Abstract
BACKGROUND
On March 23, 2014, the World Health Organization (WHO) was notified of an out-break of Ebola virus disease (EVD) in Guinea. On August 8, the WHO declared the epidemic to be a “public health emergency of international concern.”
METHODS
By September 14, 2014, a total of 4507 probable and confirmed cases, including 2296 deaths from EVD (Zaire species) had been reported from five countries in West Africa — Guinea, Liberia, Nigeria, Senegal, and Sierra Leone. We analyzed a detailed subset of data on 3343 confirmed and 667 probable Ebola cases collected in Guinea, Liberia, Nigeria, and Sierra Leone as of September 14.
RESULTS
The majority of patients are 15 to 44 years of age (49.9% male), and we estimate that the case fatality rate is 70.8% (95% confidence interval [CI], 69 to 73) among persons with known clinical outcome of infection. The course of infection, including signs and symptoms, incubation period (11.4 days), and serial interval (15.3 days), is similar to that reported in previous outbreaks of EVD. On the basis of the initial periods of exponential growth, the estimated basic reproduction numbers (R0) are 1.71 (95% CI, 1.44 to 2.01) for Guinea, 1.83 (95% CI, 1.72 to 1.94) for Liberia, and 2.02 (95% CI, 1.79 to 2.26) for Sierra Leone. The estimated current reproduction numbers (R) are 1.81 (95% CI, 1.60 to 2.03) for Guinea, 1.51 (95% CI, 1.41 to 1.60) for Liberia, and 1.38 (95% CI, 1.27 to 1.51) for Sierra Leone; the corresponding doubling times are 15.7 days (95% CI, 12.9 to 20.3) for Guinea, 23.6 days (95% CI, 20.2 to 28.2) for Liberia, and 30.2 days (95% CI, 23.6 to 42.3) for Sierra Leone. Assuming no change in the control measures for this epidemic, by November 2, 2014, the cumulative reported numbers of confirmed and probable cases are predicted to be 5740 in Guinea, 9890 in Liberia, and 5000 in Sierra Leone, exceeding 20,000 in total.
CONCLUSIONS
These data indicate that without drastic improvements in control measures, the numbers of cases of and deaths from EVD are expected to continue increasing from hundreds to thousands per week in the coming months.
AS OF SEPTEMBER 14, 2014, A TOTAL OF 4507 confirmed and probable cases of Ebola virus disease (EVD), as well as 2296 deaths from the virus, had been reported from five countries in West Africa — Guinea, Liberia, Nigeria, Senegal, and Sierra Leone. In terms of reported morbidity and mortality, the current epidemic of EVD is far larger than all previous epidemics combined. The true numbers of cases and deaths are certainly higher. There are numerous reports of symptomatic persons evading diagnosis and treatment, of laboratory diagnoses that have not been included in national databases, and of persons with suspected EVD who were buried without a diagnosis having been made.1
The epidemic began in Guinea during December 2013,2 and the World Health Organization (WHO) was officially notified of the rapidly evolving EVD outbreak on March 23, 2014. On August 8, the WHO declared the epidemic to be a “public health emergency of international concern.”3 By mid-September, 9 months after the first case occurred, the numbers of reported cases and deaths were still growing from week to week despite multinational and multisectoral efforts to control the spread of infection.1 The epidemic has now become so large that the three most-affected countries — Guinea, Liberia, and Sierra Leone — face enormous challenges in implementing control measures at the scale required to stop transmission and to provide clinical care for all persons with EVD.
Because Ebola virus is spread mainly through contact with the body fluids of symptomatic patients, transmission can be stopped by a combination of early diagnosis, contact tracing, patient isolation and care, infection control, and safe burial.1 Before the current epidemic in West Africa, outbreaks of EVD in central Africa had been limited in size and geographic spread, typically affecting one to a few hundred persons, mostly in remote forested areas.4 The largest previous outbreak occurred in the districts of Gulu, Masindi, and Mbarara in Uganda.5 This outbreak, which generated 425 cases over the course of 3 months from October 2000 to January 2001,6 was controlled by rigorous application of interventions to minimize further transmission — delivered through the local health care system, with support from international partners.5,7,8
We now report on the clinical and epidemiologic characteristics of the epidemic in Guinea, Liberia, Nigeria, and Sierra Leone during the first 9 months of the epidemic (as of September, 14, Senegal had reported only a single case). We document trends in the epidemic thus far and project expected case numbers for the coming weeks if control measures are not enhanced.
METHODS
SURVEILLANCE
Full details of the methods, along with sensitivity and uncertainty analyses, are provided in Supplementary Appendix 1, available with the full text of this article at NEJM.org; a summary is provided here. Case definitions for EVD have been reported previously by the WHO.9 In brief, a suspected case is illness in any person, alive or dead, who has (or had) sudden onset of high fever and had contact with a person with a suspected, probable, or confirmed Ebola case or with a dead or sick animal; any person with sudden onset of high fever and at least three of the following symptoms: headache, vomiting, anorexia or loss of appetite, diarrhea, lethargy, stomach pain, aching muscles or joints, difficulty swallowing, breathing difficulties, or hiccupping; or any person who had unexplained bleeding or who died suddenly from an unexplained cause. A probable case is illness in any person suspected to have EVD who was evaluated by a clinician or any person who died from suspected Ebola and had an epidemiologic link to a person with a confirmed case but was not tested and did not have laboratory confirmation of the disease. A probable or suspected case was classified as confirmed when a sample from the person was positive for Ebola virus in laboratory testing.
Clinical and demographic data were collected with the use of a standard case investigation form (see Supplementary Appendix 1) on confirmed, probable, and suspected EVD cases identified through clinical care, including hospitalization, and through contact tracing in Guinea, Liberia, Nigeria, and Sierra Leone. To create the fullest possible picture of the unfolding epidemic, these data were supplemented by information collected in informal case reports, by data from diagnostic laboratories, and from burial records. The data recorded for each case included the district of residence, the district in which the disease was reported, the patient’s age, sex, and signs and symptoms, the date of symptom onset and of case detection, the name of the hospital, the date of hospitalization, and the date of death or discharge. A subgroup of case patients provided information on potentially infectious contacts with other persons who had Ebola virus disease, including possible exposure at funerals. We present here the results from analyses of detailed data on individual confirmed and probable cases recorded by each country in databases provided to the WHO as of September 14, 2014; analyses of confirmed and probable cases, together with suspected cases, are provided in Supplementary Appendix 1.
ETHICAL CONSIDERATIONS
This study is based on data collected during surveillance and response activities for EVD in Guinea, Liberia, Nigeria, and Sierra Leone. All information on individual patients has been anonymized for presentation.
CLINICAL MANIFESTATIONS AND CASE FATALITY RATE
We report on the frequency of symptoms in patients with confirmed and probable EVD cases overall and by country. We evaluated potential risk factors for a fatal outcome, including sex, age group (<15 years, 15 to 44 years, and ≥45 years), general and hemorrhagic symptoms, and occupation (whether the patient was or was not a health care worker). We performed the analysis using logistic-regression models, with data on patients for whom there was a definitive outcome (death or recovery) by August 17, 2014.
The case fatality rate was calculated as the percentage of fatal EVD cases among reported cases with a known definitive clinical outcome (see Supplementary Appendix 1). For comparison, we also calculated a case fatality rate that was based only on the ratio of reported deaths to reported cases, including in the denominator cases for which the clinical outcome is unknown.
KEY TIME PERIODS
We investigated five key time periods that characterize the progression of infection, the detection, care, and recovery or death of a person with Ebola virus disease, and the transmission of infection: the incubation period, which is the time between infection and the onset of symptoms (information that is relevant for assessing the length of time that case contacts have to be followed up); the interval from symptom onset to hospitalization (which is indicative of the infectious period in the community); the interval from hospital admission to death and the interval from hospital admission to discharge (both of which are relevant to assessing the demand for beds in relation to hospital capacity); the serial interval, which is defined as the interval between disease onset in an index case patient and disease onset in a person infected by that index case patient; and the generation time, which is the time between infection in an index case patient and infection in a patient infected by that index case patient (required to estimate the reproduction number, or R, of the epidemic).
The incubation period was estimated retrospectively (by having patients with confirmed cases recall the likely source of infection), with a distinction made between persons with single exposures and those with multiple exposures. In the case of multiple exposures, all the times of exposure were used to fit a parametric distribution (see Supplementary Appendix 1 for a sensitivity analysis). The interval from symptom onset to hospitalization is summarized as the mean, rather than the median, number of days to reflect the average person-days of infectiousness in the community. The mean duration of hospitalization was estimated as the average number of days from hospitalization to discharge and the average number of days from hospitalization to death, weighted by the proportion of patients who died. For each statistic we calculated the mean, median, and interquartile range and fitted a gamma probability distribution to model the variation among persons (see the results in Supplementary Appendix 1). Separate estimates were obtained for health care workers and for all other adults. The serial interval was estimated from a subgroup of patients for whom information was available on the time of symptom onset in known or suspected chains of transmission. For EVD, we expect the generation time distribution to be nearly identical to the serial interval distribution (result derived in Supplementary Appendix 1).
QUANTIFICATION OF THE SPREAD OF INFECTION AND PROJECTION OF FUTURE CASES
The basic reproduction number (R0) is the average number of secondary cases that arise when one primary case is introduced into an uninfected population. These secondary cases arise after a period measured by the serial interval or by the generation time. When R0 is greater than 1, infection may spread in the population, and the rate of spread is higher with increasingly high values of R0. The doubling time (the time required for the incidence to double) was estimated on the basis of the reproduction number and the serial interval.11 After the early phase of exponential growth in case numbers, once infection has become established, the number of people still at risk declines, so the reproduction number falls from its maximum value of R0 to a smaller, net reproduction number, Rt. When Rt falls below 1, infection cannot be sustained. Estimates of R0 and Rt help in evaluating the magnitude of the effort required to control the disease, the way in which transmission rates have fluctuated through time, and the effectiveness of control measures as they are implemented.
We estimated Rt over time from the time series of incidence of cases (i.e., a plot of the number of new cases per week over the course of the epidemic) and from our estimate of the serial interval distribution.12 We then estimated R0 for the early stages of the epidemic, when transmission rates were at their highest, on the basis of the date of symptom onset. As described in Supplementary Appendix 1, average estimates of Rt for the period from July 28 to September 7, 2014, which were made on the basis of the date of report to facilitate comparison with future cases, were used to project future cases, allowing for both uncertainty in the estimates of Rt and stochastic variability in the transmission process.
RESULTS
SCALE OF THE EPIDEMIC
A total of 4507 confirmed and probable EVD cases were reported to the WHO between December 30, 2013, and September 14, 2014 — a 37-week period. A total of 718 confirmed and probable cases and 289 deaths were reported in the week of September 8 through September 14 alone. The numbers of confirmed and probable cases reported by each country over time are shown in Figures 1 and 2. Detailed information was available on 3343 confirmed and 667 probable cases; these cases were used in all our analyses, with the exception of projections (results of analyses based on confirmed, probable, and suspected cases are provided in Supplementary Appendix 1). The median age of persons with EVD was 32 years (interquartile range, 21 to 44), and there were no significant differences in the age distribution of persons with EVD among countries. The majority of persons with EVD (60.8%) were between 15 and 44 years of age (this age group makes up only 44% of the population) (Table 1). There were also no significant differences among countries in the total numbers of male and female persons with EVD reported (49.9% of the total were male patients; within-country differences have not yet been fully investigated). EVD has taken a heavy toll among health care workers in Guinea, Liberia, and Sierra Leone. By September 14, a total of 318 cases, including 151 deaths, had been reported among health care workers.
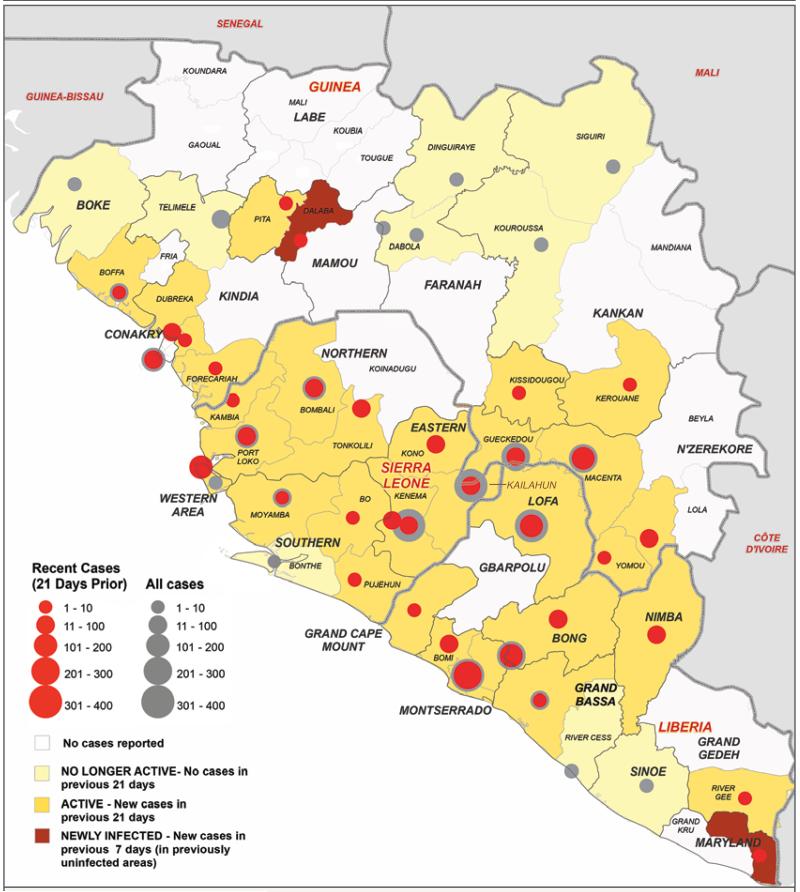
Figure 1. Districts Affected by Ebola Virus Disease in Three Countries in Africa.
The map shows the districts that have been affected by Ebola virus disease in Guinea, Liberia, and Sierra Leone. Gray circles indicate the total numbers of confirmed and probable Ebola cases reported in each affected district, and red circles the number reported during the 21 days leading up to September 14, 2014.
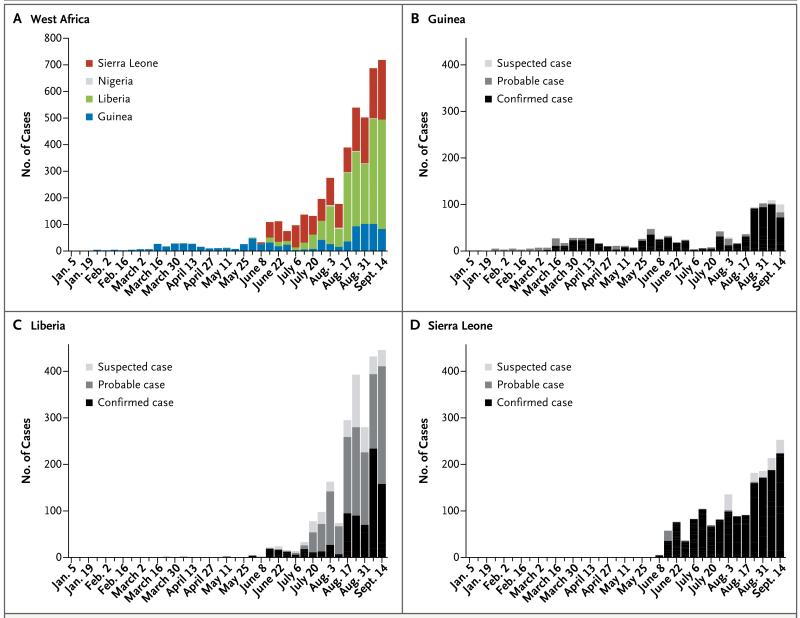
Figure 2. Weekly Incidence of Confirmed, Probable, and Suspected Ebola Virus Disease Cases.
Shown is the weekly incidence of confirmed, probable, and suspected EVD cases, according to actual or inferred week of symptom onset. A suspected case is illness in any person, alive or dead, who has (or had) sudden onset of high fever and had contact with a person with a suspected, probable, or confirmed Ebola case or with a dead or sick animal; any person with sudden onset of high fever and at least three of the following symptoms: headache, vomiting, anorexia or loss of appetite, diarrhea, lethargy, stomach pain, aching muscles or joints, difficulty swallowing, breathing difficulties, or hiccupping; or any person who had unexplained bleeding or who died suddenly from an unexplained cause. A probable case is illness in any person suspected to have EVD who was evaluated by a clinician or any person who died from suspected Ebola and had an epidemiologic link to a person with a confirmed case but was not tested and did not have laboratory confirmation of the disease. A probable or suspected case was classified as confirmed when a sample from the person was positive for Ebola virus in laboratory testing.
Table 1. Demographic Characteristics and Signs and Symptoms in Confirmed and Probable Ebola Case Patients with a Definitive Clinical Outcome in Guinea, Liberia, Nigeria, and Sierra Leone*.
| Variable | All Patients | Patients Who Died | Patients Who Recovered | Odds Ratio (95% CI)† |
|---|---|---|---|---|
| no./total no. (%) | ||||
| Demographic characteristics | ||||
| Male sex | 685/1415 (48.4) | 515/1056 (48.8) | 170/359 (47.4) | 0.93 (0.73–1.19) |
| Age group | ||||
| <15 yr | 190/1378 (13.8) | 145/1021 (14.2) | 45/357 (12.6) | 1.18 (0.83–1.71) |
| 15–44yr | 838/1378 (60.8) | 577/1021 (56.5) | 261/357 (73.1) | 0.48 (0.36–0.62) |
| ≥45 yr | 350/1378 (25.4) | 299/1021 (29.3) | 51/357 (14.3) | 2.47 (1.79–3.46) |
| Health care worker | 158/1429 (11.1) | 112/1067 (10.5) | 46/362 (12.7) | 0.86 (0.60–1.27) |
| Signs and symptoms | ||||
| General symptoms | ||||
| Fever‡ | 1002/1151 (87.1) | 746/846 (88.2) | 256/305 (83.9) | 1.34 (0.92–1.95) |
| Fatigue | 866/1133 (76.4) | 633/829 (76.4) | 233/304 (76.6) | 0.94 (0.68–1.28) |
| Loss of appetite | 681/1055 (64.5) | 498/778 (64.0) | 183/277 (66.1) | 0.92 (0.69–1.23) |
| Vomiting | 753/1114 (67.6) | 566/816 (69.4) | 187/298 (62.8) | 1.19 (0.89–1.59) |
| Diarrhea | 721/1099 (65.6) | 555/813 (68.3) | 166/286 (58.0) | 1.42 (1.06–1.89) |
| Headache | 553/1035 (53.4) | 407/757 (53.8) | 146/278 (52.5) | 1.03 (0.78–1.36) |
| Abdominal pain | 439/992 (44.3) | 311/715 (43.5) | 128/277 (46.2) | 0.85 (0.64–1.13) |
| Muscle pain | 385/990 (38.9) | 293/728 (40.2) | 92/262 (35.1) | 1.24 (0.92–1.67) |
| Joint pain | 374/950 (39.4) | 283/695 (40.7) | 91/255 (35.7) | 1.32 (0.98–1.80) |
| Chest pain | 254/686 (37.0) | 196/488 (40.2) | 58/198 (29.3) | 1.53 (1.07–2.20) |
| Cough | 194/655 (29.6) | 150/462 (32.5) | 44/193 (22.8) | 1.74 (1.18–2.61) |
| Difficulty breathing | 155/665 (23.3) | 123/472 (26.1) | 32/193 (16.6) | 1.68 (1.10–2.63) |
| Difficulty swallowing | 169/514 (32.9) | 138/375 (36.8) | 31/139 (22.3) | 2.22 (1.41–3.59) |
| Conjunctivitis | 137/658 (20.8) | 109/465 (23.4) | 28/193 (14.5) | 2.03 (1.29–3.29) |
| Sore throat | 102/467 (21.8) | 82/339 (24.2) | 20/128 (15.6) | 1.94 (1.13–3.46) |
| Confusion | 84/631 (13.3) | 68/446 (15.2) | 16/185 (8.6) | 2.00 (1.14–3.71) |
| Hiccups | 108/947 (11.4) | 91/699 (13.0) | 17/248 (6.9) | 2.15 (1.27–3.82) |
| Jaundice | 65/627 (10.4) | 52/443 (11.7) | 13/184 (7.1) | 1.83 (0.99–3.63) |
| Eye pain | 48/622 (7.7) | 39/438 (8.9) | 9/184 (4.9) | 1.95 (0.95–4.40) |
| Rash | 37/642 (5.8) | 30/453 (6.6) | 7/189 (3.7) | 1.90 (0.86–4.83) |
| Coma or unconsciousness | 37/627 (5.9) | 34/445 (7.6) | 3/182 (1.6) | 4.59 (1.61–19.34) |
| Unexplained bleeding | 168/932 (18.0) | 140/693 (20.2) | 28/239 (11.7) | 1.83 (1.20–2.90) |
| Hematemesis | 26/670 (3.9) | 20/503 (4.0) | 6/167 (3.6) | 1.07 (0.44–3.01) |
| Blood in stool | 48/843 (5.7) | 35/614 (5.7) | 13/229 (5.7) | 0.98 (0.52–1.96) |
| Bleeding gums | 19/837 (2.3) | 18/608 (3.0) | 1/229 (0.4) | 6.69 (1.35–121.32) |
| Bloody nose | 16/836 (1.9) | 15/610 (2.5) | 1/226 (0.4) | 8.02 (1.54–148.62) |
| Bloody cough | 20/831 (2.4) | 16/605 (2.6) | 4/226 (1.8) | 1.63 (0.58–5.82) |
| Other bleeding | 8/657 (1.2) | 5/493 (1.0) | 3/164 (1.8) | 0.45 (0.11–2.23) |
| Bleeding at injection site | 20/833 (2.4) | 19/605 (3.1) | 1/228 (0.4) | 6.51 (1.32–118.04) |
| Blood from vagina§ | 14/431 (3.2) | 13/290 (4.5) | 1/126 (0.8) | 6.0 (1.11–112.4) |
| Blood in urine | 10/827 (1.2) | 9/601 (1.5) | 1/226 (0.4) | 5.14 (0.90–98.73) |
| Bleeding under skin | 5/827 (0.6) | 5/604 (0.8) | 0/223 | NA |
Data are as of September 14, 2014. Patients with date of onset up to August 17, 2014, were included. Total numbers are the numbers of patients with data on the variable in question. NA denotes not applicable.
Odds ratios are adjusted for country. CI denotes confidence interval.
Fever was defined as a body temperature above 38°C; however, in practice, health care workers at the district level often do not have a medical thermometer and simply ask whether the person’s body temperature is more elevated than usual.
Percentages reflect only female patients.
GEOGRAPHIC ORIGIN AND THE SPREAD OF INFECTION
In December 2013, the first cases occurred in Guéckédou and Macenta districts, the focus of the epidemic in Guinea. During March 2014, a rise in the numbers of cases in these two districts, in addition to the first reports from Lofa and other districts in Liberia, was followed by the discovery of cases in the capital, Conakry. A second increase in case incidence in Guinea — first in Guéckédou and Macenta and then in the capital — occurred in May and June.
During May, the focus of the epidemic in Guinea expanded to the neighboring districts of Kenema and Kailahun in Sierra Leone, and in June further cases were reported in Lofa district in Liberia. These five districts have remained the focus of transmission in the border areas of the three countries. From July onward, there were sharp increases in case numbers at the epidemic foci in all three countries, at other sites away from the epicenter, and in the capital cities of Conakry, Freetown, and Monrovia (Fig. 1, and animated map and timeline at NEJM.org). However, although EVD has spread to many parts of Guinea, Liberia, and Sierra Leone, it has not been reported in all districts in the countries: among the total of 67 districts in the three countries, only 43 have reported one or more confirmed, probable, or suspected cases, and more than 90% of cases have been reported from just 14 districts.
CLINICAL MANIFESTATIONS AND CASE FATALITY RATE
Table 1 provides information on demographic characteristics and symptom frequency in patients with confirmed or probable EVD with a definitive outcome in Guinea, Liberia, Nigeria, and Sierra Leone. The most common symptoms reported between symptom onset and case detection included fever (87.1%), fatigue (76.4%), loss of appetite (64.5%), vomiting (67.6%), diarrhea (65.6%), headache (53.4%), and abdominal pain (44.3%). Specific hemorrhagic symptoms were rarely reported (in <1% to 5.7% of patients). “Unexplained bleeding,” however, was reported in 18.0% of cases. These patterns are similar in each country (see Supplementary Appendix 1).
Assessing the case fatality rate during this epidemic is complicated by incomplete information on the clinical outcomes of many cases, both detected and undetected. Estimates of the case fatality rate (Table 2) derived by calculating the ratio of all reported deaths to all reported cases to date are low in comparison with historical outbreaks and are highly variable among the affected countries. However, estimating the case fatality rate using only the 46% of cases with definitive recorded clinical outcomes gives higher estimates that show no significant variation among countries (Table 2). This analysis shows that by September 14, a total of 70.8% (95% confidence interval [CI], 68.6 to 72.8) of case patients with definitive outcomes have died, and this rate was consistent among Guinea, Liberia, and Sierra Leone (Table 2). The case fatality rate in Nigeria was lower (45.5%), though this estimate is based on only 11 recent cases. The case fatality rate among hospitalized case patients was 64.3% (95% CI, 61.5 to 67.0) lower than that among all patients with definitive outcomes and was consistent among countries. The case fatality rate among health care workers ranged from 56.1% (95% CI, 41.0 to 70.1) in Guinea to 80.0% (95% CI, 68.7 to 87.9) in Liberia (Table 2). Risk factors for a fatal outcome, after adjustment for country, are provided in Table 1. Significant risk factors for death include an age of 45 years or older as compared with 44 years of age or younger (odds ratio, 2.47; 95% CI, 1.79 to 3.46) and a number of general symptoms (diarrhea, conjunctivitis, difficulty breathing or swallowing, confusion or disorientation, and coma) and hemorrhagic symptoms (unexplained bleeding, bleeding gums, bloody nose, bleeding at the injection site, and bleeding from the vagina) (odds ratios and 95% confidence intervals for these factors are provided in Table 1).
Table 2. Estimates of Epidemiologic Variables for Confirmed and Probable Ebola Cases, According to Country, as of September 14, 2014*.
| Variable | All Countries | Guinea | Liberia | Nigeria | Sierra Leone | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| no. of days | no. of patients with data | no. of days | no. of patients with data | no. of days | no. of patients with data | no. of days | no. of patients with data | no. of days | no. of patients with data | |
| Incubation period | ||||||||||
| Single-day exposures | ||||||||||
| Observed† | 9.4±7.4 | 500 | 10.7±8.7 | 35 | 9.5±6.6 | 259 | NC | <10 | 9.0±8.1 | 201 |
| Fitted‡ | 9.1±7.3 | 500 | 9.9±9.8 | 35 | 9.4±6.7 | 259 | NC | <10 | 8.5±7.6 | 201 |
| Multi-day exposures | ||||||||||
| Observed† | 11.4±NA | 155 | 10.9±NA | 20 | 11.7±NA | 79 | NC | <10 | 10.8±NA | 48 |
| Fitted‡ | 9.7±5.5 | 155 | 8.3±4.5 | 20 | 9.9±5.7 | 79 | NC | <10 | 9.9±5.6 | 48 |
| Serial interval§ | ||||||||||
| Observed | 15.3±9.1 | 92 | 19.0±11.0 | 40 | 13.1±6.6 | 26 | NC | <10 | 11.6±5.6 | 25 |
| Fitted¶ | 15.3±9.3 | 92 | 19.0±11.2 | 40 | 13.1±7.8 | 26 | NC | <10 | 11.6±6.3 | 25 |
| R0 ∥ | ||||||||||
| Mean (95% CI) | — | 1.71 (1.44–2.01) | 1.83 (1.72–1.94) | 1.2 (0.67–1.96) | 2.02(1.79–2.26) | |||||
| Doubling time — days (95% CI) | — | 17.53 (13.18–26.64) | 15.78 (14.4–17.37) | 59.75 (13.27–∞) | 12.84 (10.92–15.66) | |||||
| R ** | ||||||||||
| Mean (95% CI) | — | 1.81 (1.60–2.03) | 1.51 (1.41–1.60) | 1.38(1.27–1.51) | ||||||
| Doubling time — days (95% CI) | — | 15.7(12.9–20.3) | 23.6 (20.2–28.2) | NC | 30.2(23.6–42.3) | |||||
| Interval from symptom onset | ||||||||||
| To hospitalization | 5.0±4.7 | 1135 | 5.3±4.3 | 484 | 4.9±5.1 | 245 | 4.1±1.4 | 11 | 4.6±5.1 | 395 |
| To hospital discharge | 16.4±6.5 | 267 | 16.3±6.1 | 152 | 15.4±8.2 | 41 | NC | <10 | 17.2±6.2 | 70 |
| To death | 7.5±6.8 | 594 | 6.4±5.3 | 248 | 7.9±8.0 | 212 | NC | <10 | 8.6±6.9 | 128 |
| To WHO notification | 6.1±8.5 | 2185 | 7.5±10.4 | 743 | 6.0±8.7 | 797 | 3.9±2.3 | 11 | 4.5±5.0 | 634 |
| Interval from WHO notification | ||||||||||
| To hospital discharge | 11.8±7.2 | 312 | 11.1±5.8 | 164 | 11±8.0 | 41 | NC | <10 | 12.7±8.4 | 102 |
| To death | −3.0±13.8 | 584 | −4.4±14.4 | 300 | −1.8±13.6 | 221 | NC | <10 | −1.6±9.2 | 58 |
| Interval from hospitalization | ||||||||||
| To hospital discharge | 11.8±6.1 | 290 | 11±5.4 | 159 | 12.8±8.1 | 40 | NC | <10 | 12.4±5.8 | 86 |
| To death | 4.2±6.4 | 121 | 2.5±3.4 | 36 | 4.5±6.0 | 63 | NC | <10 | 4.4±6.0 | 17 |
| Duration of hospital stay — days†† | 6.42 | 4.99 | 6.72 | NC | 6.88 | |||||
| rate (95% CI) | no. of patients with data | rate (95% CI) | no. of patients with data | rate (95% CI) | no. of patients with data | rate (95% CI) | no. of patients with data | rate (95% CI) | no. of patients with data | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case fatality rate | ||||||||||
| All cases, based on current | 37.7 (36.1–39.2) | 3747 | 57.5 (53.7–61.1) | 677 | 34.7 (32.4–37.1) | 1616 | 40.0 (19.8–64.3) | 15 | 31.6 (29.3–34.1) | 1439 |
| status | ||||||||||
| All cases, based on definitive | 70.8 (68.6–72.8) | 1737 | 70.7 (66.7–74.3) | 542 | 72.3 (68.9–75.4) | 739 | 45.5 (21.3–72.0) | 11 | 69.0 (64.5–73.1) | 445 |
| outcome | ||||||||||
| Before August 18 | 71.3 (68.7–73.7) | 1244 | 68.7 (64.3–72.8) | 454 | 79.8 (75.7–83.4) | 416 | 50.0 (23.7–76.3) | 10 | 65.4 (60.4–70.1) | 364 |
| August 18–September 14 | 59.9 (54.7–64.9) | 354 | 80.7 (71.2–87.6) | 88 | 41.1 (34.3–48.2) | 190 | NC | <10 | 84.0 (74.1–90.6) | 75 |
| All hospitalized cases, based on definitive outcome | 64.3 (61.5–67.0) | 1153 | 64.7 (60.1–68.9) | 450 | 67.0 (62.0–71.7) | 361 | 40.0 (16.8–68.7) | 10 | 61.4 (56.1–66.5) | 332 |
| According to sex | ||||||||||
| Male | 72.2 (69.1–75.1) | 874 | 68.5 (62.6–73.9) | 254 | 74.9 (70.4–79.0) | 395 | NC | <10 | 71.9 (65.7–77.5) | 221 |
| Female | 69.9 (66.7–73.0) | 818 | 72.7 (67.3–77.6) | 286 | 71.6 (66.4–76.3) | 317 | NC | <10 | 64.4 (57.7–70.6) | 208 |
| According to age group | ||||||||||
| <15 yr | 73.4 (67.2–78.8) | 218 | 78.1 (67.3–86.0) | 73 | 70.7 (60.1–79.5) | 82 | NC | <10 | 71.4 (59.3–81.1) | 63 |
| 15–44 yr | 66.1 (63.1–69.0) | 1012 | 64.9 (59.5–69.9) | 319 | 70.6 (66.1–74.8) | 422 | NC | <10 | 61.4 (55.4–67.0) | 264 |
| ≥45 yr | 80.4 (76.2–84.0) | 398 | 78.6 (71.1–84.6) | 140 | 81.1 (74.4–86.4) | 164 | NC | <10 | 82.2 (73.1–88.8) | 90 |
| According to occupation | ||||||||||
| Health care worker | 69.4 (62.1–75.8) | 170 | 56.1 (41.0–70.1) | 41 | 80.0 (68.7–87.9) | 65 | NC | <10 | 68.4 (55.5–79.0) | 57 |
| Non–health care worker | 70.9 (68.6–73.1) | 1567 | 71.9 (67.8–75.6) | 501 | 71.5 (68.0–74.8) | 674 | NC | <10 | 69.1 (64.3–73.5) | 388 |
Plus–minus values are means ±SD. NA denotes not available, NC not calculated, and WHO World Health Organization.
Contacts on day 0 (i.e., on the day of symptom onset) were excluded.
Contacts on day 0 (i.e., on the day of symptom onset) were excluded. Gamma probability distributions were fitted to confirmed and probable cases.
The serial interval is the interval between disease onset in an index case patient and disease onset in a person infected by that index case patient. In this category, the number of patients with data is the number of epidemiologically linked pairs in which the later case patient reported only one direct contact.
Gamma probability distributions were fitted to confirmed and probable cases.
The basic reproduction number (Ro) is the average number of secondary cases that arise when one primary case is introduced into an uninfected population. We estimated the Ro and associated mean doubling time, using a serial interval of 15.3 days, for the period up to March 30, 2014, for Guinea; up to August 24, 2014, for Liberia and Nigeria; and up to July 6, 2014, for Sierra Leone. This number was estimated for individual countries only and not for the combined data.
We estimated R, the mean value of Rt, (the estimated net reproduction number), and associated mean doubling time, using a serial interval of 15.3 days, for the period of July 21 to August 31, 2014. This number was estimated for individual countries only and not for the combined data.
The mean duration of hospital stay was calculated as the weighted average of the observed means from the hospitalization-to-discharge and hospitalization-to-death distributions. This variable was not calculated in Nigeria because there were fewer than 10 case patients with data.
KEY TIME PERIODS
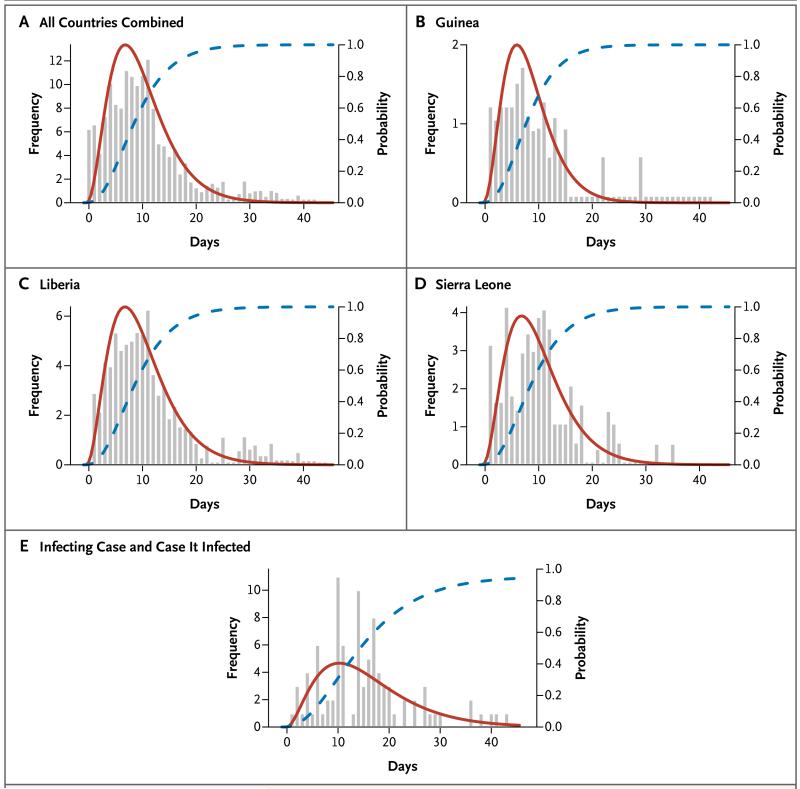
The mean incubation period was 11.4 days (Table 2 and Fig. 3A), and did not vary by country (Fig. 3B, 3C, and 3D). Approximately 95% of the case patients had symptom onset within 21 days after exposure (Fig. 3A), which is the recommended period for follow-up of contacts. The estimated mean (±SD) serial interval was 15.3±9.3 days (Table 2 and Fig. 3E), which is the same as the estimated mean generation time (see Supplementary Appendix 1). The mean time from the onset of symptoms to hospitalization, a measure of the period of infectiousness in the community, was 5.0±4.7 days (Table 2), and was no shorter for health care workers than for other case patients. The mean time to death after admission to the hospital was 4.2±6.4 days, and the mean time to discharge was 11.8±6.1 days. The mean length of stay in hospital was 6.4 days in Guinea, Liberia, and Sierra Leone (Table 2).
Figure 3. Time between Exposure and Disease Onset.
Panel A through D show the observed times (>0) between exposure and disease onset for all countries, Guinea, Liberia, and Sierra Leone, respectively, including only cases with multiple exposure days (histograms in gray), best-fit (gamma) probability density function (red curves) and cumulative distribution for the incubation period (blue curves). Panel E shows the observed times between disease onset in an index case patient and disease onset in the person infected by the index case patient (histograms in gray) and best-fit (gamma) probability density function (red curve) and cumulative distribution (blue curve) for the serial interval.
QUANTIFICATION OF THE SPREAD OF INFECTION AND PROJECTION OF FUTURE CASES
Estimates of the basic reproduction number, R0,were 1.71 (95% CI, 1.44 to 2.01) for Guinea, 1.83 (95% CI, 1.72 to 1.94) for Liberia, 1.20 (95% CI, 0.67 to 1.96) for Nigeria, and 2.02 (95% CI, 1.79 to 2.26) for Sierra Leone (Table 2, and Fig. S7 in Supplementary Appendix 1). Although R0 reflects the maximum potential for growth in case incidence, Figure S7 in Supplementary Appendix 1 shows the variation in the estimated net reproduction number, Rt, during the course of the epidemic. Between March and July 2014, the Rt for Guinea fluctuated around the threshold value of 1 but appeared to increase again in August, reflecting the rise in case incidence in Macenta district. In Sierra Leone, the value of Rt dropped between June and August as the case incidence stabilized in Kenema and Kailahun. In Liberia, the Rt remained above 1 for most of the period between March and August, reflecting the consistent increase in case incidence (Fig. S9) in that country.
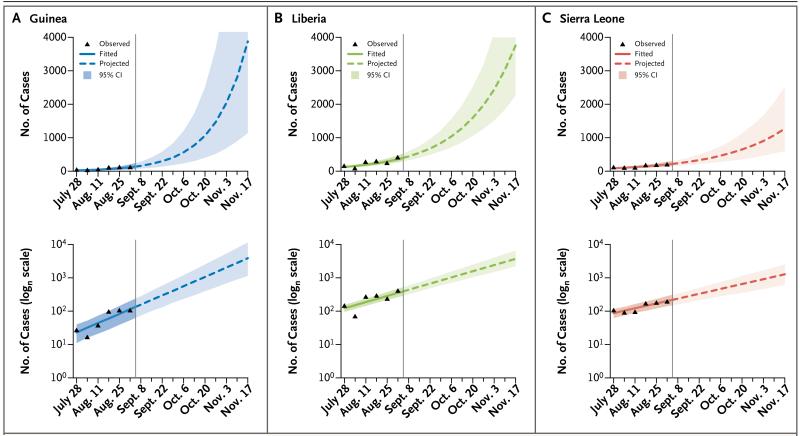
The growing numbers of cases reported from Guinea, Liberia, and Sierra Leone in August and early September suggest that the Rt remains above 1 in a still-expanding epidemic (reliable estimates of Rt could be obtained only to early September owing to reporting delays). As of September 14, the doubling time of the epidemic was 15.7 days in Guinea, 23.6 days in Liberia, and 30.2 days in Sierra Leone (Table 2). We estimate that, at the current rate of increase, assuming no changes in control efforts, the cumulative number of confirmed and probable cases by November 2 (the end of week 44 of the epidemic) will be 5740 in Guinea, 9890 in Liberia, and 5000 in Sierra Leone, exceeding 20,000 cases in total (Fig. 4, and Table S8 in Supplementary Appendix 2). The true case load, including suspected cases and undetected cases, will be higher still.
Figure 4. Observed and Projected Case Incidence.
Observed and projected weekly case incidence in Guinea (Panel A), Liberia (Panel B), and Sierra Leone (Panel C) are shown on linear (upper panels) and logarithmic (lower panels) scales
DISCUSSION
Although the current epidemic of EVD in West Africa is unprecedented in scale, the clinical course of infection and the transmissibility of the virus are similar to those in previous EVD outbreaks. The incubation period, duration of illness, case fatality rate, and R0 are all within the ranges reported for previous EVD epidemics.7,13-18 Our estimates of R0 are similar to other recent estimates for this West Africa epidemic.19-23 The combination of signs and symptoms recorded between symptom onset and clinical presentation is also similar to that in other reports.14,17,24-26 We infer that the present epidemic is exceptionally large, not principally because of the biologic characteristics of the virus, but rather because of the attributes of the affected populations and because control efforts have been insufficient to halt the spread of infection.
Certain characteristics of the affected populations may have led to the rapid geographic dissemination of infection. The populations of Guinea, Liberia, and Sierra Leone are highly interconnected, with much cross-border traffic at the epicenter and relatively easy connections by road between rural towns and villages and between densely populated national capitals. The large intermixing population has facilitated the spread of infection, but a large epidemic was not inevitable. In Nigeria, the number of cases has so far been limited, despite the introduction of infection into the large cities of Lagos (approximately 20 million people) and Port Harcourt (>1 million people). The critical determinant of epidemic size appears to be the speed of implementation of rigorous control measures.
Previous experience with EVD outbreaks, though they have been limited in size and geographic spread, suggests that transmission can be interrupted, and case incidence reduced, within 2 to 3 weeks after the introduction of control measures.1,5,7,14-17,24,27-31 This view is re-inforced by the estimates of case reproduction number presented in this analysis. We estimate the R0 to have varied between 1.71 (upper boundary of the 95% confidence interval, 2.01) in Guinea to 2.02 (upper boundary of the 95% confidence interval, 2.26) in Sierra Leone. This means that transmission has to be a little more than halved to achieve control of the epidemic and eventually to eliminate the virus from the human population. Considering the prospects for a novel Ebola vaccine, an immunization coverage exceeding 50% would have the same effect. Greater reductions in transmission would, of course, be desirable, but minimum requirements for the containment of EVD are far less severe than for the containment of more contagious diseases, such as measles. Between March and July 2014, the reproduction number in Guinea fluctuated around the threshold value of 1, suggesting that modest further intervention efforts at that point could have achieved control.
The analyses in this paper can be used to inform recommendations regarding control measures. The measured duration of the incubation period, and its variation, imply that the advice to follow case contacts for 21 days1 is appropriate. To curtail transmission in the community, the period from symptom onset to hospitalization (a mean of 5 days but a maximum of >40 days) clearly needs to be reduced. Surprisingly, the mean was not shorter among health care workers, who are at risk both of acquiring and transmitting the infection to others. The average length of hospital stay of about 1 week (6.4 days) means that the number of beds required to treat EVD patients is roughly equal to the rising weekly case incidence. Even without allowing for underreporting, 995 patients with confirmed, probable, or suspected infection were known to need clinical care in the week of September 8 through 14 alone, which far exceeds the present bed capacity in Guinea, Liberia, and Sierra Leone (approximately 610 beds in total).
The data used in these analyses were collected in the field by various field teams across Guinea, Liberia, Nigeria, and Sierra Leone. Although they provide an excellent opportunity to better understand the current EVD epidemic in Africa, they understate the magnitude of the problem. It is likely that many cases have not been detected, and for those cases that have been reported, case records are often incomplete. Therefore, interpretation of the available case data requires care. We recognize, however, that data are being collected under extreme conditions, and the top priorities are patient care, contact tracing, and limiting transmission in the community, rather than epidemiologic investigations. In addition, in this initial assessment it was not possible to consider all the sources of heterogeneity (e.g., geographic and health care-related) affecting the development of this epidemic. Thus the future projections provided here should be regarded as indicative of likely future trends more than precise predictions. Despite these limitations and the resulting uncertainties, the results presented here help us to understand the spread of infection and the potential for control.
Some details of the current analysis remain to be confirmed by further investigation. For example, our estimate of 15.3 days for the serial interval is slightly longer than past estimates.32,33 This may reflect the difficulties of collecting temporally unbiased data on exposure through contact tracing, either in the current outbreak or during previous outbreaks. Alternatively, a longer serial interval may indicate that case isolation has been less effective in the current epidemic, resulting in a higher proportion of transmission events occurring late in the course of illness.
Case fatality is among the most important topics for further investigation. Our estimates of case fatality are consistent in Guinea (70.7%), Liberia (72.3%), and Sierra Leone (69.0%) when estimates are derived with data only for patients with recorded definitive clinical outcomes (1737 patients). Estimates for hospitalized patients with recorded definitive clinical outcomes are also consistent across countries but are lower than those for all patients with definitive clinical outcomes. In contrast, simply taking the ratio of reported deaths to reported cases gives estimates that differ among countries (Table 2). These discrepancies perhaps reflect the challenges of clinical follow-up and data capture. The lower case fatality rate among hospitalized patients than among all persons with EVD could indicate that hospitalization increased survival, that cases of EVD in nonhospitalized persons were more likely to be detected if they were fatal, or that some persons died before they could be admitted to the hospital. In each of the countries studied, the case fatality rate is lowest among persons 15 to 44 year of age, and highest among persons 45 years of age or older, and some limited variation in the case fatality rate among health care workers was observed among countries. The reasons for this variation are not yet known. Moreover, the case fatality rate among hospitalized patients may differ from that among patients who are never seen by a physician. Liberia has reported an unusually high proportion of deaths among patients with suspected (but not probable or confirmed) EVD cases (58% [440 of 754 patients]), as compared with Guinea (13% [4 of 30 patients]) and Sierra Leone (35% [74 of 213 patients]). The implication is that many true EVD case patients in Liberia may have died before receiving a definitive diagnosis.
Notwithstanding the geographic variation in case incidence within and among Guinea, Liberia, and Sierra Leone, the current epidemiologic outlook is bleak. Forward projections suggest that unless control measures — including improvements in contact tracing, adequate case isolation, increased capacity for clinical management, safe burials, greater community engagement, and support from international partners — improve quickly, these three countries will soon be reporting thousands of cases and deaths each week, projections that are similar to those of the Centers for Disease Control and Prevention. Experimental therapeutics and vaccines offer promise for the future but are unlikely to be available in the quantities needed to make a substantial difference in control efforts for many months, even if they are proved to be safe and effective. Furthermore, careful assessment of the most effective means of utilizing such interventions (e.g., vaccination or treatment of contacts versus health care workers) will be required while stocks remain limited. For the medium term, at least, we must therefore face the possibility that EVD will become endemic among the human population of West Africa, a prospect that has never previously been contemplated. The risk of continued epidemic expansion and the prospect of endemic EVD in West Africa call for the most forceful implementation of present control measures and for the rapid development and deployment of new drugs and vaccines.
Supplementary Material
Acknowledgments
Supported by the Medical Research Council, the Bill and Melinda Gates Foundation, the Models of Infectious Disease Agent Study of the National Institute of General Medical Sciences (National Institutes of Health), the Health Protection Research Units of the National Institute for Health Research, European Union PREDEMICS consortium, Wellcome Trust, and Fogarty International Center.
We thank Caitlin Collins for help with data management.
Appendix
The authors (and members of the WHO Ebola Response team who contributed to this article, in alphabetic order) are as follows: Bruce Aylward, M.D., M.P.H., Philippe Barboza, M.P.H., Luke Bawo, B.Pharm., M.P.H., Eric Bertherat, M.D., Pepe Bilivogui, Isobel Blake, Ph.D., Rick Brennan, Sylvie Briand, M.D., Jethro Magwati Chakauya, Kennedy Chitala, Roland M. Conteh, Anne Cori, Ph.D., Alice Croisier, M.D., Jean-Marie Dangou, Boubacar Diallo, M.D., Christl A. Donnelly, Sc.D., Christopher Dye, D.Phil., Tim Eckmanns, Neil M. Ferguson, D.Phil., Pierre Formenty, D.V.M., M.P.H., Caroline Fuhrer, M.Sc., Keiji Fukuda, Tini Garske, Ph.D., Alex Gasasira, M.B., Ch.B., M.P.H., Stephen Gbanyan, Peter Graaff, M.Sc., M.B.A., Emmanuel Heleze, Amara Jambai, Thibaut Jombart, Ph.D., Francis Kasolo, Albert Mbule Kadiobo, Sakoba Keita, Daniel Kertesz, Moussa Koné, Chris Lane, Jered Markoff, B.B.A., Moses Massaquoi, Harriet Mills, Ph.D., John Mike Mulba, Emmanuel Musa, Joel Myhre, M.A., Abdusalam Nasidi, Eric Nilles, M.D., Pierre Nouvellet, Ph.D., Deo Nshimirimana, Isabelle Nuttall, M.D., M.P.H., Tolbert Nyenswah, Olushayo Olu, M.B., B.S., M.P.H., Scott Pendergast, M.Econ., William Perea, Jonathan Polonsky, M.Sc., Steven Riley, D.Phil., Olivier Ronveaux, M.D., M.P.H., Keita Sakoba, Ravi Santhana Gopala Krishnan, Mikiko Senga, Ph.D., M.P.H., Faisal Shuaib, M.B., B.S., M.P.H., Dr.P.H., Maria D. Van Kerkhove, Ph.D., Rui Vaz, M.D., M.P.H., Niluka Wijekoon Kannangarage, M.B., B.S., E.P.H., M.P.H., and Zabulon Yoti.
The authors’ affiliations are as follows: World Health Organization (WHO), Geneva (B.A., P.B., E.B., R.B., S.B., J.M.C., K.C., A. Crosier, J.-M.D., C.D., T.E., P.F., C.F., K.F., A.G., P.G., F.K., A.M.K., D.K., M.K., J. Markoff, E.M., J. Myhre, E.N., D.N., I.N., O.O., S.P., W.P., J.P., O.R., R.S.G.K., M.S., R.V., N.W.K., Z.Y.); Ministry of Health, Liberia (L.B., S.G., S.K., M.K., M.M., J.M.M., T.N.); Ministry of Health, Guinea (P.B., B.D., E.H., K.S.); Ministry of Health, Nigeria (A.N., F.S.); Ministry of Health, Sierra Leone (R.M.C., A.J.); and the Medical Research Council Centre for Outbreak Analysis and Modelling, WHO Collaborating Centre for Infectious Disease Modelling, Department of Infectious Disease Epidemiology, Imperial College London, London (I.B., A. Cori, C.A.D., T.G., H.M., P.N., S.R., M.D.V.K.), and Public Health England (C.L.) — both in the United Kingdom.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.World Health Organization Ebola virus disease: Cuban medical team heading for Sierra Leone. http://www.who.int/csr/disease/ebola/en/
- 2.Briand S, Bertherat E, Cox P, et al. The international Ebola emergency. N Engl J Med. doi: 10.1056/NEJMp1409858. DOI: 10.1056/NEJMp1409858. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization WHO statement on the meeting of the International Health Regulations Emergency Committee regarding the 2014 ebola outbreak in West Africa. http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/
- 4.Centers for Disease Control and Prevention Ebola outbreaks 2000-2014. 2014 http://www.cdc.gov/vhf/ebola/resources/outbreaks.html.
- 5.Okware SI, Omaswa FG, Zaramba S, et al. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002;7:1068–75. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 6.Malaria risk for travellers to Africa. Wkly Epidemiol Rec. 2001;76:25–7. [PubMed] [Google Scholar]
- 7.Borchert M, Mutyaba I, Van Kerkhove MD, et al. Ebola haemorrhagic fever outbreak in Masindi District, Uganda: outbreak description and lessons learned. BMC Infect Dis. 2011;11:357. doi: 10.1186/1471-2334-11-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raabe VN, Mutyaba I, Roddy P, Lut-wama JJ, Geissler W, Borchert M. Infection control during filoviral hemorrhagic fever outbreaks: preferences of community members and health workers in Masindi, Uganda. Trans R Soc Trop Med Hyg. 2010;104:48–50. doi: 10.1016/j.trstmh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Case definition recommendations for ebola or Marburg virus diseases. http://www.who.int/csr/resources/publications/ebola/ebola-case-definition-contact-en.pdf?ua=1.
- 10.Garske T, Legrand J, Donnelly CA, et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 11.Wallinga J, Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160:509–16. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–12. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breman JG, Piot P, Johnson KM, et al. The epidemiology of Ebola hemorrhagic fever in Zaire, 1976. In: Pattyn SR, editor. Ebola virus haemorrhagic fever. Elsevier Science; Amsterdam: 1978. pp. 85–97. [Google Scholar]
- 14.Roddy P, Howard N, Van Kerkhove MD, et al. Clinical manifestations and case management of Ebola haemorrhagic fever caused by a newly identified virus strain, Bundibugyo, Uganda, 2007-2008. PLoS One. 2012;7(12):e52986. doi: 10.1371/journal.pone.0052986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 16.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S87–S91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 17.Ndambi R, Akamituna P, Bonnet MJ, Tukadila AM, Muyembe-Tamfum JJ, Colebunders R. Epidemiologic and clinical aspects of the Ebola virus epidemic in Mosango, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S8–S10. doi: 10.1086/514297. [DOI] [PubMed] [Google Scholar]
- 18.Team RoaWIS Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 19.Althaus CL. Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa. Plos Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.91afb5e0f279e7f29e7056095255b288. http://currents.plos.org/outbreaks/article/estimating-the-reproduction-number-of-zaire-ebolavirus-ebov-during-the-2014-outbreak-in-west-africa/ [DOI] [PMC free article] [PubMed]
- 20.Fisman D, Khoo E, Tuite A. Early epidemic dynamics of the West African 2014 Ebola outbreak: estimates derived with a simple two-parameter model. Plos Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.89c0d3783f36958d96ebbae97348d571. http://currents.plos.org/outbreaks/article/obk-14-0036-early-epidemic-dynamics-of-the-west-african-2014-ebola-outbreak-estimates-derived-with-a-simple-two-parameter-model/ [DOI] [PMC free article] [PubMed]
- 21.Gire SK, Goba A, Andersen KG, et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345:1369–72. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes MFC, Rossi L, et al. Assessing the international spreading risk associated with the 2014 West African Ebola outbreak. Plos Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. http://currents.plos.org/outbreaks/article/assessing-the-international-spreading-risk-associated-with-the-2014-west-african-ebola-outbreak/ [DOI] [PMC free article] [PubMed]
- 23.Nishiura H, Chowell G. Early transmission dynamics of Ebola virus disease (EVD), West Africa, March to August 2014. Euro Surveill. 2014 Sep 11;19(36):20894. doi: 10.2807/1560-7917.es2014.19.36.20894. [DOI] [PubMed] [Google Scholar]
- 24.Sureau PH. Firsthand clinical observations of hemorrhagic manifestations in Ebola hemorrhagic fever in Zaire. Rev Infect Dis. 1989;11(Suppl 4):S790–S793. doi: 10.1093/clinids/11.supplement_4.s790. [DOI] [PubMed] [Google Scholar]
- 25.Georges A-J, Leroy EM, Renaut AA, et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994-1997: epidemiologic and health control issues. J Infect Dis. 1999;179(Suppl 1):S65–S75. doi: 10.1086/514290. [DOI] [PubMed] [Google Scholar]
- 26.Bray M, Mahanty S. Ebola hemorrhagic fever and septic shock. J Infect Dis. 2003;188:1613–7. doi: 10.1086/379727. [DOI] [PubMed] [Google Scholar]
- 27.Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ. 1983;61:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 28.Branch SP, Division V, Control D, Eradication S. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 29.Parkes-Ratanshi R, Elbireer A, Mbambu B, Mayanja F, Coutinho A, Merry C. Ebola outbreak response; experience and development of screening tools for viral haemorrhagic fever (VHF) in a HIV center of excellence near to VHF epicentres. PLoS One. 2014;9(7):e100333. doi: 10.1371/journal.pone.0100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Towner JS, Sealy TK, Khristova ML, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4(11):e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wamala JF, Lukwago L, Malimbo M, et al. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007-2008. Emerg Infect Dis. 2010;16:1087–92. doi: 10.3201/eid1607.091525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S76–S86. doi: 10.1086/514306. [DOI] [PubMed] [Google Scholar]
- 33.Muyembe T, Kipasa M. International Scientific and Technical Committee and WHO Collaborating Centre for Haemorrhagic Fevers. Ebola haemorrhagic fever in Kikwit, Zaire. Lancet. 1995;345:1448. doi: 10.1016/s0140-6736(95)92640-2. [DOI] [PubMed] [Google Scholar]
- 34.Estimating the future number of cases in the Ebola epidemic — Liberia and Sierra Leone, 2014–2015. Morb Mortal Wkly Rep. 2014 Sep 23; Epub ahead of print. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.