Abstract
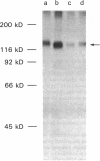
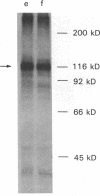
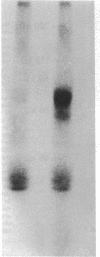
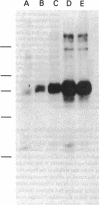
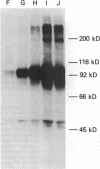
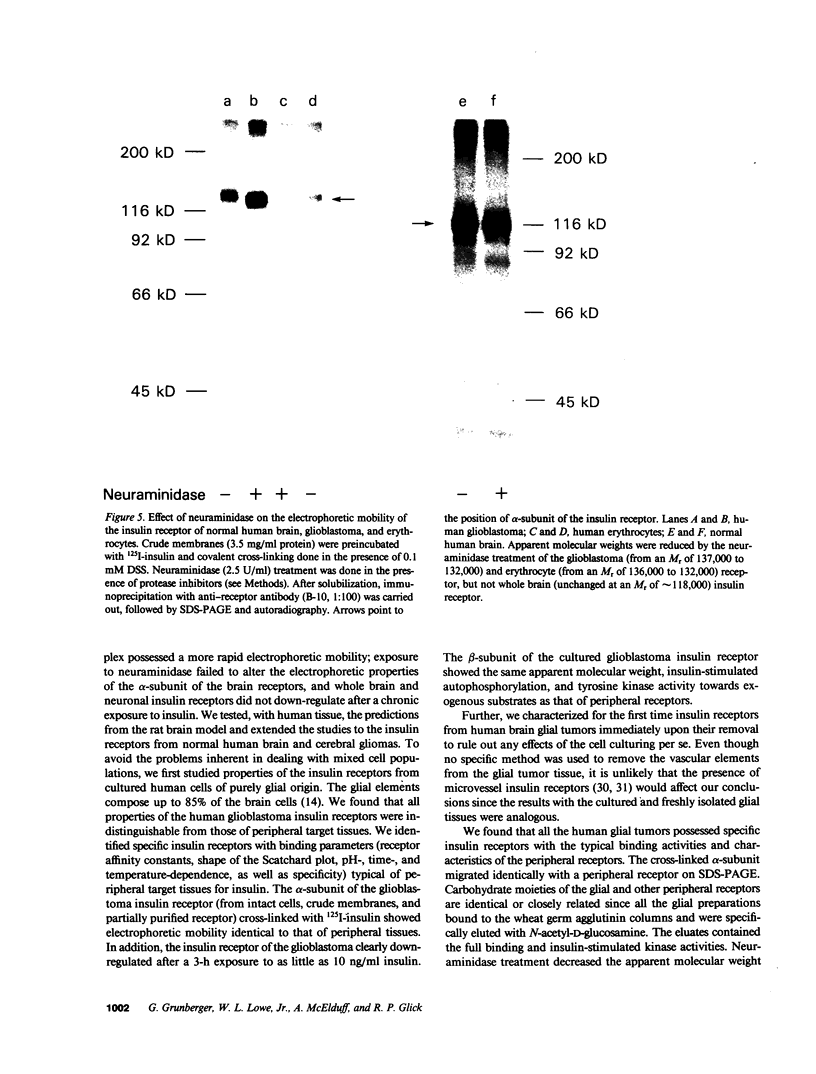
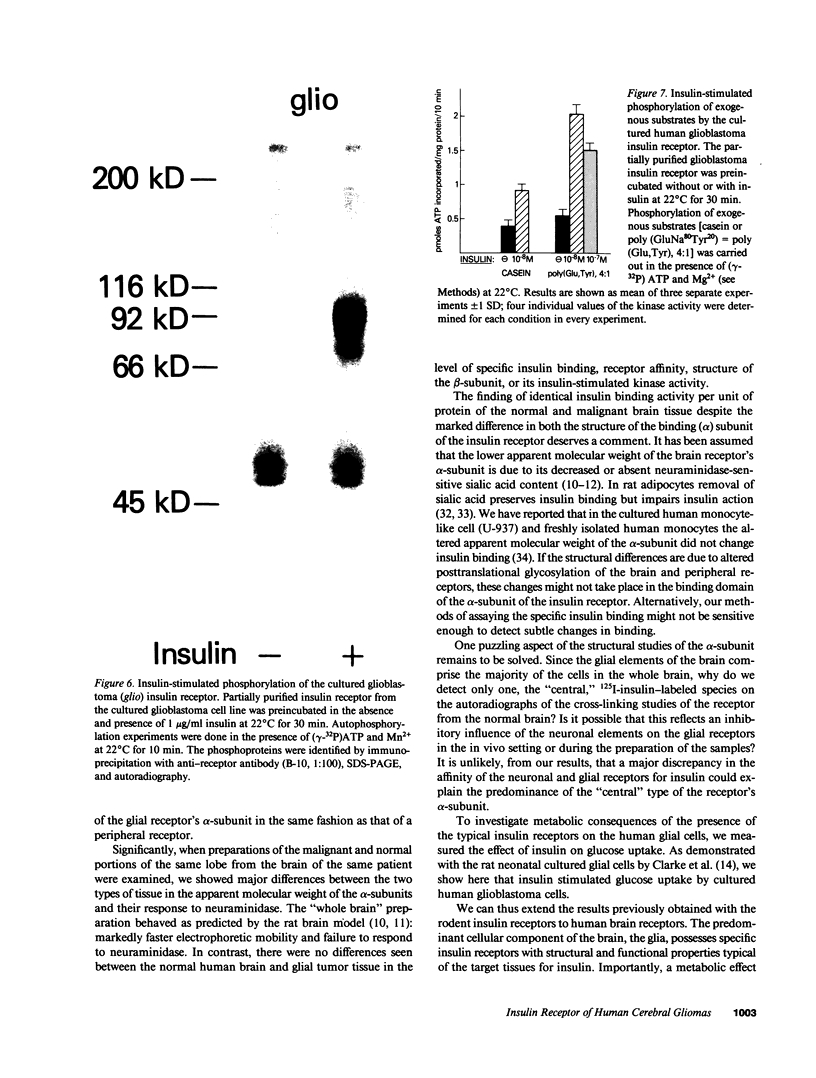
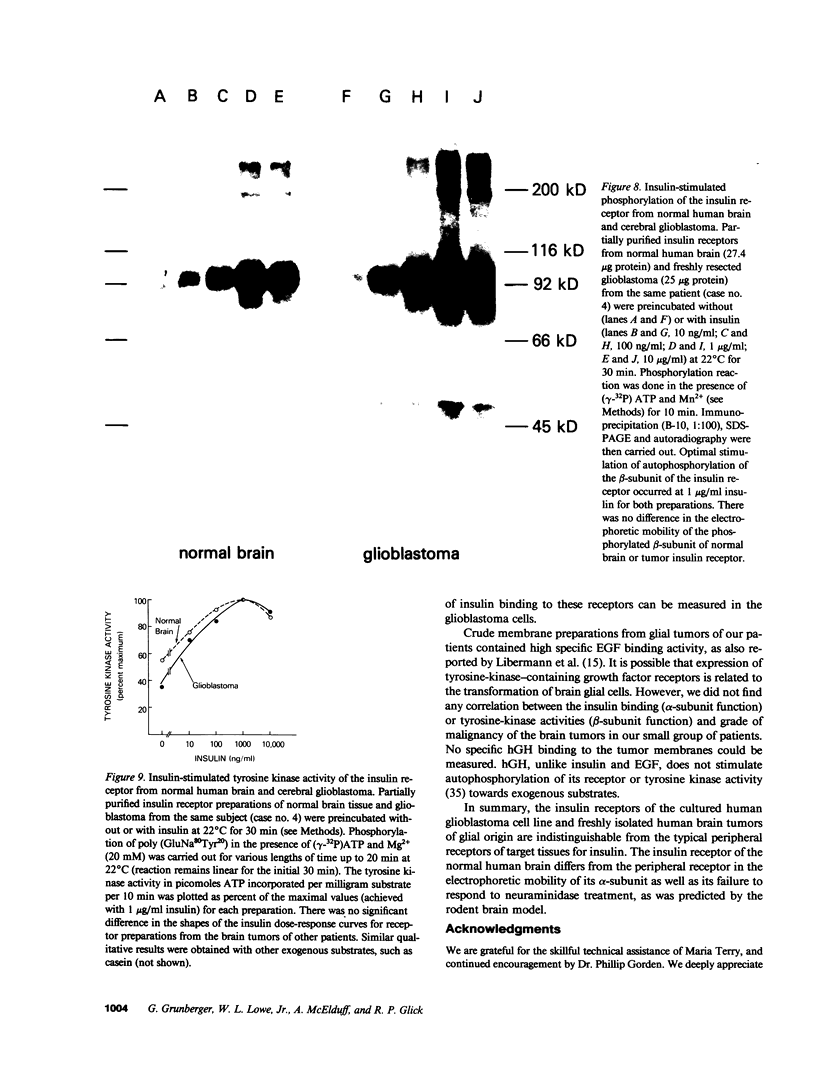
The insulin receptor from human brain tumors of glial origin was examined for the first time using intact cells (from an established cultured human glioblastoma cell line) and partially purified solubilized membranes (from cultured cells and freshly isolated human brain tumors). The structure of the glial insulin receptor subunits was assessed by affinity cross-linking of 125I-insulin with the alpha-subunit of the receptor, neuraminidase treatment of the cross-linked receptor, behavior of the receptor on lectin columns, and electrophoretic mobility of the phosphorylated beta-subunit. The functions of the insulin receptor were examined by measuring specific 125I-insulin binding (receptor concentration, affinity, specificity, pH-, time-, and temperature dependence), insulin-induced down-regulation of the receptor, insulin-stimulated autophosphorylation of the beta-subunit, and phosphorylation of exogenous substrates as well as insulin-stimulated glucose uptake in glioblastoma cells. All of these properties were typical for the insulin receptor from target tissues for insulin action. The insulin receptor of the normal human brain showed the altered electrophoretic mobility and lack of neuraminidase sensitivity of its alpha-subunit previously reported for the rat brain receptor. There was no difference, however, in the functions of the receptor subunits (binding, phosphorylation) from the normal brain tissue and the eight human gliomal tumors. Since the glial elements compose a majority of the brain cells, the "normal" structure and function of their insulin receptor might provide a key to understanding the role of insulin in the carbohydrate metabolism of the human central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa K., Grunberger G., McElduff A., Gorden P. Polypeptide hormone receptor phosphorylation: is there a role in receptor-mediated endocytosis of human growth hormone? Endocrinology. 1985 Aug;117(2):631–637. doi: 10.1210/endo-117-2-631. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Jr, Raizada M. K. Effects of insulin and tunicamycin on neuronal insulin receptors in culture. Am J Physiol. 1983 Sep;245(3):C283–C287. doi: 10.1152/ajpcell.1983.245.3.C283. [DOI] [PubMed] [Google Scholar]
- Cherqui G., Caron M., Capeau J., Picard J. Carbohydrate determinants involved in both the binding and action of insulin in rat adipocytes. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):627–643. doi: 10.1016/0303-7207(82)90151-4. [DOI] [PubMed] [Google Scholar]
- Clarke D. W., Boyd F. T., Jr, Kappy M. S., Raizada M. K. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J Biol Chem. 1984 Oct 10;259(19):11672–11675. [PubMed] [Google Scholar]
- Cuatrecasas P., Illiano G. Membrane sialic acid and the mechanism of insulin action in adipose tissue cells. Effects of digestion with neuraminidase. J Biol Chem. 1971 Aug 25;246(16):4938–4946. [PubMed] [Google Scholar]
- Devaskar S. U., Holekamp N. Insulin downregulates neonatal brain insulin receptors. Biochem Biophys Res Commun. 1984 Apr 30;120(2):359–367. doi: 10.1016/0006-291x(84)91262-2. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Kowalski A., Fehlmann M., van Obberghen E. Insulin receptors in rat brain: insulin stimulates phosphorylation of its receptor beta-subunit. FEBS Lett. 1984 Jun 25;172(1):87–90. doi: 10.1016/0014-5793(84)80879-0. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Comi R. J., Taylor S. I., Gorden P. Tyrosine kinase activity of the insulin receptor of patients with type A extreme insulin resistance: studies with circulating mononuclear cells and cultured lymphocytes. J Clin Endocrinol Metab. 1984 Dec;59(6):1152–1158. doi: 10.1210/jcem-59-6-1152. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Gorden P. Affinity alteration of insulin receptor induced by a phorbol ester. Am J Physiol. 1982 Oct;243(4):E319–E324. doi: 10.1152/ajpendo.1982.243.4.E319. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Roth J., Gorden P. Protein kinase activity of the insulin receptor in human circulating and cultured mononuclear cells. Biochem Biophys Res Commun. 1983 Sep 15;115(2):560–566. doi: 10.1016/s0006-291x(83)80181-8. [DOI] [PubMed] [Google Scholar]
- Haskell J. F., Meezan E., Pillion D. J. Identification of the insulin receptor of cerebral microvessels. Am J Physiol. 1985 Jan;248(1 Pt 1):E115–E125. doi: 10.1152/ajpendo.1985.248.1.E115. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. J. Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest. 1979 Aug;64(2):636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978 Apr 27;272(5656):827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Heidenreich K. A., Zahniser N. R., Berhanu P., Brandenburg D., Olefsky J. M. Structural differences between insulin receptors in the brain and peripheral target tissues. J Biol Chem. 1983 Jul 25;258(14):8527–8530. [PubMed] [Google Scholar]
- Hendricks S. A., Agardh C. D., Taylor S. I., Roth J. Unique features of the insulin receptor in rat brain. J Neurochem. 1984 Nov;43(5):1302–1309. doi: 10.1111/j.1471-4159.1984.tb05387.x. [DOI] [PubMed] [Google Scholar]
- Kosmakos F. C., Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980 Oct 25;255(20):9860–9869. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Razon N., Bartal A. D., Yarden Y., Schlessinger J., Soreq H. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984 Feb;44(2):753–760. [PubMed] [Google Scholar]
- McElduff A., Grunberger G., Gorden P. An alteration in apparent molecular weight of the insulin receptor from the human monocyte cell line U-937. Diabetes. 1985 Jul;34(7):686–690. doi: 10.2337/diab.34.7.686. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Pillion D. J., Haskell J. F., Meezan E. Cerebral cortical microvessels: an insulin-sensitive tissue. Biochem Biophys Res Commun. 1982 Jan 29;104(2):686–692. doi: 10.1016/0006-291x(82)90691-x. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Hendricks S. A., Quarum M., Roth J. The insulin receptor of rat brain is coupled to tyrosine kinase activity. J Biol Chem. 1984 Mar 25;259(6):3470–3474. [PubMed] [Google Scholar]
- Rhoads D. E., DiRocco R. J., Osburn L. D., Peterson N. A., Raghupathy E. Stimulation of synaptosomal uptake of neurotransmitter amino acids by insulin: possible role of insulin as a neuromodulator. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1198–1204. doi: 10.1016/0006-291x(84)90903-3. [DOI] [PubMed] [Google Scholar]
- Robert A., Grunberger G., Carpenter J. L., Dayer J. M., Orci L., Gorden P. The insulin receptor of a human monocyte-like cell line: characterization and function. Endocrinology. 1984 Jan;114(1):247–253. doi: 10.1210/endo-114-1-247. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schravendijk C. F., Hooghe-Peters E. L., De Meyts P., Pipeleers D. G. Identification and characterization of insulin receptors on foetal-mouse brain-cortical cells. Biochem J. 1984 May 15;220(1):165–172. doi: 10.1042/bj2200165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Moule M. L., Yeung C. W. Characterization of insulin receptor subunits in brain and other tissues by photoaffinity labeling. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1671–1678. doi: 10.1016/0006-291x(80)91366-2. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Goens M. B., Hanaway P. J., Vinych J. V. Characterization and regulation of insulin receptors in rat brain. J Neurochem. 1984 May;42(5):1354–1362. doi: 10.1111/j.1471-4159.1984.tb02795.x. [DOI] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]