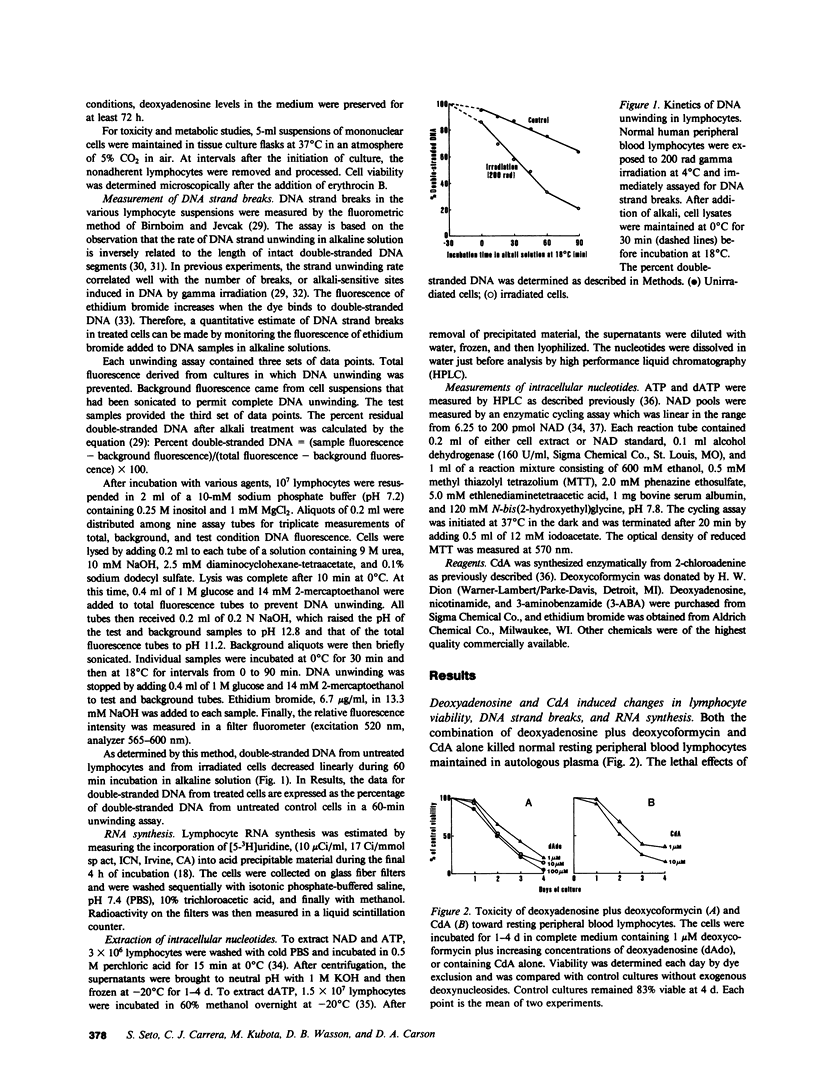
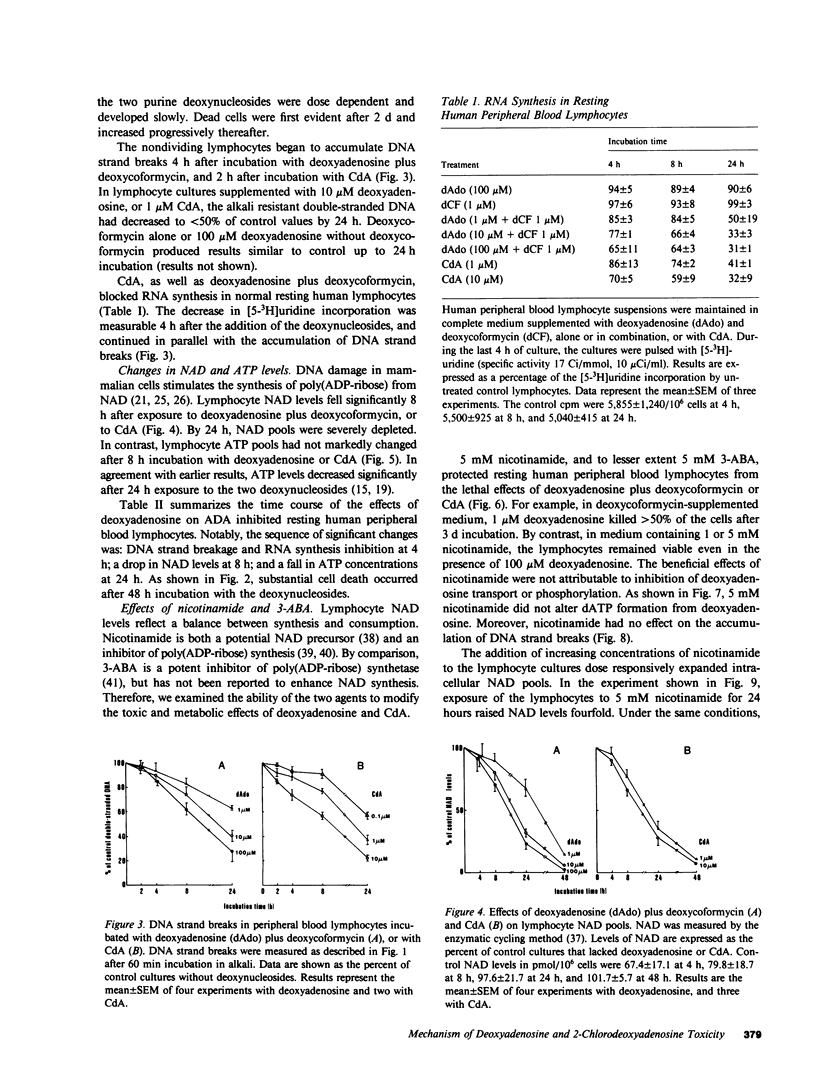
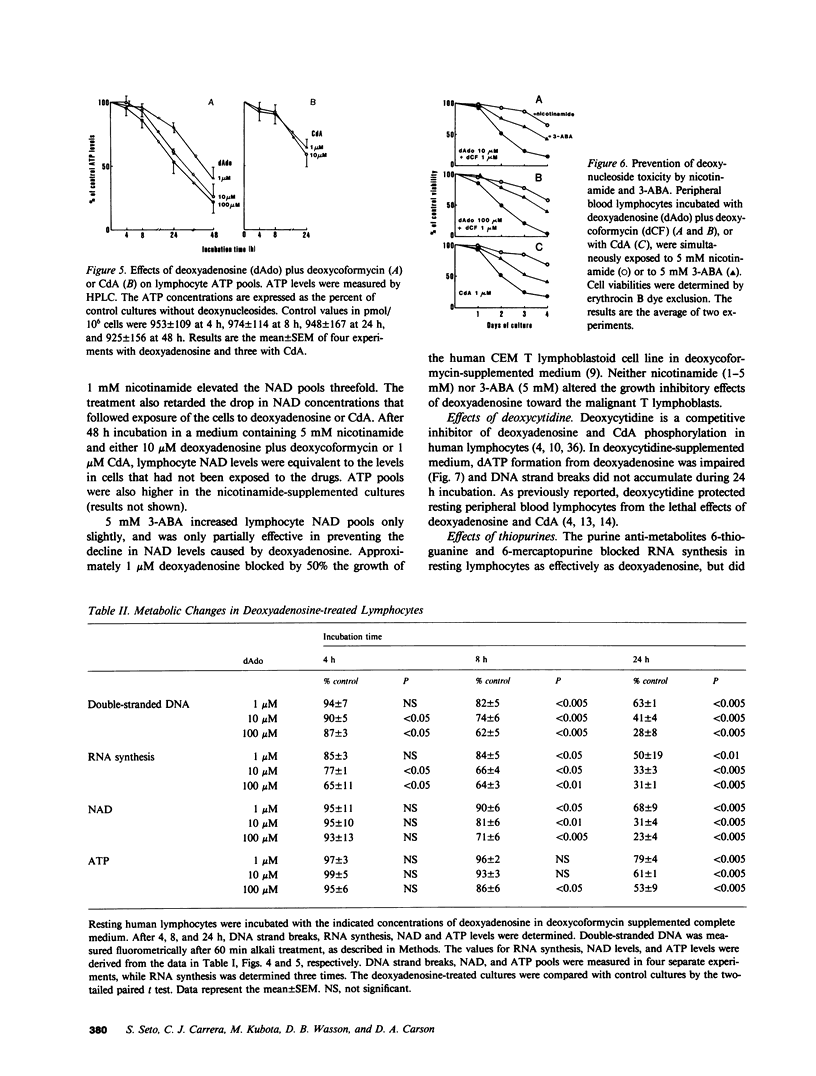
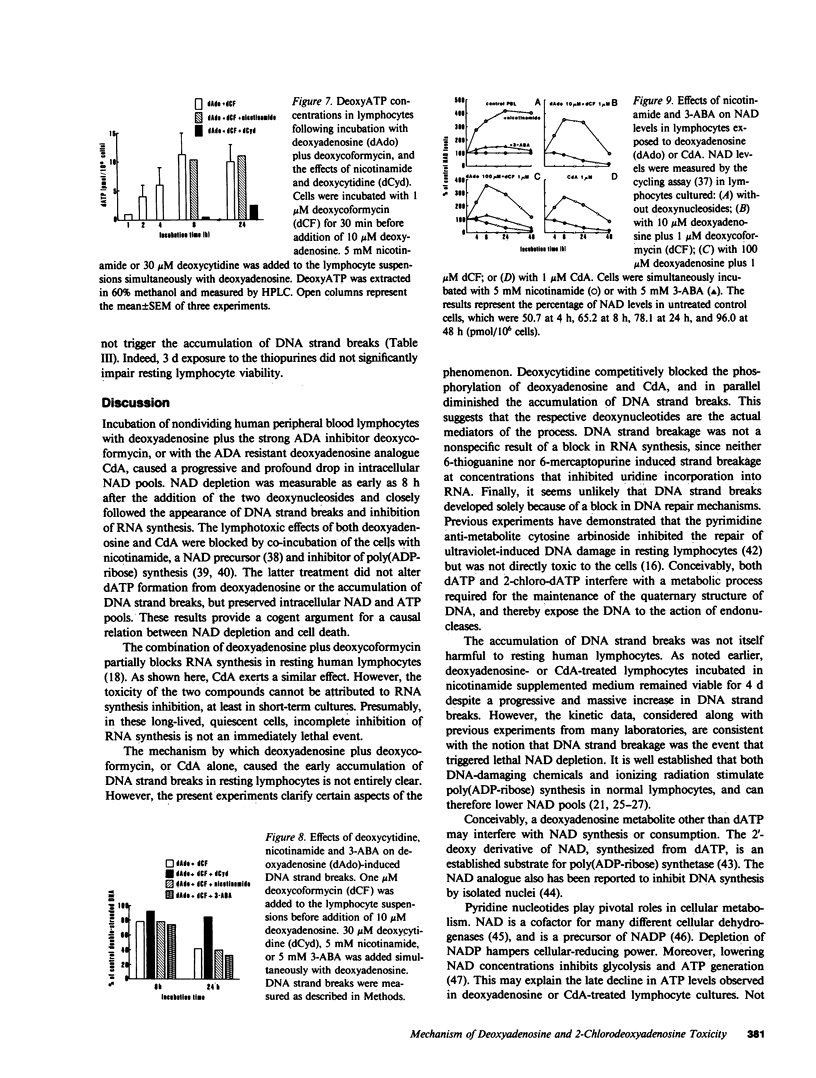
Abstract
Deoxyadenosine has been implicated as the toxic metabolite causing profound lymphopenia in immunodeficient children with a genetic deficiency of adenosine deaminase (ADA), and in adults treated with the potent ADA inhibitor deoxycoformycin. However, the biochemical basis for deoxyadenosine toxicity toward lymphocytes remains controversial. The present experiments have examined in detail the sequential metabolic changes induced in nondividing human peripheral blood lymphocytes by incubation with deoxyadenosine plus deoxycoformycin, or with 2-chlorodeoxyadenosine (CdA), an ADA resistant deoxyadenosine congener with anti-leukemic and immunosuppressive properties. The lymphotoxic effect of deoxyadenosine and CdA required their phosphorylation, and was inhibited by deoxycytidine. As early as 4 h after exposure to the deoxynucleosides, strand breaks in lymphocyte DNA began to accumulate, and RNA synthesis decreased. These changes were followed by a significant fall in intracellular NAD levels at 8 h, a drop in ATP pools at 24 h, and cell death by 48 h. Incubation of the lymphocytes with 5 mM nicotinamide, a NAD precursor and an inhibitor of poly(ADP-ribose) synthetase, prevented NAD depletion. The nicotinamide treatment also rendered the lymphocytes highly resistant to deoxyadenosine and CdA toxicity, without altering dATP formation or the accumulation of DNA strand breaks. The poly(ADP-ribose) synthetase inhibitor 3-aminobenzamide exerted a similar although less potent effect. These results suggest that NAD depletion, probably triggered by poly(ADP-ribose) formation, is the principle cause of death in normal resting human lymphocytes exposed to deoxyadenosine plus deoxycoformycin, or to CdA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W. Nicotinamide stimulates repair of DNA damage in human lymphocytes. Biochem Biophys Res Commun. 1980 Jul 16;95(1):67–72. doi: 10.1016/0006-291x(80)90705-6. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Association of poly(adenosine diphosphoribose) synthesis with DNA damage and repair in normal human lymphocytes. J Clin Invest. 1979 Jun;63(6):1164–1171. doi: 10.1172/JCI109410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernofsky C., Swan M. An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem. 1973 Jun;53(2):452–458. doi: 10.1016/0003-2697(73)90094-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Brox L., Ng A., Pollock E., Belch A. DNA strand breaks induced in human T-lymphocytes by the combination of deoxyadenosine and deoxycoformycin. Cancer Res. 1984 Mar;44(3):934–937. [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Beutler E. Antileukemic and immunosuppressive activity of 2-chloro-2'-deoxyadenosine. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2232–2236. doi: 10.1073/pnas.81.7.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Kaye J., Ullman B., Martin D. W., Jr, Robins R. K., Montgomery J. A. Deoxycytidine kinase-mediated toxicity of deoxyadenosine analogs toward malignant human lymphoblasts in vitro and toward murine L1210 leukemia in vivo. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6865–6869. doi: 10.1073/pnas.77.11.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Lakow E., Kamatani N. Possible metabolic basis for the different immunodeficient states associated with genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3848–3852. doi: 10.1073/pnas.79.12.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Taetle R., Yu A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood. 1983 Oct;62(4):737–743. [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Donofrio J., Coleman M. S., Hutton J. J., Daoud A., Lampkin B., Dyminski J. Overproduction of adenine deoxynucleosides and deoxynucletides in adenosine deaminase deficiency with severe combined immunodeficiency disease. J Clin Invest. 1978 Oct;62(4):884–887. doi: 10.1172/JCI109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Greenbaum A. L., Pinder S. The pathway of biosynthesis of nicotinamide-adenine dinucleotide in rat mammary gland. Biochem J. 1968 Mar;107(1):55–62. doi: 10.1042/bj1070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer W. L., Kaplan J. G. DNA strand breaks in murine lymphocytes: induction by purine and pyrimidine analogues. Biochem Biophys Res Commun. 1983 Sep 30;115(3):834–840. doi: 10.1016/s0006-291x(83)80010-2. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Fetter J. E., Small W. C., Bagnara A. S., Williams S. R., Ullman B., Martin D. W., Jr, Wasson D. B., Carson D. A. Effects of mutational loss of adenosine kinase and deoxycytidine kinase on deoxyATP accumulation and deoxyadenosine toxicity in cultured CEM human T-lymphoblastoid cells. J Biol Chem. 1982 Jun 10;257(11):6380–6386. [PubMed] [Google Scholar]
- Hillyard D., Rechsteiner M. C., Olivera B. M. Pyridine nucleotide metabolism in mammalian cells in culture. J Cell Physiol. 1973 Oct;82(2):165–179. doi: 10.1002/jcp.1040820205. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Ratech H. Genetic deficiencies of adenosine deaminase and purine nucleoside phosphorylase and their implications for therapy of leukemias. Curr Top Hematol. 1983;4:1–35. [PubMed] [Google Scholar]
- Jacobson E. L., Jacobson M. K. Pyridine nucleotide levels as a function of growth in normal and transformed 3T3 cells. Arch Biochem Biophys. 1976 Aug;175(2):627–634. doi: 10.1016/0003-9861(76)90553-1. [DOI] [PubMed] [Google Scholar]
- Jeffery J. Kinetic aspects of soluble dehydrogenases requiring nicotinamide coenzymes. Experientia Suppl. 1980;36:1–39. doi: 10.1007/978-3-0348-5419-1_1. [DOI] [PubMed] [Google Scholar]
- Kefford R. F., Fox R. M. Deoxycoformycin-induced response in chronic lymphocytic leukaemia: deoxyadenosine toxicity in non-replicating lymphocytes. Br J Haematol. 1982 Apr;50(4):627–636. doi: 10.1111/j.1365-2141.1982.tb01963.x. [DOI] [PubMed] [Google Scholar]
- Kefford R. F., Fox R. M. Purine deoxynucleoside toxicity in nondividing human lymphoid cells. Cancer Res. 1982 Jan;42(1):324–330. [PubMed] [Google Scholar]
- Kohn K. W., Erickson L. C., Ewig R. A., Friedman C. A. Fractionation of DNA from mammalian cells by alkaline elution. Biochemistry. 1976 Oct 19;15(21):4629–4637. doi: 10.1021/bi00666a013. [DOI] [PubMed] [Google Scholar]
- Kwan D. K., Norman A. Radiosensitivity of human lymphocytes and thymocytes. Radiat Res. 1977 Jan;69(1):143–151. [PubMed] [Google Scholar]
- Lichtenwalner D. M., Suhadolnik R. J. Adenosine diphosphate ribosylation of histone and nonhistone chromosomal proteins with oxidized nicotinamide adenine dinucleotide and 2'-deoxynicotinamide adenine dinucleotide using nuclei isolated from rat liver and HeLa cells. Biochemistry. 1979 Aug 21;18(17):3749–3755. doi: 10.1021/bi00584a016. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Matsumoto S. S., Yu J., Yu A. L. Inhibition of RNA synthesis by deoxyadenosine plus deoxycoformycin in resting lymphocytes. J Immunol. 1983 Dec;131(6):2762–2766. [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Pulleyblank D. E. Native and denatured DNA, cross-linked and palindromic DNA and circular covalently-closed DNA analysed by a sensitive fluorometric procedure. Biochem Biophys Res Commun. 1974 Nov 27;61(2):396–403. doi: 10.1016/0006-291x(74)90970-x. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen B., Tyrsted G., Dupont B. The deoxyribonucleoside 5'-triphosphate (dATP and dTTP) pool in phytohemagglutinin-stimulated and non-stimulated human lymphocytes. Exp Cell Res. 1973 Jun;79(2):249–256. doi: 10.1016/0014-4827(73)90442-4. [DOI] [PubMed] [Google Scholar]
- Prosser J. S. Survival of human T and B lymphocytes after X-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1976 Nov;30(5):459–465. doi: 10.1080/09553007614551271. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROITT I. M. The inhibition of carbohydrate metabolism in ascites-tumour cells by ethyleneimines. Biochem J. 1956 Jun;63(2):300–307. doi: 10.1042/bj0630300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin P. W., Jacobson M. K., Mitchell V. R., Busbee D. L. Reduction of nicotinamide adenine dinucleotide levels by ultimate carcinogens in human lymphocytes. Cancer Res. 1980 Jun;40(6):1803–1807. [PubMed] [Google Scholar]
- Rydberg B. The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat Res. 1975 Feb;61(2):274–287. [PubMed] [Google Scholar]
- Siaw M. F., Mitchell B. S., Koller C. A., Coleman M. S., Hutton J. J. ATP depletion as a consequence of adenosine deaminase inhibition in man. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6157–6161. doi: 10.1073/pnas.77.10.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. L., Sikorski G. W., Catino D. M., Berger S. J., Berger N. A. Poly(adenosinediphosphoribose) polymerase inhibitors stimulate unscheduled deoxyribonucleic acid synthesis in normal human lymphocytes. Biochemistry. 1982 Apr 13;21(8):1813–1821. doi: 10.1021/bi00537a017. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Suhadolnik R. J., Baur R., Lichtenwalner D. M., Uematsu T., Roberts J. H., Sudhakar S., Smulson M. ADP-ribosylation of isolated nuclei from HeLa cells, rat liver, fetal rat liver, and Novikoff hepatoma. Effect of nicotinamide adenine dinucleotide analogs on template activity for DNA synthesis, incorporation into nuclear proteins, and a new 1''-3' osidic linkage. J Biol Chem. 1977 Jun 25;252(12):4134–4144. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Yew F. F., Johnson R. T. Ultraviolet-induced DNA excision repair in human B and T lymphocytes. II. Effect of inhibitors and DNA precursors. Biochim Biophys Acta. 1979 Apr 26;562(2):240–251. doi: 10.1016/0005-2787(79)90170-9. [DOI] [PubMed] [Google Scholar]



