Abstract
Introduction
Germ-line mutations in the micro-ribonucleic acid processing gene DICER1 have been shown to predispose to a subset of benign tumors susceptible to malignant transformation, including ovarian Sertoli-Leydig cell tumor, nontoxic multinodular goiter, multilocular cystic nephroma and pleuropulmonary blastoma, which can occur in children and young adults. This may be due to reduced Dcr-1 homolog expression in carriers of germline mutations, which causes impairment of micro-ribonucleic acid processing and deregulates the growth and differentiation of target cells, leading to an increased risk of tumorigenesis. Many carriers of germ-line DICER1 mutations remain unaffected, but development of tumors within carriers is associated with varying prognoses.
Case presentation
Despite the Dcr-1 homolog syndrome phenotype being incompletely defined, a DICER1 mutation was suspected when a girl (case 1 patient) of Danish ethnicity presented with both an ovarian Sertoli-Leydig cell tumor and a multinodular goiter at the age of 13 years. In addition, family history included a male sibling (case 2 patient) who also had a multinodular goiter and had undergone a hemithyroidectomy at the age of 14 years. Subsequent DICER1 screening of the girl identified two novel mutations in exon 21 - a nonsense (c.3647C>A, p.Ser1216*) and a missense (c.3649T>A, p.Tyr1217Asn) mutation. The siblings had inherited the mutations from their father and paternal grandfather, which both currently were asymptomatic, indicating reduced penetrance of the nonsense mutation. Analysis of the parents revealed that the mutations were present in cis, making the contribution of the missense mutation less significant.
Conclusion
We report a novel pathogenic DICER1 mutation (p.Ser1216*) in a Danish family associated with ovarian Sertoli-Leydig cell tumor and a multinodular goiter. A multinodular goiter was diagnosed in the siblings during childhood. Clinicians should be aware of a potential germ-line DICER1 mutation when evaluating multinodular goiter in young patients with or without a family history of thyroid diseases.
Keywords: DICER1, Germ-line mutation, Multinodular goiter, Ovarian Sertoli-Leydig cell tumor
Introduction
Dcr-1 homolog (DICER1) is a member of the ribonuclease type III family and plays an important role in the processing and maturation of micro-ribonucleic acids (miRNAs) [1]. miRNAs are small (20 to 25 nucleotides), double-stranded, non-coding, endogenous RNA molecules that modulate gene expression at the post-transcriptional level by imperfect base pairing to the complementary sequences on target messenger RNAs (mRNAs). miRNA genes are transcribed by RNA polymerase II or III into primary miRNA transcripts termed pri-miRNAs. Pri-miRNAs are subsequently cleaved by the Drosha-DGCR8 complex to release hairpin-shaped pre-miRNAs. Pre-miRNAs are exported to the cytoplasm where DICER1 cuts their terminal loop and generates short miRNA duplexes. A single strand of the small RNA duplexes is finally incorporated into the RNA-induced silencing complex, and in this position, miRNAs bind to their target mRNAs, modulating protein expression [1].
Impaired DICER1 expression and subsequent altered miRNA processing have a substantial impact on the dysregulation of target oncogenes, leading to enhanced tumorigenesis [2]. DICER1 mutations have previously been associated with ovarian Sertoli-Leydig cell tumor (SLCT), nontoxic multinodular goiter (MNG) and multilocular cystic nephroma. These conditions generally follow a benign course [3,4]. In addition, DICER1 mutations predispose to a rare type of lung cancer most often seen in children, known as pleuropulmonary blastoma [5]. Recently, DICER1 mutations were also suggested to be associated with diseases, such as Wilms’ tumor, cervix embryonal rhabdomyosarcoma and pineoblastoma [6-8].
Here we report a novel germ-line DICER1 nonsense mutation in a pair of siblings with MNG, as well as SLCT in the index case.
Case presentation
Case 1 patient was a 13-year-old girl of Danish ethnicity (proband), who presented with swelling of the neck, as well as a deep voice, hirsutism and acne vulgaris in the beard area of the face. She was subsequently diagnosed with MNG and ultrasonic examination identified 13 nodules ranging from 6 to 12mm in size. Examination of her hormonal status revealed increased levels of androstenedione (26nmol/l) and testosterone (total: 6.8nmol/l and free: 0.146nmol/l). Follicle-stimulating hormone and luteinizing hormone levels were normal, as was the Synacthen test. A computed tomography scan identified a tumor in her left ovary. She immediately underwent unilateral oophorectomy and subsequent histopathological examination detected encapsulated tumor tissue, including strings of immature and slightly atypical Sertoli cells together with accumulations of Leydig cells. There were only a few mitoses and no necrosis. The final histopathological diagnosis was reported as an encapsulated SLCT of intermediate degree of differentiation. Immunohistochemical analyses showed positive staining for vimentin and inhibin, whereas α-fetoprotein gave a negative result. Follow-up included an ultrasonic-guided examination of the ovary and measurement of hormonal status and serum inhibin B level for five years with an increasing interval.
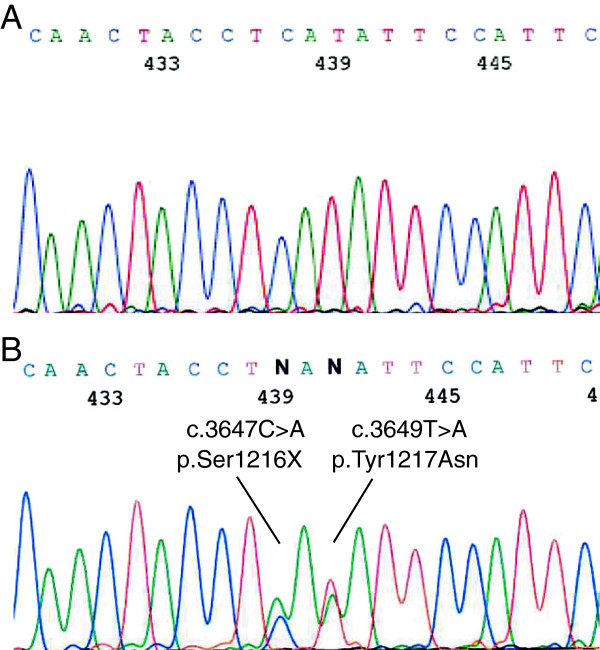
Since our proband, had an ovarian SLCT as well as MNG, the pediatricians suspected a DICER1 mutation and referred the girl for genetic counseling. Blood samples were collected, genomic deoxyribonucleic acid (DNA) was purified, and the entire coding region and the exon-intron boundaries of DICER1 were screened. The analysis identified two mutations in exon 21 - a nonsense mutation (c.3647C>A, p.Ser1216*) and a missense mutation (c.3649T>A, p.Tyr1217Asn) of unknown significance (Figure 1, panel B).
Figure 1.
Identification of theDICER1c.3647C>A, p.Ser1216* and the c.3649T>A, p.Tyr1217Asn mutations. Deoxyribonucleic acid was purified from blood samples from a wild-type and the proband. The DICER1 gene was amplified using intronic primer pairs flanking each exon, followed by sequencing. The analysis revealed a DICER1 nucleotide c.3647C>A, p.Ser1216* mutation in exon 21 and a DICER1 nucleotide c.3649T>A, p.Tyr1217Asn mutation also in exon 21 in the proband (panel B) not present in the wild-type (panel A).
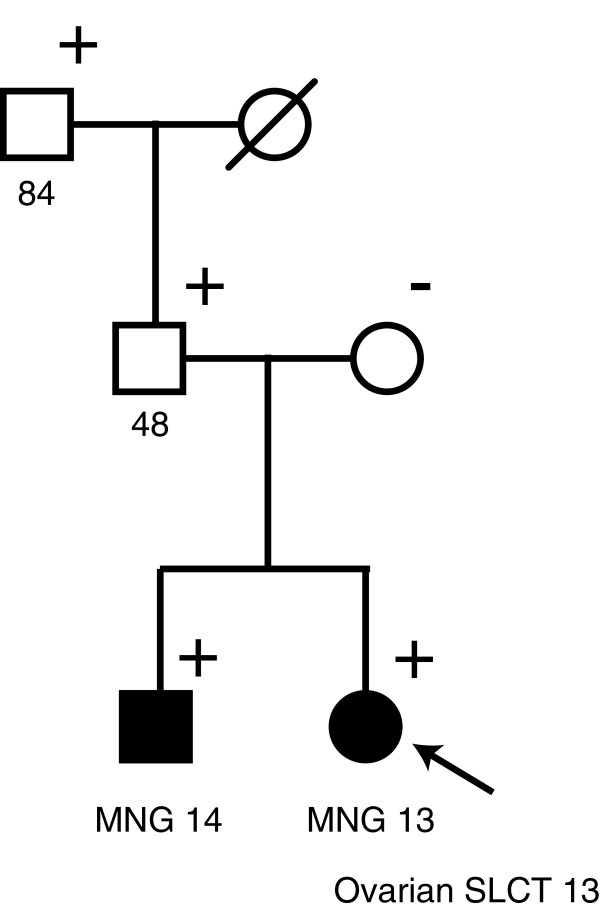
Since a pathogenic DICER1 mutation was identified, first and second degree relatives of the proband were screened for the mutation. The analyses revealed that the brother, the father and the paternal grandfather all carried the pathogenic DICER1 mutation as well as the missense mutation (Figure 2), indicating that the two mutations are present in cis on the same allele.
Figure 2.
Family pedigree. Multinodular goiter and ovarian Sertoli-Leydig cell tumor are indicated as well as the age at diagnosis. Diagonal slash indicates deceased, while the proband is indicated with an arrow. Mutation-positive individuals are indicated with +. MNG, multinodular goiter; SLCT, Sertoli-Leydig cell tumor.
Case 2 patient is a five-year older brother of the proband who had an MNG and underwent hemithyroidectomy at the age of 14. Ultrasound (US)-guided fine-needle aspirate from the dominating hypoechoic cold nodule measuring (4×3.5×2.5cm) yielded follicular neoplasia. Subsequently, the brother underwent a right-sided hemi-thyroidectomy and the histopathological examination revealed an encapsulated follicular adenoma with papillary hyperplasia. The brother is at present time in his early 20s and remains euthyroid without any pressure symptoms. He has annual consultations regarding the MNG of the left lobe to monitor potential growth in the cold nodules.
The father, at the age of 50, has no chronic illnesses and no medical history of any thyroid symptoms. A recent thyroid check-up confirmed the euthyroid status and a normal palpatory examination of the thyroid and neck area. The medical history of the paternal grandfather reveals a remarkably fit patient in his mid-80s who is being treated for hypertension and hypercholesterolemia. The grandfather remains euthyroid and palpatoric examination of the thyroid and neck area did not reveal any goiter, nodules or enlarged lymph nodes.
Discussion
Ovarian SLCTs are rare androgen-producing sex cord-gonadal stromal tumors that account for less than 1% of ovarian tumors, occurring most commonly in younger women while MNG is a common disease likely caused by low iodine intake but also with a heritable predisposition. In this study, a girl was diagnosed with MNG and ovarian SLCT at the age of 13 years. Subsequently, a pathogenic DICER1 mutation was identified. To date, 45 different pathogenic germ-line DICER1 mutations have been reported in 53 probands with various neoplasms worldwide, including frameshift, nonsense, splicing and missense mutations scattered throughout the gene, as well as large genomic rearrangements (Table 1) [3-5,7-13]. The novel nonsense mutation identified in the present study resides in exon 21, the largest exon of DICER1, where currently 11 other pathogenic germ-line mutations and deletions have been identified. Hence, exon 21 of the DICER1 gene has the highest frequency of disease-causing mutations, followed by exons 8 and 23 with five pathogenic mutations each. Exon 21 is of particular interest as it encodes a large part of the ribonuclease (RNase) IIIa domain. The RNase IIIa domain is highly important for the production of miRNAs from the 3’ arm of precursors and forms, together with RNase IIIb, the core catalytic element of Dicer1.
Table 1.
Previously reported germ-line mutations in the DICER1 gene
| Region | Nucleotide change | Amino acid change | Reference |
|---|---|---|---|
| Exon 4 |
c.328_338dupGTGTCAGCTGT |
p.Arg114Cysfs*18 |
Slade et al.[11] |
| Exon 7 |
c.876_879delAAAG |
p.Arg293Ilefs*4 |
Rio Frio et al.[4] |
| Exon 8 |
c.912_919dupAGACTGTC |
p.Arg307Glnfs*8 |
Foulkes et al.[7] |
| Exon 8 |
c.1128_1132delAGTAA |
p.Lys376Asnfs*11 |
Sabbaghian et al.[8] |
| Exon 8 |
c.1153delC |
p.Arg385Alafs*73 |
Slade et al.[11] |
| Exon 8 |
c.1196_1197dupAG |
p.Trp400Serfs*59 |
Slade et al.[11] |
| Exon 8 |
c.1306dupT |
p.Ser436Phefs*41 |
Foulkes et al.[7] |
| Exon 9 |
c.1507G>T |
p.Glu503* |
Hill et al.[5] |
| Exon 10 |
c.1525C>T |
p.Arg509* |
Darrat et al. 2013 |
| Exon 10 |
c.1684_1685delAT |
p.Met562Valfs*11 |
Hill et al.[5] |
| Exon 10 |
c.1716delT |
p.Phe572Leufs*15 |
Slade et al.[11] |
| Exon 12 |
c.1910dupA |
p.Tyr637* |
Doros et al.[10]; Hill et al.[5] |
| Exon 12 |
c.1966C>T |
p.Arg656* |
Foulkes et al.[7]; Slade et al.[11]; Hill et al.[5] (reported in four individuals) |
| Intron 12 |
c.2040+1G>C |
p.? |
Slade et al.[11] |
| Intron 13 |
c.2117-1G>A |
p.? |
Foulkes et al.[7] |
| Exon 14 |
c.2245_2248dupTACC |
p.Pro750Leufs*12 |
Hill et al.[5] |
| Exon 14 |
c.2247C>A |
p.Tyr749* |
Doros et al.[10]; Hill et al.[5] |
| Exon 15 |
c.2268_2271delTTTG |
p.Cys756* |
Slade et al.[11] |
| Exon 15 |
c.2392dupA |
p.Thr798Asnfs*33 |
Hill et al.[5] |
| Exon 16 |
c.2457C>G |
p.Ile813_Tyr819del |
Rio Frio et al.[4] |
| r.2437_2457del21 | |||
| Exon 16 |
c.2516C>T |
p.Ser839Phe |
Rio Frio et al.[4] |
| Intron 17 |
c.2805-1G>T |
p.Tyr936_Arg996del |
Rio Frio et al.[4] |
| r.2805_2987del183 | |||
| Exon 18 |
c.2830C>T |
p.Arg944* |
Hill et al.[5] |
| Intron 18 |
c.2988-2_2988-1delAGinsCT |
p.? |
Slade et al.[11] |
| Exon 21 |
c.3270-6_4051 – 1280delinsG |
p.Tyr1091Ser*28 |
Sabbaghian et al.[13] |
| Exon 21 |
c.3288_3289insTTTC |
p.Gly1097Phefs*8 |
Slade et al.[11] |
| Exon 21 |
c.3505delT |
p.Ser1169Glnfs*23 |
Slade et al.[11] |
| Exon 21 |
c.3540C>A |
p.Tyr1180* |
Hill et al.[5] |
| Exon 21 |
c.3583_3584delGA |
p.Asp1195Leufs*39 |
Slade et al.[11] |
| Exon 21 |
c.3611_3616delACTACAinsT |
p.Tyr1204Leufs*29 |
Foulkes et al.[7] |
| Exon 21 |
c.3647C>A |
p.Ser1216* |
This study |
| Exon 21 |
c.3665delT |
p.Leu1222Tyrfs*17 |
Slade et al.[11] |
| Exon 21 |
c.3726C>A |
p.Tyr1242* |
Slade et al.[11] |
| Exon 21 |
c.3793delA |
p.Thr1265Glnfs*37 |
Slade et al.[11] |
| Exon 21 |
c.3907_3908delCT |
p.Leu1303Valfs*4 |
Foulkes et al.[7] |
| Intron 21 |
c.4050+1delG |
p.? |
Foulkes et al.[7] |
| Exon 23 |
c.4309_4312delGACT |
p.Asp1437Metfs*16 |
Bahubeshi et al.[3]; Doros et al.[10] |
| Exon 23 |
c.4403_4406delCTCT |
p.Ser1468Phefs*21 |
Slade et al.[11] |
| Exon 23 |
c.4740G>T |
p.Gln1580His |
Slade et al.[11] |
| Exon 23 |
c.4748T>G |
p.Leu1583Arg |
Hill et al.[5] |
| Exon 23 |
c.5018_5021delTCAA |
p.Ile1673Thrfs*31 |
Rio Frio et al.[4] |
| Exon 24 |
c.5104C>T |
p.Gln1702* |
Doros et al.[10]; Dehner et al.[9] |
| Exon 24 |
c.5122_5128delGGAGATG |
p.Gly1708Argfs*7 |
Slade et al.[11] |
| Exon 25 |
c.5465A>T |
p.Asp1822Val |
Slade et al.[11] |
| Exon 25 | c.5477C>A | p.Ser1826* | Bahubeshi et al.[3] |
Some of the information is obtained from [https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=DICER1]. NM177438.2 was used as a reference sequence.
Our data indicate reduced penetrance of the c.3647C>A DICER1 mutation. At the time of our study, two of the adult family members showed no signs of neoplasms, whereas the mutation was associated with characteristic tumors at an early age in the proband and her brother. The relatively low penetrance in the described family is in line with other reported familial DICER1 mutations [3,4,14]. The low penetrance of DICER1 mutations could relate to a decisive role of miRNAs in the differentiation of stem cells [15], assuming a significant intrauterine selection pressure against the mutations in combination with variations in genetic background. Although the level of penetrance of DICER1 mutations seems modest, mutational screening of DICER1 in children and young persons with MNG as well as rare tumors of the ovary, eyes, lungs or kidneys could still be useful. Such screening might also be important in cases devoid of a family history. However, the apparent modest level of penetrance makes the issue of intrauterine testing for the mutation ethically debatable. Genetic counseling of parents of children with a recognized DICER1 mutation who intend to have more children would benefit from a more precise knowledge of the tumorigenic penetrance of the different DICER1 mutations. Therefore, clinical follow-up studies of patients with pathogenic DICER1 mutations with and without neoplastic diseases are required for improved counseling and treatment.
Conclusions
Very few clinicians couple the rare SLCT with the more common MNG and it is therefore reasonable to assume that the syndromes associated with DICER1 mutations are under diagnosed. Based on the findings in the present case report, future awareness of SLCT and MNG should reveal a more accurate incidence. Moreover, clinicians should be aware of a potential germ-line DICER1 mutation when evaluating multinodular goiter in young patients with or without a family history of thyroid diseases.
Consent
Written informed consent was obtained from the first patient's (Case 1) legal guardian and all other involved patients for publication of this case report and accompanying images. Copies of the written consents are available for review by the Editor-in-Chief of this journal.
Abbreviations
mRNAs: Messenger RNAs; miRNAs: microRNAs; MNG: Multinodular goiter; SLCT: Sertoli-Leydig cell tumor; US: Ultrasound.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MR, TvOH and FCN were involved in the genetic screening of the patients. AJ, CR, MRu and AMG performed the clinical examinations and the genetic counseling of the patients. MR and TvOH drafted the manuscript, while FCN, AJ, CR, MRu and AMG were involved in the revision of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Maria Rossing, Email: rossing.maria@gmail.com.
Anne-Marie Gerdes, Email: Anne-Marie.Gerdes@regionh.dk.
Anders Juul, Email: anders.juul@rh.regionh.dk.
Catherine Rechnitzer, Email: Catherine.Rechnitzer@regionh.dk.
Martin Rudnicki, Email: pmru@regionsjaelland.dk.
Finn C Nielsen, Email: finn.cilius.nielsen@regionh.dk.
Thomas vO Hansen, Email: Thomas.Van.Overeem.Hansen@regionh.dk.
Acknowledgements
We thank Stine Østergaard and Shehrukh Jamil for technical assistance. We also thank the patients reported in this case report for their consent to publish the findings.
References
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- Bahubeshi A, Bal N, Rio Frio T, Hamel N, Pouchet C, Yilmaz A, Bouron-Dal Soglio D, Williams GM, Tischkowitz M, Priest JR, Foulkes WD. Germline DICER1 mutations and familial cystic nephroma. J Med Genet. 2010;47:863–866. doi: 10.1136/jmg.2010.081216. [DOI] [PubMed] [Google Scholar]
- Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O'Brien PK, Serfas K, Broderick P, Houlston RS, Lesueur F, Bonora E, Muljo S, Schimke RN, Bouron-Dal Soglio D, Arseneau J, Schultz KA, Priest JR, Nguyen VH, Harach HR, Livingston DM, Foulkes WD, Tischkowitz M. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, Goodfellow PJ. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong CS, Priest JR, Foulkes WD. Exploring the endocrine manifestations of DICER1 mutations. Trends Mol Med. 2012;18:503–505. doi: 10.1016/j.molmed.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, Choong CS, Charles A, Frieder RP, Dishop MK, Graf N, Ekim M, Bouron-Dal Soglio D, Arseneau J, Young RH, Sabbaghian N, Srivastava A, Tischkowitz MD, Priest JR. Extending the phenotypes associated with DICER1 mutations. Hum Mutat. 2011;32:1381–1384. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- Sabbaghian N, Hamel N, Srivastava A, Albrecht S, Priest JR, Foulkes WD. Germline DICER1 mutation and associated loss of heterozygosity in a pineoblastoma. J Med Genet. 2012;49:417–419. doi: 10.1136/jmedgenet-2012-100898. [DOI] [PubMed] [Google Scholar]
- Dehner LP, Jarzembowski JA, Hill DA. Embryonal rhabdomyosarcoma of the uterine cervix: a report of 14 cases and a discussion of its unusual clinicopathological associations. Mod Pathol. 2012;25:602–614. doi: 10.1038/modpathol.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doros L, Yang J, Dehner L, Rossi CT, Skiver K, Jarzembowski JA, Messinger Y, Schultz KA, Williams G, Andre N, Hill DA. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer. 2012;59:558–560. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR. et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48:273–278. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- Darrat I, Bedoyan JK, Chen M, Schuette JL, Lesperance MM. Novel DICER1 mutation as cause of multinodular goiter in children. Head Neck. 2013;35:E369–371. doi: 10.1002/hed.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbaghian N, Srivastava A, Hamel N, Plourde F, Gajtko-Metera M, Niedziela M, Foulkes WD. Germ-line deletion in DICER1 revealed by a novel MLPA assay using synthetic oligonucleotides. Eur J Hum Genet. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Schultz KA, Pacheco MC, Yang J, Williams GM, Messinger Y, Hill DA, Dehner LP, Priest JR. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: a report from the International Pleuropulmonary Blastoma Registry. Gynecol Oncol. 2011;122:246–250. doi: 10.1016/j.ygyno.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]