Abstract
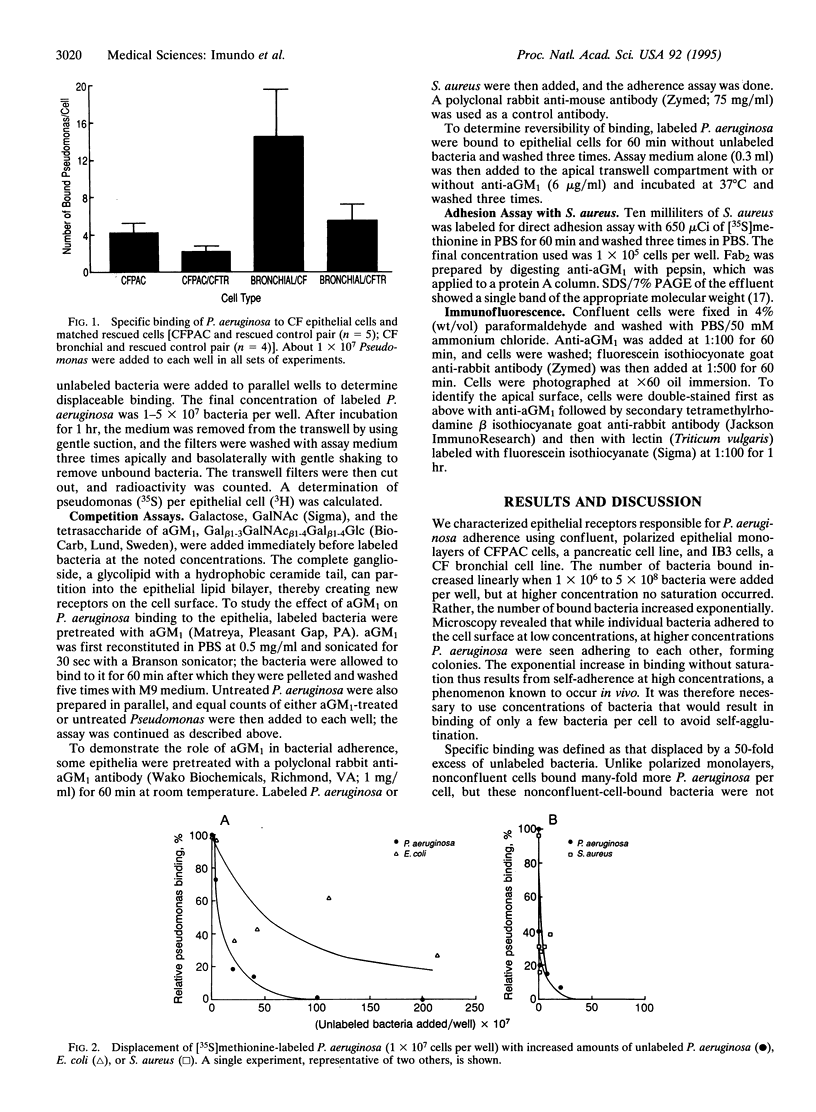
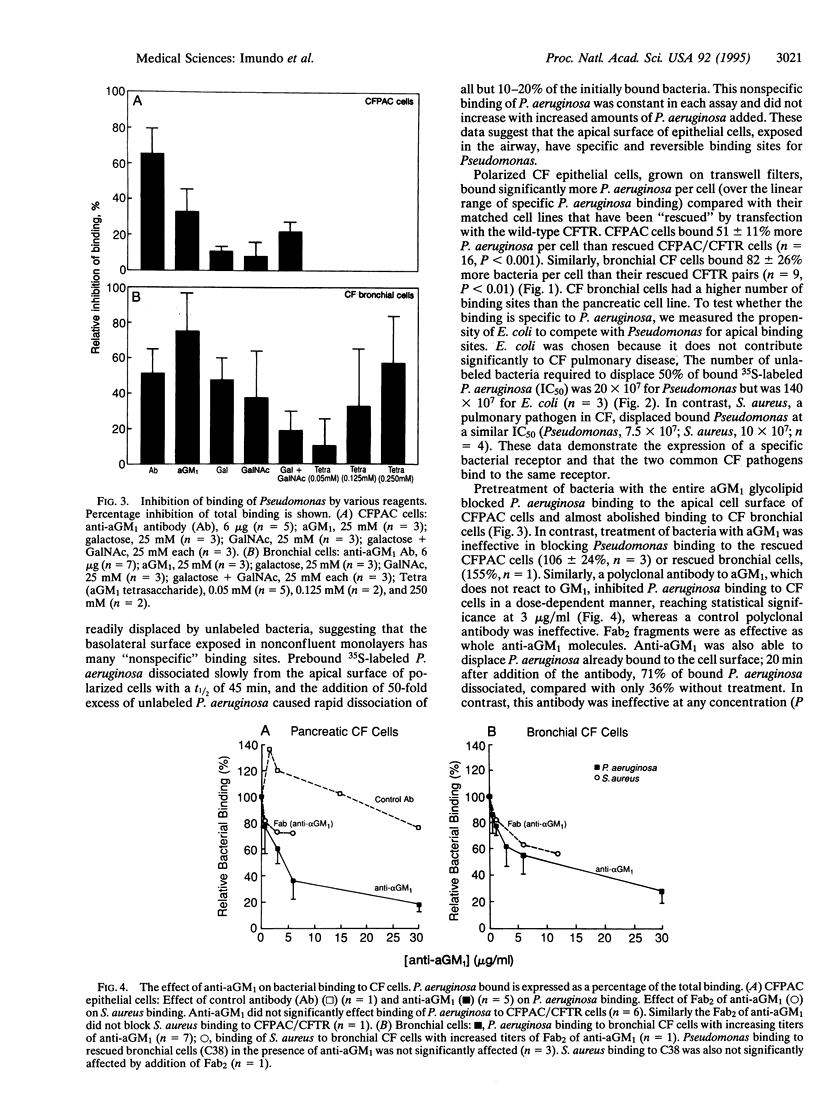
Chronic colonization and infection of the lung with Pseudomonas aeruginosa is the major cause of morbidity and mortality in cystic fibrosis (CF) patients. We found that polarized CF bronchial and pancreatic epithelia bound P. aeruginosa in a reversible and dose-dependent manner. There was significantly greater binding to CF bronchial and pancreatic cells than to their matched pairs rescued with the wild-type CF transmembrane conductance regulator. Bound P. aeruginosa were easily displaced by unlabeled P. aeruginosa but not by Escherichia coli, an organism that does not cause significant pulmonary disease in CF. In contrast, Staphylococcus aureus, a frequent pathogen in CF, could effectively displace bound P. aeruginosa from its receptor. We found undersialylation of apical proteins and a higher concentration of asialoganglioside 1 (aGM1) in apical membranes of CF compared with rescued epithelia. Incubation of P. aeruginosa with aGM1 reduced its binding, as did treatment of the epithelia with the tetrasaccharide moiety of this ganglioside (Gal beta 1-3GalNAc beta 1-4Gal beta 1-4Glc). Finally, an antibody to aGM1 effectively displaced P. aeruginosa from its binding site and blocked binding of S. aureus to CF cells but not to rescued cells. These results show that the tetrasaccharide of aGM1 is a receptor for P. aeruginosa and S. aureus and that its increased abundance in the apical membrane of CF epithelia makes it a likely contributor to the pathogenesis of bacterial infections in the CF lung.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abman S. H., Ogle J. W., Harbeck R. J., Butler-Simon N., Hammond K. B., Accurso F. J. Early bacteriologic, immunologic, and clinical courses of young infants with cystic fibrosis identified by neonatal screening. J Pediatr. 1991 Aug;119(2):211–217. doi: 10.1016/s0022-3476(05)80729-2. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Barasch J., al-Awqati Q. Defective acidification of the biosynthetic pathway in cystic fibrosis. J Cell Sci Suppl. 1993;17:229–233. doi: 10.1242/jcs.1993.supplement_17.32. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W. Biochemistry of airway mucus secretions. Fed Proc. 1980 Nov;39(13):3067–3074. [PubMed] [Google Scholar]
- Borén T., Falk P., Roth K. A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993 Dec 17;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- Crowe J. E., Jr, Murphy B. R., Chanock R. M., Williamson R. A., Barbas C. F., 3rd, Burton D. R. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1386–1390. doi: 10.1073/pnas.91.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Sonoda F., Hornick D. B. Emergence and persistence of Pseudomonas aeruginosa in the cystic fibrosis airway. Semin Respir Infect. 1992 Sep;7(3):168–178. [PubMed] [Google Scholar]
- Flotte T. R., Afione S. A., Solow R., Drumm M. L., Markakis D., Guggino W. B., Zeitlin P. L., Carter B. J. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993 Feb 15;268(5):3781–3790. [PubMed] [Google Scholar]
- Hazlett L. D., Masinick S., Barrett R., Rosol K. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect Immun. 1993 Dec;61(12):5164–5173. doi: 10.1128/iai.61.12.5164-5173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubesch P., Dörk T., Wulbrand U., Kälin N., Neumann T., Wulf B., Geerlings H., Weissbrodt H., von der Hardt H., Tümmler B. Genetic determinants of airways' colonisation with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993 Jan 23;341(8839):189–193. doi: 10.1016/0140-6736(93)90062-l. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Pittet J. F., Matthay M. A., Pier G., Grady M., Wiener-Kronish J. P. Pseudomonas aeruginosa-induced lung and pleural injury in sheep. Differential protective effect of circulating versus alveolar immunoglobulin G antibody. J Clin Invest. 1993 Sep;92(3):1221–1228. doi: 10.1172/JCI116693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Saiman L., Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993 Oct;92(4):1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. O. Immunology of cystic fibrosis. Br Med Bull. 1992 Oct;48(4):893–911. doi: 10.1093/oxfordjournals.bmb.a072584. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993 Jul 2;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- Zar H., Saiman L., Quittell L., Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995 Feb;126(2):230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]




