully extended and landed in a similarly extended position. The CJ eliminated any active prestretch of the musculature and thus only utilized a concentric contraction (Power et al., 2004). The subjects were allocated at least 10s rest between jumps. Vertical ground reaction forces (vGRF) and surface electromyography (sEMG) of both gastrocnemius lateralis (GL) and vastus lateralis (VL) were simultaneously acquired at 2000Hz.
Measures
Surface electromyography recording (sEMG)
The participants’ skin was prepared before placement of the EMG electrodes. Hair at the site of electrode placement was shaved and the skin was cleaned with alcohol. Bipolar active disposable dual Ag/AgCl snap electrodes 1cm in diameter for each circular conductive area and 2cm center-to-center spacing were placed unilaterally (dominant lower limb) over the longitudinal axes of the GL and VL. For the GL, the electrodes were placed in the direction of the line between the head of the fibula and the heel and for VL at 2/3 on the line from the anterior spina iliaca superior to the lateral side of the patella, according to the SENIAM/ISEKI protocol. The sEMG signals of the GLs of both lower limbs were recorded by an electromyographic acquisition system (EMG832C, EMG system Brasil, São José dos Campos, Brazil) with sampling rate of 2000 Hz using a commercially designed software program (DATAQ Instruments Hardware Manager, DATAQ Instruments, Inc., OH, USA). EMG activity was amplified (bi-polar differential amplifier, input impedance = 2MΩ, common mode rejection ratio > 100 dB min (60 Hz), gain x 20, noise > 5 µV), and analog-to-digitally converted (12 bit). A ground electrode was placed on the right clavicle.
Shoulder Joint Range of Motion (ROM)
Subjects adopted a supine position with knees bent, supporting the lumbar spine at the bench. The fleximeter was placed on the dominant arm, above the elbow, and the hands were placed together to set zero degrees of the fleximeter. Then, each subject performed a passive ROM for a horizontal abduction movement of the shoulder joint. The maximal passive shoulder ROM was evaluated before and after the SS protocol with a fleximeter, with a sensitivity of 1° (Sanny, Brazil).
Intervention
Static-stretching protocol (SS)
The subjects remained seated on a chair, with the hands behind the head, arms raised above the shoulder joint. Then, a researcher moved both arms (shoulder joint), at the same time, passively, to the maximal ROM of the horizontal abduction position, bringing the elbows back as far as possible. An extensive passive stretching protocol was employed consisting of ten stretches of 30 seconds, with 15 seconds of rest between each stretch (Behm et al., 2004). The intensity was 70-90% of the point of discomfort (POD), where 0="no stretch discomfort at all" and 100%="the maximum imaginable stretch discomfort" (Behm and Chaouachi, 2011; Behm and Kibele, 2007). The POD intensity was required from the subjects during all stretches and the ROM was readjusted at 70-90% POD as necessary. The SS protocol was applied and controlled (POD) by the same strength and conditioning researcher.
Data analysis
All the vGRF and sEMG data were analyzed only during the propulsive CJ phase (defined by vGRF) by a customized Matlab routine (MathWorks Inc., USA). Then, the mean of the three maximal jumps were calculated for each variable.
CJ performance analysis
For each CJ task, the vertical GRF data was filtered with a fourth-order 80Hz low-pass zero-lag Butterworth filter. We computed the following variables during the propulsive phase of the CJ: (a) propulsive time, (b) peak of force, (c) impulse (vGRF area).
sEMG analysis
The digitized sEMG data was first band-pass filtered at 20-400 Hz by using a fourth order Butterworth filter with a zero lag. For the time domain analysis, we calculated the peak of RMS, using a maximal value of the EMG RMS (150ms moving window) for each muscle, and each EMG RMS data was integrated (IEMG).
Statistical analyses
The normality and homogeneity of variances within the data were confirmed with the Shapiro-Wilk and Levene tests, respectively. A mixed model ANOVA (2x2) was used for all ROM, vGRF and sEMG variables, with factors being group (stretched and control) and repeated measures for condition (pre- and post-stretching). Post-hoc comparisons were performed with the Bonferroni test. Cohen’s formula for effect size (ES) was calculated, and the results were based on the following criteria: <0.35 trivial effect; 0.35-0.80 small effect; 0.80-1.50 moderate effect; and >1.5 large effect, for recreationally trained according to Rhea (2004). Test-retest reliability (ICC) was calculated and ranged between 0.76 and 0.98 for all dependent variables. An alpha of 5% was used for all statistical tests.
Results
A significant interaction for passive ROM of the shoulder joint revealed significant increases between the measurements taken before and those taken after the SS protocol (mean±SD: 134.9 ± 12.4° to 141.9 ± 14.4°, p < 0.001, ES = 0.54, Δ% = 5.93%). There were no significant differences with pre- to post-SS measures for the control group (mean±SD: 135 ± 12° to 139 ± 14°, p = 0.704). There were no significant main effects between the groups for shoulder joint passive ROM.
Regarding sEMG variables, there were no significant differences between groups (control versus stretched) or condition (pre-stretching versus post-stretching) for the peak amplitude of RMS and IEMG for both muscles (VL and GL) (Table 1).
Table 1.
Mean (±standard deviation) of the peak amplitude of the root mean square (RMS) and integrated electromyography (IEMG) before and after the static stretching (SS) protocol for vastus lateralis (VL) and gastrocnemius lateralis (GL) and groups (control and stretched).
| Variable | Muscle | Control Group | Stretched Group | ||
|---|---|---|---|---|---|
| Pre- Intervention | Post- Intervention | Pre- Intervention | Post- Intervention | ||
| Peak RMS (Volts) | VL | .91 (.37) | .87 (.18) | .94 (.22) | .99 (.25) |
| GL | .53 (.11) | .59 (.12) | .59 (.15) | .60 (.15) | |
| IEMG (V.s) | VL | 780 (334) | 746 (214) | 787 (210) | 842 (204) |
| GL | 424 (110) | 445 (92) | 457 (111) | 468 (118) | |
A significant interaction demonstrated a decrease for peak force (Figure 1a) and an increased peak propulsion duration (Figure 1b) between pre- and post-stretching only for stretched group (p = 0.021, ES = 1.41, Δ% = 25% and p = 0.024, ES = 1.32, Δ%=23.6%, respectively). Additionally, there was a significant main effect between groups (stretched and control group) for peak force (p = 0.045, ES = 1.38, Δ% = 28.4%). For the impulse, there were no significant differences between pre-stretching and post-stretching for both stretched and control group.
Figure 1.
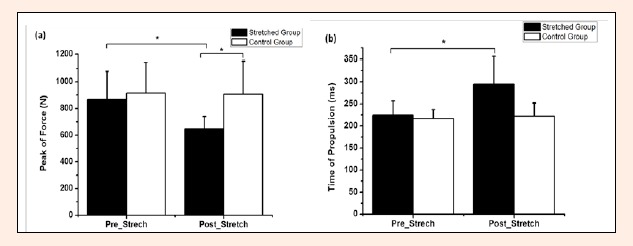
Mean and standard deviation of the (a) peak of force and (b) time of propulsion before and after static stretching (SS) for both groups stretched and control. * p < 0.05.
Discussion
The purpose of the present study was to evaluate the acute effects of an upper limb SS protocol on the maximal concentric jump performance. It was hypothesized that extensive upper body SS would impair lower body jump performance.
The main findings of the present study were the evidence of impairment on the maximal concentric jump performance (increase of propulsion duration and decrease of peak force) and the increase of shoulder ROM after an acute upper limb SS protocol. The total volume (300s) and high intensity (70-90% POD) of SS used in the present study increased the passive shoulder joint ROM by 5.9%. However, the significant increases in shoulder joint passive ROM after the SS protocol only occurred with the stretched group.
Related to the force variables, we observed an increase of peak of propulsion phase and a decrease for peak force between pre- and post-stretching only for the stretched group. The non-local impairments associated with extensive upper body exercise on lower body performance is in accord with Halperin et al.(2014) who found a reduction in quadriceps muscle force and activation following a 100s isometric elbow flexor fatigue protocol. Similarly, Kennedy et al. (2013) reported 23% and 9% deficits in plantar flexors MVC after maximal and submaximal fatiguing protocols of the forearms. The decline in jumping performance of lower limbs after upper limb SS protocol suggests a central nervous system mechanism (Kennedy et al., 2013), taking into account the neural coupling between upper and lower-limb neural networks which might modulate the muscle activation, reflexes and coordination of muscles in a different extremity (Huang and Ferris, 2004; 2009). Halperin et al. (2014) detected a crossover fatigue effect from the fatigued elbow flexors and knee extensors to the non-exercised knee extensors but no significant effect on the non-exercised elbow flexors. They suggested the knee extensors were more susceptible than the elbow flexors to non-local or crossover muscle fatigue effects regardless of the muscle group being fatigued. It would be interesting in a subsequent investigation to examine whether the upper body to lower body SS deficits found in the present study would also occur with lower body SS influences upon upper body performance.
The effects of prolonged and intense SS on the joint receptors might lead to inhibitory inputs from afferent Type III (mechanoreceptor) and IV (nociceptor) afferents and Golgi tendon organ discharge, thereby limiting neural drive to the primary motor cortex. Long loop reflexes from muscle spindle afferents (Marsden et al., 1983) as well as skin and subcutaneous afferents (Corden et al., 2000) can influence cortical activation of the affected and non-local muscles (Kagamihara et al., 2003). Since intrafusal stretch receptor discharge frequency from Ia afferents can contribute up to 30% of the motoneuron excitation (Gandevia, 2001), SS-induced reductions in afferent excitability could adversely affect muscle force. As there is no horizontal connectivity within the primary motor cortex between arm and leg representations, the decline in supraspinal drive may be attributed to mechanisms that originate upstream of primary motor cortex, where arm and leg representations overlap considerably (Aboodarda et al., 2014; Byblow et al., 2007; Trajano et al., 2013). Gandevia (2001) provides extensive evidence of supraspinal inhibition indicating that muscle feedback concerning force-generating status can impair cortical sites.
Considering the sEMG variables, such as peak of EMG or IEMG, we did not observe significant differences between pre-stretching and post-stretching for both muscles (VL and GL) and groups. Perhaps this non-significant response can be explained by the non-linear force-EMG relationship (Solomonow et al., 1990). The earliest studies on the force-EMG relationship reported a linear relationship (Lippold, 1952) however; contemporary studies have illustrated a non-linear or curvilinear force-EMG relationships with the quadriceps during isometric (Alkner et al., 2000) and dynamic (Fujita et al., 2011) contractions. A non-linear force-EMG relationship indicates that the sEMG is not sensitive to all changes in force output, and it is not sensitive enough to capture the differences between conditions, which were relatively small (~5%). In addition, we can relate the high variability of the data (sEMG) with the inter-subject differences of the SS protocol intensity.
We recognize that this study has some limitations. The placement of the sEMG electrodes over the VL and GL might have led to cross-talk from adjacent muscles, such as the vastus intermedius, rectus femoris, soleus, tibialis, and peroneal muscles. We chose to use the most extensive SS protocol in the literature that included subjective information about the stretching intensity. However, we do recognize that the intensity of the stretching might not be commonly utilized during warm-ups to activity or during the rehabilitation processes.
Conclusion
In conclusion, an acute extensive SS protocol can increase the shoulder ROM, and negatively affect both the propulsion duration and peak force of the maximal concentric jump, without providing significant changes in muscle activation. These non-local effects should be taken into consideration by athletes, coaches, fitness professionals and rehabilitation specialists when stretching one body part and subsequently training another muscle. SS-induced deficits are not localized to only the stretched muscle and may affect subsequent power performance in other muscle groups.
Biographies

Paulo H. MARCHETTI
Employment
Associated Professor
Degree
Ph.D
Research interests
Postural control, biomechanics, resistance training, stretching
E-mail: dr.pmarchetti@gmail.com

Fernando H. D. de O. SILVA
Employment
Physical Educator
Degree
Master
Research interests
Biomechanics
E-mail: ferpkfr@gmail.com

Enrico G. SOARES
Employment
Physical Educator
Degree
Master
Research interests
Biomechanics
E-mail: emaildoenrico@gmail.com

Érica P. SERPA
Employment
Physical Educator
Degree
Master
Research interests
Biomechanics
E-mail: erica_serpa@hotmail.com

Priscyla S.M. NARDI
Employment
Physiotherapist and Physical Educator
Degree
Specialist
Research interests
Sports physiotherapy, biomechanics
E-mail: priscylanardi@gmail.com

Guanis de B.VILELA Junior
Employment
Assistant Professor
Degree
PhD
Research interests
biomechanics
E-mail: guanis@gmail.com

David G. BEHM
Employment
Associated Dean Professor
Degree
PhD
Research interests
Biomechanics, motor control
E-mail: dbehm@mun.ca
References
- Aboodarda S.J., Copithorne D.B., Power K.E., Drinkwater E.J., Behm D.G. (2014) Elbow flexor fatigue modulates central excitability of the knee extensors. Medicine & Science in Sports & Exercise 1, 1-28. [DOI] [PubMed] [Google Scholar]
- Alkner B.A., Tesch P.A., Berg H.E. (2000) Quadriceps emg/force relationship in knee extension and leg press. Medicine & Science in Sports & Exercise 32, 459-463. [DOI] [PubMed] [Google Scholar]
- Avela J., Finni T., Liikavainio T., Niemela E., Komi P.V. (2004) Neural and mechanical responses of the triceps surae muscle group after 1h of repeated fast passive stretches. Journal of Applied Physiology 96, 2325-2332. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Bambury A., Cahill F., Power K. (2004) Effect of acute static stretching on force, balance, reaction time, and movement time. Medicine & Science in Sports & Exercise 36(8), 1397-1402. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Chaouachi A. (2011). A review of the acute effects of static and dynamic stretching on performance. European Journal of Applied Physiology 111, 2633-2651. [DOI] [PubMed] [Google Scholar]
- Behm D.G., Kibele A. (2007) Effects of differing intensities of static stretching on jump performance. European Journal of Applied Physiology 101, 587-594. [DOI] [PubMed] [Google Scholar]
- Brandenburg J., Pitney W.A., Luebbers P.E., Veera A., Czajka A. (2007) Time course of changes in vertical-jumping ability after static stretching. International Journal of Sports Physiology and Performance 2, 170-181. [DOI] [PubMed] [Google Scholar]
- Byblow W.D., Coxon J.P., Stinear C.M., Fleming M.K., Williams G., Müller J.F., Ziemann U. (2007) Functional connectivity between secondary and primary motor areas underlying handfoot coordination. Journal of Neurophysiology 98(1), 414-422. [DOI] [PubMed] [Google Scholar]
- Carroll T.J., Herbert R.D., Munn J., Lee M., Gandevia S.C. (2006) Contralateral effects of unilateral strength training: evidence and possible mechanisms. Journal of Applied Physiology 101(5), 1514-1522. [DOI] [PubMed] [Google Scholar]
- Corden D.M., Lippold O.C., Buchanan K., Norrigton C. (2000) Long-latency component of the stretch reflex in human muscle is not mediated by intramuscular stretch receptors. Journal of Neurophysiology 84(1), 184-188. [DOI] [PubMed] [Google Scholar]
- Doix A.C.M., Lefevre F., Colson S.S. (2013). Time Course of the Cross-Over Effect of Fatigue on the Contralateral Muscle after Unilateral Exercise. PLoS One 8(5), 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J. (2003) Sample Size Estimation: How many individuals should be studied? Radiology 227(2), 309-313. [DOI] [PubMed] [Google Scholar]
- Farthing J.P. (2009) Cross-education of strength depends on limb dominance: Implications fot theory and application. Exercise and Sports Science Review 37, 179-187. [DOI] [PubMed] [Google Scholar]
- Farthing J.P., Chilibeck P.D., Binsted G. (2005) Cross-education of arm muscular strength is unidirectional in right-handed individuals. Medicine & Science in Sports & Exercise 37(9), 1594-1600. [DOI] [PubMed] [Google Scholar]
- Fowles J.R., Sale D.G., MacDougall J.D. (2000) Reduced strength after passive stretch of the human plantarflexors. Journal of Applied Physiology 89, 1179-1188. [DOI] [PubMed] [Google Scholar]
- Fujita E., Kanehisa H., Yoshitake Y., Fukunaga T., Nishizono H. (2011) Association between knee extensor strength and emg activities during squat movement. Medicine & Science in Sports & Exercise 43, 2328-2334. [DOI] [PubMed] [Google Scholar]
- Gandevia S.C. (2001) Spinal and supraspinal factors in human muscle fatigue. Physiological Reviews 81(4), 1725-1776. [DOI] [PubMed] [Google Scholar]
- Haddad M., Dridi A., Chtara M., Chaouachi A., Wong D.P., Behm D., Chamari K. (2014) Static stretching can impair explosive performance for at least 24 hours. Journal of Strength and Conditioning Research 28(1), 140-146. [DOI] [PubMed] [Google Scholar]
- Halperin I., Aboodarda S.J., Behm D.G. (2014) Knee extension fatigue attenuates repeated force production of the elbow flexors. European Journal of Applied Physiology 25, 1-7. [DOI] [PubMed] [Google Scholar]
- Huang H.J., Ferris D.P. (2004) Neural coupling between upper and lower limbs during recumbent stepping. Journal of Applied Physiology 97(4), 1299-1308. [DOI] [PubMed] [Google Scholar]
- Huang H.J., Ferris D.P. (2009) Upper and lower limb muscle activation is bidirectionally and ipsilaterally coupled. Medicine & Science in Sports & Exercise 41(9), 1778-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagamihara Y., Hayashi A., Masakado Y., Kouno Y. (2003) Long-loop reflex from arm afferents to remote muscles in normal man. Experimental Brain Research 151(1), 136-144. [DOI] [PubMed] [Google Scholar]
- Kennedy A., Hug F., Sveistrup H., Guével A. (2013) Fatiguing handgrip exercise alters maximal force-generating capacity of plantar-flexors. European Journal of Applied Physiology 113(3), 559-566. [DOI] [PubMed] [Google Scholar]
- Lee M., Carroll T.J. (2007) Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Medicine 37(1), 1-14. [DOI] [PubMed] [Google Scholar]
- Lima B.N., Lucareli P.R.L., Gomes W.A., Silva J.J., Bley A.S., Hartigan E.H., Marchetti P.H. (2014) The acute effects of unilateral ankle plantar flexors static- stretching on postural sway and gastrocnemius muscle activity during single-leg balance tasks. Journal of Sports Science and Medicine 13(3), 564-570. [PMC free article] [PubMed] [Google Scholar]
- Lippold O.C.J. (1952) The relation between integrated action potentials in a human muscle and its isometric tension. The Journal of Physiology 117, 492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti P.H., Uchida M.C. (2011) Influence of unilateral fatigue of lower limbs on the bilateral vertical jump. Revista Brasileira de Medicina do Esporte 17(6), 401-404. [Google Scholar]
- Marsden C.D., Meadows J.C., Merton P.A. (1983) "Muscular wisdom" that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Advances in Neurology 39, 169-211. [PubMed] [Google Scholar]
- Maulder P., Cronin J. (2005) Horizontal and vertical jump assessment: reliability, symmetry, discriminative and predictive ability. Physical Therapy in Sport 6(2), 74-82. [Google Scholar]
- Nelson A.G., Kokkonen J., Winchester J.B., Kalani W., Peterson K., Kenly M.S., Arnall D.A. (2012) A 10-week stretching program increases strength in the contralateral muscle. Journal of Strength and Conditioning Research 26(3), 832-836. [DOI] [PubMed] [Google Scholar]
- Pacheco L., Balius R., Aliste L., Pujol M., Pedret C. (2011) The acute effects of different stretching exercises on jump performance. Journal of Strength and Conditioning Research 25(11), 2991-2998. [DOI] [PubMed] [Google Scholar]
- Power K., Behm D., Cahill F., Carroll M., Young W. (2004) An acute bout of static stretching of static stretching: Effects on force and jumping performance. Medicine & Science in Sports & Exercise 36(8), 1389-1396. [DOI] [PubMed] [Google Scholar]
- Rattey J., Martin P.G., Kay D., Cannon J., Marino F.E. (2006) Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross-over effect. Pflügers Archiv European Journal of Physiology 452, 199-207. [DOI] [PubMed] [Google Scholar]
- Regueme S.C., Barthelemy J., Nicol C. (2007) Exhaustive stretch-shortening cycle exercise: no contralateral effects on muscle activity in maximal motor performances. Scandinavian Journal of Medicine & Science in Sports 17, 547-555. [DOI] [PubMed] [Google Scholar]
- Rhea M.R. (2004) Determining the magnitude of treatment effects in strength training research through the use of the effect size. Journal of Strength and Conditioning Research 18(4), 918-920. [DOI] [PubMed] [Google Scholar]
- Rubini E.C., Costa A.L.L., Gomes S.C. (2007). The effects of stretching on strength performance. Sports Medicine 37(3), 213-224. [DOI] [PubMed] [Google Scholar]
- Sariyildiz M., Karacan I., Rezvani A., Ergin O., Cidem M. (2011) Cross-education of muscle strength: cross-training effects are not confined to untrained contralateral homologous muscle. Scandinavian Journal of Medicine & Science in Sports 21(6), 359-364. [DOI] [PubMed] [Google Scholar]
- Serpa E.P., Vilela Junior G.B., Marchetti P.H. (2014) Aspectos biomecânicos da unidade músculo-tendínea sob efeito do alongamento. Revista CPAQV – Centro de Pesquisas Avançadas em Qualidade de Vida 6(1), 1-14 (In Portuguese). [Google Scholar]
- Shima N., Ishida K., Katayama K., Morotome Y., Sato Y., Miyamura M. (2002) Cross education of muscular strength during unilateral resistance training and detraining. European Journal of Applied Physiology 86(4), 287-294. [DOI] [PubMed] [Google Scholar]
- Solomonow M., Baten C., Smit J., Baratta R., Hermens H., D‘Ambrosia R., Shoji H. (1990) Electromyogram power spectra frequencies associated with motor unit recruitment strategies. Journal of Applied Physiology 68(3), 1177-1185. [DOI] [PubMed] [Google Scholar]
- Strang A.J., Berg W.B., Hieronymus M. (2009) Fatigue-induced early onset of anticipatory postural adjustments in non-fatigued muscles: support for a centrally mediated adaptation. Experimental Brain Research 197, 245-254. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Maruyama A., Hirakoba K., Maeda M., Etoh S., Kawahira K., Rothwell J.C. (2011) Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the nonexercised upper limb. Brain Stimulation 4(2), 90-96. [DOI] [PubMed] [Google Scholar]
- Todd G., Petersen N.T., Taylor J.L., Gandevia S.C. (2003) The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Experimental Brain Research 150, 308-313. [DOI] [PubMed] [Google Scholar]
- Trajano G.S., Seitz L., Nosaka K., Blazevich A.J. (2013) Contribution of central vs. peripheral factors to the force loss induced by passive stretch of the human plantar flexors. Journal of Applied Physiology 115, 212-218. [DOI] [PubMed] [Google Scholar]
- Wallmann H.W., Mercer J.A., McWhorter W. (2005) Surface electromyougraphic assessment of the effects of static stretching of the gastrocnemius on vertical jump performance. Journal of Strength and Conditioning Research 19(3), 684-688. [DOI] [PubMed] [Google Scholar]
- Zhou S. (2000) Chronic neural adaptations to unilateral exercise: Mechanisms of cross education. Exercise and Sport Sciences Reviews 28, 177-184. [PubMed] [Google Scholar]
- Martin P.G., Weerakkody N., Gandevia S.C., Taylor J.L. (2008) Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans.. Journal of Physiology 586(pt 5), 1277-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]


