Abstract
Background
It has been theorised that sperm competition promotes the strategic usage of costly sperm. Although sperm competition is thought to be an important driving force of reproductive traits in simultaneous hermaphrodites as well as in species with separate sexes, empirical studies on strategic ejaculation in simultaneous hermaphrodites are scarce.
Results
In the present study, we tested whether the simultaneously hermaphroditic land snail Euhadra quaesita adjusts the number of sperm donated according to the condition of the mate and whether the pattern of strategic ejaculation is in line with previously suggested theories. We found that individuals donated much more sperm when they copulated with a virgin mate than when they copulated with a non-virgin.
Conclusion
The virgin-biased pattern of ejaculation matches the theoretical prediction and suggests that sperm competition significantly influence the reproductive traits of simultaneously hermaphroditic land snails.
Keywords: Sperm competition, Strategic ejaculation, Sperm allocation, Simultaneous hermaphrodites, Land snails
Background
If a female copulates with more than one male and stores the resulting sperm, sexual selection is likely to continue after copulation via sperm competition and cryptic female choice. Although the outcome of sperm competition can be influenced by non-numerical factors such as sperm length and seminal fluid composition [1,2], the relative number of sperm transferred by competing males is a key predictor of fertilization success under post-copulatory reproductive conditions in several species [3-5]. Because sperm cells are relatively small and require less energy to produce than eggs, males are expected to evolve an undiscriminating eagerness to copulate and transfer their sperm, whereas females are expected to show a discriminating passivity [6]. However, accumulating evidence has suggested that sperm and seminal fluids are, in fact, costly to produce [7-9]. Selection would therefore favour males that adjust their ejaculate expenditure according to the indicators of expected fertility in a mating event, which include mate condition and sperm competition levels [10,11]. Indeed, such strategic ejaculation has been reported in many animals, including insects, fish, and mammals [12]. As in species with separate sexes, post-copulatory sexual selection is thought to be an important evolutionary force leading to morphological and behavioral diversification in simultaneous hermaphrodites, which have male and female functions at the same time [13,14]. Moreover, previous empirical studies have shown that such organisms are careful about the use of resources for reproduction and can adjust the allocation of resources between their male and female functions [15]. Therefore, simultaneous hermaphrodites are also expected to be prudent with their limited male reserves. However, the literature on this point has been dominated by studies on species with separate sexes.
Several factors are known to be involved in the strategic usage of limited male reserves. Among these factors, sperm competition risk and intensity have been well studied from both theoretical [16-18] and empirical perspectives [19-21]. The sperm competition risk model considers the probability that sperm competition occurs in a focal female [18]. Alternatively, the sperm competition intensity model applies the number of competitors when females mate with more than two males [17]. For example, animals use the number of rivals and the mating history of their mates as indicators in these cases. Theoretical studies have shown that the properties of the mates with which males should spend more ejaculate are species or population specific [11]. Engqvist & Reinhold [22] have shown that when ejaculates from more than two males compete to fertilize ova (i.e., the sperm competition intensity model), the remating frequency of the mates and the fertilization pattern of the sperm from several mates determine the evolutionary consequences of strategic ejaculation. Their model has predicted that a strategy in which larger ejaculations are invested in favour of non-virgin mates is evolutionary stable whenever the remating rate is low. On the other hand, when the remating rate is high, last-male sperm precedence (i.e., the fertilization priority of sperm from the latter male) is necessary for the evolution of the strategy focusing on non-virgins. Conversely, first-male sperm precedence or random fertilization lead to strategic ejaculation that prioritizes virgin mates. Simultaneous hermaphrodites that have a habit of mating with reciprocal intromission (i.e., they play both male and female roles and exchange sperm in a single mating event) are expected to satisfy the conditions of sperm competition intensity models. Such hermaphroditic animals are thought to be intensely promiscuous because the fertilization success of their male function is expected to increase with an increasing number of matings [23], but see [24], which leads to high remating rates and sperm competition among several individuals. In hermaphrodites, therefore, the strategy of ejaculation should be determined by the sperm priority pattern according to the theoretical models proposed by Engqvist & Reinhold [22]. Contrary to the theoretical prediction, however, the simultaneously hermaphroditic earthworm Eisenia andrei, in which the fertilization pattern is thought to be random, exhibits greater ejaculate expenditure during copulation with a non-virgin mate than that with a virgin mate [25].
Other factors have been proposed as important for the allocation of the costly ejaculate of males. Female quality (e.g., fecundity) is thought to influence ejaculate expenditure [26-28]. Moreover, because of their unique reproductive systems, another potential factor affects ejaculate size in simultaneous hermaphrodites. When there is a difference in the expected fitness gain between sex roles, individuals show a preference for a particular sex role, and conflict over the preferred sex role arises [13,29]. Some studies have proposed that such gender conflict could be solved by sperm trading, in which sperm transfer from one mate is conditional upon the other mate’s sperm transfer [30]. When individuals reciprocally intromit their penises in a single mating event, they may alter the amount of sperm transferred to their mates (autosperm) based on the amount of sperm received (allosperm). Although a pattern of strategic ejaculation according to the mate’s mating status (virgin or non-virgin) was reported in a simultaneously hermaphroditic freshwater snail, there remains the possibility that the patterns of resource allocation to a female function differ between virgin and non-virgin mates, and thus the effect of mate’s fecundity was unclear [31]. Moreover, although large individuals of the hermaphroditic land snail Succinea putris adjust their ejaculate expenditures according to the body sizes of their mates, this result may be explainable in terms of sperm transfer number of the mates because body size is significantly correlated with ejaculation size in this species [32]. Therefore, experiments that examine these factors at the same time are needed to test the theories of sperm competition intensity in simultaneous hermaphrodites. Although only a limited literature exists on strategic ejaculation in simultaneous hermaphrodites, Baur et al. [33] examined it and found no evidence of strategic ejaculation according to either mate mating status, mate body size, and mate sperm transfer number in the simultaneously hermaphroditic land snail Arianta arbustorum. However, it has been suggested that sexual selection including sperm competition is weak in Arianta arbustorum[34-36]. Moreover, although Anthes et al. [37] found that copulation duration was correlated with sperm competition intensity in a simultaneously hermaphroditic sea slug, Lange et al. [38] revealed that copulation duration was not a reliable proxy for sperm transfer number in that species. Therefore, whether the pattern of strategic ejaculation in simultaneous hermaphrodites is in line with the theories of sperm competition games remains an unclear, controversial issue.
In this study, we examined the effects of the mating history of the mate (virgin or non-virgin) and other potential factors (quality and sperm transfer of mates) on ejaculate size in the simultaneously hermaphroditic land snail Euhadra quaesita. In contrast to the reproductive traits of A. arbustorum, those of E. quaesita have been suggested to be influenced by sexual selection [39]. In Cornu aspersum, it has been determined that allosperm near the internal wall of the allosperm storage organ of a mate can survive longer and may have a better chance of successful fertilisation [40]. Therefore, allosperm from the first donor would reach this limited hospitable location and have priority for fertilization. Indeed, several studies have reported this sperm precedence pattern [41-43], but see [44]. Moreover, species from the Helicoidea including E. quaesita and C. aspersum have similar basic morphology of genitalia. Therefore, it is expected that E. quaesita snail will value virgin mates more highly and donate larger ejaculates to such mates.
Results
We collected spermatophores from 30 pairs of two non-virgin snails and 20 non-virgin / virgin pairs. Although the number of sperm transferred was highly variable (range 1.05 × 105 – 2.11 × 107 for snails in the non-virgin mate treatment; 5.37 × 105 – 1.95 × 107 for the virgin mate treatment) [Additional file 1], similar variabilities have been reported in other species of simultaneously hermaphroditic land snails [33,45,46]. The stepwise deletion of the fixed effects from the linear models produced a model including an effect of mate mating status (Table 1). In contrast, the other two factors associated with the mates (mate body size and mate sperm transfer number) did not affect the number of sperm transferred to the mate. Neither the body size nor the epiphallus length of the snails affected their own sperm transfer. Thus, even when controlling for other mate-associated factors, our model analyses showed that mate mating status significantly affected the number of sperm that a snail transferred to its mate. The snails donated more sperm to virgin mates than to non-virgin mates (Figure 1). On average, the snails that mated with virgins invested 2.20-fold more sperm in the mating (mean = 1.43 × 106 for the non-virgin mate treatment; 3.24 × 106 for the virgin mate treatment).
Table 1.
Results of stepwise deletion of effects from the initial model for the sperm transfer number
| Variable | Coefficient (± SE) | Log-likelihood ratio | d. f. | p -value |
|---|---|---|---|---|
| Intercept |
14.17 ± 0.24 |
|
|
|
| Body size |
|
0.17 |
1 |
0.68 |
| Epiphallus length |
|
0.49 |
1 |
0.48 |
|
Mate mating status (A) |
0.82 ± 0.38 |
4.72 |
1 |
0.03 |
| Mate body size (B) |
|
2.32 |
1 |
0.13 |
| Mate sperm transfer Number |
|
1.51 |
1 |
0.22 |
| A × B | 0.70 | 1 | 0.40 |
Significant effects are in bold.
Figure 1.
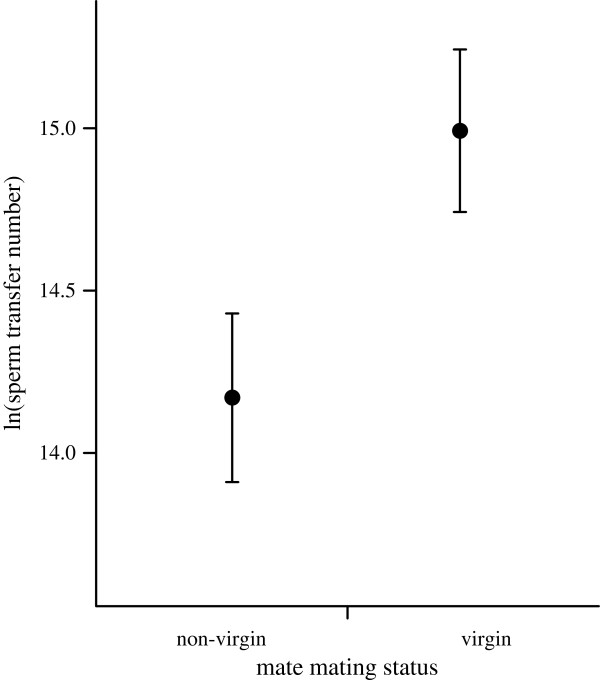
The numbers of sperm transferred (mean ± SE) to virgin and non-virgin mates. Sperm transfer number was log (natural) transformed.
Discussion
In this study, we found that the number of sperm transferred by the focal snails differed according to the mating status of the mate in Euhadra quaesita. We also found that the pattern of the differences in sperm transfer number was biased toward virgin mates, as was consistent with the prediction of the sperm competition theory under high remating rate and first-male sperm precedence proposed by Engqvist & Reinhold [22]. An explanation other than sperm competition theory, however, may account for this result: if sperm recipients can actively control the transfer of allosperm by the sperm donor, the virgin-biased pattern of ejaculation may be caused by the active influence of the virgin recipients, which do not have any allosperm for cross-fertilization and would have a greater motivation to receive allosperm. However, even if virgins receive the same amount of allosperm as non-virgins, virgins are likely able to fertilize all eggs laid during their lifetimes with this amount of sperm. Moreover, although an excess of allosperm may be used by its recipient as a nutrient source after digestion and absorption, it is also thought to facilitate polyspermy [47,48] and thus lead to decrease in hatched eggs. The risk of polyspermy would be particularly high in the species of the Helicoidea including E. quaesita because the donors of these species manipulate the physiology of the recipients and interrupt the digestion of the sperm by the transfer of specific substances [45,49] K. Kimura et al. 2009, unpublished]. Therefore, the difference in allosperm requirement between virgin and non-virgin recipients is unlikely to explain our findings although further experiments are necessary to determine the effect of sperm recipients on the transfer of allosperm. Instead, our results suggested that snails modulate their behavior to use sperm economically in the presence of different sperm competition levels between virgin and non-virgin mates, as expected by sperm competition theory. This likely results in greater fertilization opportunity, although further experiments are needed to confirm this hypothesis. These findings also provided strong support for the prediction that post-copulatory sexual selection has a considerable influence on the evolution of behavioral reproductive traits in simultaneous hermaphrodites [13,23]. It remains unclear which cues snails use to discriminate the mating status of their mates. However, chemosensory cues may play an important role in this discrimination, because it is reported that snails of E. peliomphala, a species that is closely related to E. quaesita, communicate with air-borne chemicals [50]. Body contact during courtship may also provide an opportunity to communicate and discriminate mating status. How precisely snails can recognize mating status also remains unclear. Engqvist & Reinhold [22] have shown that when animals can discriminate precisely and categorize mates as virgin, singly mated, or multiply mated, a strategy evolves in which animals invest more sperm into singly mated individuals than into virgins. However the relationship between the amount of sperm investment into virgin and multiply mated individuals is approximately same as in a model that assumes only virgin and non-virgin categories (i.e., the model previously discussed). In the present study, we used non-virgin snails that had mated freely in the wild until capture. Because E. quaesita is thought to repeat mating in interval of 1–2 weeks [39], the majority of the non-virgins used in this study were most likely multiply mated. Therefore, the facts suggest that regardless of the snails’ ability to discriminate, the virgin-biased pattern found in this study is consistent with the theoretical prediction under high remating rate and first-male sperm precedence by Engqvist & Reinhold [22].
As theoretically predicted for simultaneous hermaphrodites [23], snails of E. quaesita exhibit promiscuity and engage in multiple matings between oviposition [51], K. Kimura 2012, unpublished]. Moreover, similar to Bradybaena fruticum[52], E. quaesita has a simple and unbranched allosperm storage organ, which would not allow sperm recipients to strictly control the use of allosperm stored from several individuals [K. Kimura 2011, unpublished]. Therefore, although further experiments are needed to confirm this hypothesis in our study species, the allosperm of the first mate would occupy a location that is associated with greater survival, as previously shown in Cornu aspersum[40], and thus contribute more to fertilization than the last male’s sperm (i.e., first-male sperm precedence). These findings suggest that E. quaesita exhibits a high remating rate and sperm priority toward the former donor, which are the conditions required for the evolution of strategic ejaculation in which virgin status is regarded as valuable [22].
Sperm donors are expected to allocate more sperm to more fecund recipients [11,26]. However, we found no evidence that snails adjust sperm transfer number according to the body size of their mates (Table 1). Although the relationship between body size and fecundity in our study species remains unclear, large size tends to be correlated with high fecundity in various species of pulmonate land snails that lay eggs in batches, which would include E. quaesita[53]. Factors other than body size may also serve as indicators of fecundity. For example, several studies have reported that males adjust their ejaculation expenditure according to the ages of their mates [54,55]. However, this behavior does not seem to occur in our study species. Virgin individuals of E. quaesita are thought to be 2–3 years old, while non-virgins are thought to be 2–6 years old [K. Kimura 2011, unpublished]. In our laboratory experiment, the age variation was expected to be greater in non-virgin mates than in virgins. Therefore, if E. quaesita adjusts sperm transfer number according to mate age, sperm transfer number should have varied to a greater degree in the non-virgin mate treatment than in the virgin mate treatment. However, we found that the variations in sperm transfer number were similar between snails that copulated with either mate type (Figure 1). Although further experiments are needed to confirm the effect of mate age, these findings suggest that snails do not behave prudently in terms of mate quality. Our experiment also showed that E. quaesita snails select a sperm transfer number independently of the number of sperm received from the mate. Although theoretical studies have proposed that gender conflict between hermaphroditic individuals can be solved by sperm trading [30], the generality of gender conflict and sperm trading is unclear due to a lack of empirical data. Therefore, additional experiments are needed to examine gender conflict in E. quaesita. Moreover, how to transfer sperm into a mate may help to understand the independence of sperm transfer number found in this study. E. quaesita transfers sperm enclosed in a spermatophore into a mate and this strategy would be against allosperm digestion by the mate [56,57]. Sperm trading has been thought to be associated with the uncertainty of male fertilization success resulting from the allosperm digestion. Therefore, although further experiments are needed to test the hypothesis, such species producing spermatophores may not adopt the strategy of sperm trading.
Conclusion
In conclusion, our results show that simultaneously hermaphroditic land snails would execute strategic ejaculation according to the mating status of their mates. The pattern of this adjustment was virgin-biased, matching the theoretical prediction. Thus, sperm competition in these land snails strongly influences their behavioral reproductive traits and is more important for snails than the detection of high quality mates.
Methods
Study species
We investigated the simultaneously hermaphroditic land snail Euhadra quaesita in this study. When individuals of this species reach sexual maturity, they stop their shell growth and form a reflected lip at the shell aperture. E. quaesita lives for several years after sexual maturation and undergoes multiple matings. E. quaesita forms a spermatophore consisting of a body and a tail. Similar to many other stylommatophorans, the body part of the spermatophore, which is produced in an epiphallus, contains sperm, whereas the tail part, which is produced in the flagellum, does not contain sperm. Although simultaneously hermaphroditic land snails with internal fertilization have divergent reproductive organs [58,59], even within the same species [57,60], the effects of these differences in genitalia have been overlooked in the context of strategic ejaculation in land snails [32,33,61]. In this study, therefore, we investigated the epiphallus length of snails to control for these effects. The mating process of E. quaesita consists of courtship behavior and copulation. Two snails that are sexually aroused show courtship behavior, which consists of rubbing on and licking the potential mate. The duration of this courtship behavior is approximately 5–10 min [39]. The snails then simultaneously and reciprocally intromit their penises. The duration of copulation in this species is 100–150 min. During copulation, the spermatophore is introduced into the oviduct of the sperm recipient. Within five minutes of the spermatophore donation, the donor retracts its penis. Although spermatophore donation is not always synchronous, the second donation is usually performed successfully. The sperm then leave the spermatophore and migrate into the allosperm storage organ.
Adult and semi-adult snails were collected in the summer of 2012 from Hachijo Island, Japan and kept individually in plastic pots (450 ml) at 22 degrees Celsius and approximately 65% RH. The snails were fed a powder consisting of barley, protein, and calcium ad libitum and their housings were cleaned every 2 weeks. The semi-adults matured within 1–2 weeks after collection and were considered as virgin adults in our laboratory experiments. We categorized the individuals collected as adults as non-virgin snails because a preliminary field investigation had revealed that all adults (N > 20) had already stored allosperm in their bodies. Although the virgin snails were grown under laboratory conditions, their shell sizes did not significantly differ from those of the non-virgins (Welch’s t test, t = 0.62, df = 26, p = 0.54). In addition to mating status (i.e., virgin or non-virgin), three aspects potentially differ between non-virgins and virgins with respect to reproduction. First, the amount of autosperm reserves may be different in non-virgins and virgins because of the matings preceding capture in the non-virgins. Second, the sex allocation pattern (i.e., resource allocation between male and female functions) may also differ, because information on the presence of conspecifics can influence this allocation in simultaneous hermaphrodites [62] and the virgins were isolated before sexual maturation, in contrast with the non-virgins. Third, the age structures may differ: virgins are thought to be 2–3 years old, while non-virgins are thought to be 2–6 years old [K. Kimura 2011, unpublished]. To control the first and second aspects, both non-virgin and virgin snails experienced more than 8 weeks of isolation before the laboratory experiments. Although the interval between matings has been shown to affect the number of transferred sperm [61,63], 8 weeks is likely a sufficient interval to replenish autosperm in E quaesita. Because isolation is thought to alter sex allocation [64,65], this period also likely minimise the potential difference in sex allocation patterns between non-virgins and virgins. The impact of age structure is considered above in the Discussion. Moreover, although a decrease in the already stored allosperm would occur over this isolation period, a preliminary investigation has revealed that non-virgins (N = 5) can lay hatchable eggs after one year of isolation. Therefore, sperm competition was expected to occur in non-virgins.
Experimental procedures
Focal non-virgin snails were selected. These non-virgins were paired with either a non-virgin or a virgin potential mate. Each pair was placed in a small container (2000 ml) and given the opportunity to mate. The individuals of the successfully mated pairs were frozen with liquid nitrogen shortly after mating. The frozen snails were dissected after defrosting. Photographs of the entire reproductive system were taken with a Nikon COOLPIX P7000 camera. The epiphallus lengths of all the snails were measured using Image J software (National Institutes of Health, Bethesda, MD, USA). The spermatophore delivered in the focal mating was pulled out gently. The number of sperm in the spermatophore was counted for each mated snail. The sperm counting procedure was performed as described by Locher & Baur [66], except that the duration and strength of sonication was changed, and DNA staining was not performed. A preliminary investigation revealed that our sonication strength (38 kHz) did not destroy the head of the sperm even after 80 hours of sonication and that the sperm head could be clearly identified using light microscopy due to its large size. A 30 μl sample of the obtained sperm suspension was transferred to a counting chamber (MATSUNAMI MPC-200). The sperm heads in 10 randomly selected cells (1 cell = 25 nl) were counted, and the mean number of heads was used to calculate the total number of sperm in the spermatophore. To evaluate body size, the shell diameter and height of all snails were measured using a vernier caliper.
Statistical analyses
Linear models were used to test whether sperm transfer number was affected by body size, epiphallus length, mate body size, mate sperm transfer number, or mate mating status (virgin or non-virgin). Both body size (shell diameter × shell diameter × shell height) and epiphallus length were log (natural) transformed prior to the linear model analyses. Sperm transfer number was also log (natural) transformed because this transformation supported the assumption of a Gaussian distribution (Kolmogorov-Smirnov test, D = 0.11, p = 0.53). In the linear models, the number of sperm transferred by the non-virgins was treated as a dependent variable. Body size, epiphallus length, mate body size, mate sperm transfer number, and mate mating status were treated as fixed effects. The two-way interaction between mate body size and mate mating status was also included in the linear models as a fixed effect. The significance of the fixed effects in the linear models was assessed with an analysis of deviance (likelihood ratio tests using a chi-square approximation). The models were simplified by the stepwise deletion of non-significant (p > 0.05) fixed effects, beginning with the effect showing the highest p-value [67]. The linear model analyses were conducted in R 2.15.1 [68].
Availability of supporting data
The data set supporting the results of this article is included within the article and its additional file.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KK designed the study, performed the experiments, analyzed the data, and drafted the manuscript. SC participated in the design of the study and drafted the manuscript. Both authors read and approved the final manuscript.
Supplementary Material
Sperm transfer number of focal snails.
Contributor Information
Kazuki Kimura, Email: k.kimura.000@gmail.com.
Satoshi Chiba, Email: schiba@biology.tohoku.ac.jp.
Acknowledgements
We express our sincere gratitude to T. Kazama for help with the experimental work and to Y. Kameda, T. Hirano, and K. Shibuya for collecting materials. We are also grateful to three anonymous referees for improving the manuscript. This study was financially supported by The Global COE Program J03 (Ecosystem management adapting to global change) of the MEXT, Japan.
References
- Snook RR. Sperm in competition: not playing by the numbers. Trends Ecol Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Pizzari T. Sexual selection: sperm in the fast lane. Curr Biol. 2009;19:R292–R294. doi: 10.1016/j.cub.2009.02.026. [DOI] [PubMed] [Google Scholar]
- Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. The effect of ratios and numbers of spermatozoa mixed from two males on proportions of offspring. Reproduction. 1974;39:251–258. doi: 10.1530/jrf.0.0390251. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Møller AP. Sperm competition and sexual selection. London: Academic Press; 1998. [Google Scholar]
- Gage M, Morrow E. Experimental evidence for the evolution of numerous, tiny sperm via sperm competition. Curr Biol. 2003;13:754–757. doi: 10.1016/S0960-9822(03)00282-3. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Ejaculate cost and male choice. Am Nat. 1982;119:601–610. doi: 10.1086/283938. [DOI] [Google Scholar]
- Nakatsuru K, Kramer DL. Is sperm cheap? limited male fertility and female choice in the lemon tetra (Pisces, characidae) Science. 1982;216:753–755. doi: 10.1126/science.216.4547.753. [DOI] [PubMed] [Google Scholar]
- Olsson M, Madsen T, Shine R. Is sperm really so cheap? costs of reproduction in male adders, vipera berus. Proc R Soc B. 1997;264:455–459. doi: 10.1098/rspb.1997.0065. [DOI] [Google Scholar]
- Wedell N, Gage MJG, Parker GA. Sperm competition, male prudence and sperm-limited females. Trends Ecol Evol. 2002;17:313–320. doi: 10.1016/S0169-5347(02)02533-8. [DOI] [Google Scholar]
- Parker GA, Pizzari T. Sperm competition and ejaculate economics. Biol Rev. 2010;85:897–934. doi: 10.1111/j.1469-185X.2010.00140.x. [DOI] [PubMed] [Google Scholar]
- Kelly CD, Jennions MD. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol Rev. 2011;86:863–884. doi: 10.1111/j.1469-185X.2011.00175.x. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Simultaneous hermaphroditism and sexual selection. Proc Natl Acad Sci USA. 1979;76:2480–2484. doi: 10.1073/pnas.76.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels NK. In: Sperm competition and sexual selection. Birkhead TR, Møller AP, editor. London: Academic Press; 1998. Mating conflicts and sperm competition in simultaneous hermaphrodites; pp. 219–254. [Google Scholar]
- Schärer L. Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution. 2009;63:1377–1405. doi: 10.1111/j.1558-5646.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition games: raffles and roles. Proc R Soc B. 1990;242:120–126. doi: 10.1098/rspb.1990.0114. [DOI] [Google Scholar]
- Parker GA, Ball MA, Stockley P, Gage MJ. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc R Soc B. 1996;263:1291–1297. doi: 10.1098/rspb.1996.0189. [DOI] [Google Scholar]
- Parker GA, Ball MA, Stockley P, Gage MJ. Sperm competition games: a prospective analysis of risk assessment. Proc R Soc B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage MJG. Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit fly. Anim Behav. 1991;42:1036–1037. doi: 10.1016/S0003-3472(05)80162-9. [DOI] [Google Scholar]
- Pilastro A, Scaggiante M, Rasotto MB. Individual adjustment of sperm expenditure accords with sperm competition theory. Proc Natl Acad Sci USA. 2002;99:9913–9915. doi: 10.1073/pnas.152133499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBarco-Trillo J, Ferkin MH. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- Engqvist L, Reinhold K. Theoretical influence of female mating status and remating propensity on male sperm allocation patterns. J Evol Biol. 2006;19:1448–1458. doi: 10.1111/j.1420-9101.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- Michiels NK, Koene JM. Sexual selection favors harmful mating in hermaphrodites more than in gonochorists. Integr Comp Biol. 2006;46:473–480. doi: 10.1093/icb/icj043. [DOI] [PubMed] [Google Scholar]
- Sprenger D, Faber J, Michiels NK, Anthes N. Natural female mating rate maximizes hatchling size in a marine invertebrate. J Anim Ecol. 2008;77:696–701. doi: 10.1111/j.1365-2656.2008.01376.x. [DOI] [PubMed] [Google Scholar]
- Velando A, Eiroa J, Domínguez J. Brainless but not clueless: earthworms boost their ejaculates when they detect fecund non-virgin partners. Proc R Soc B. 2008;275:1067–1072. doi: 10.1098/rspb.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold K, Kurtz J, Engqvist L. Cryptic male choice: sperm allocation strategies when female quality varies. J Evol Biol. 2002;15:201–209. doi: 10.1046/j.1420-9101.2002.00390.x. [DOI] [Google Scholar]
- Mallard S, Barnard C. Competition, fluctuating asymmetry and sperm transfer in male gryllid crickets (Gryllus bimaculatus and Gryllodes sigillatus) Behav Ecol Sociobiol. 2003;53:190–197. [Google Scholar]
- Cornwallis CK, Birkhead TR. Changes in sperm quality and numbers in response to experimental manipulation of male social status and female attractiveness. Am Nat. 2007;170:758–770. doi: 10.1086/521955. [DOI] [PubMed] [Google Scholar]
- Leonard J. Sexual conflict and the mating systems of simultaneously hermaphroditic gastropods. Amer Malacol Bull. 1991;9:45–58. [Google Scholar]
- Anthes N, Putz A, Michiels NK. Sex role preferences, gender conflict and sperm trading in simultaneous hermaphrodites: a new framework. Anim Behav. 2006;72:1–12. doi: 10.1016/j.anbehav.2005.09.017. [DOI] [Google Scholar]
- Loose MJ, Koene JM. Sperm transfer is affected by mating history in the simultaneously hermaphroditic snail Lymnaea stagnalis. Invertebr Biol. 2008;127:162–167. doi: 10.1111/j.1744-7410.2007.00121.x. [DOI] [Google Scholar]
- Dillen L, Jordaens K, Van Dongen S, Backeljau T. Effects of body size on courtship role, mating frequency and sperm transfer in the land snail Succinea putris. Anim Behav. 2010;79:1125–1133. doi: 10.1016/j.anbehav.2010.02.010. [DOI] [Google Scholar]
- Baur B, Locher R, Baur A. Sperm allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Anim Behav. 1998;56:839–845. doi: 10.1006/anbe.1998.0855. [DOI] [PubMed] [Google Scholar]
- Baminger H, Haase M. Variation in spermathecal morphology and amount of sperm stored in populations of the simultaneously hermaphroditic land snail Arianta arbustorum. J Zool. 1999;249:165–171. doi: 10.1111/j.1469-7998.1999.tb00754.x. [DOI] [Google Scholar]
- Baminger H, Haase M. Variation of distal genitalia in the simultaneously hermaphroditic land snail Arianta arbustorum (Pulmonata, Stylommatophora) caused by sexual selection? Biol J Linnean Soc. 2000;71:599–613. doi: 10.1111/j.1095-8312.2000.tb01280.x. [DOI] [Google Scholar]
- Bojat NC, Haase M. Sperm storage in the simultaneously hermaphroditic land snail Arianta arbustorum. J Zool. 2002;258:497–503. doi: 10.1017/S0952836902001656. [DOI] [Google Scholar]
- Anthes N, Putz A, Michiels NK. Hermaphrodite sex role preferences: the role of partner body size, mating history and female fitness in the sea slug Chelidonura sandrana. Behav Ecol Sociobiol. 2006;60:359–367. doi: 10.1007/s00265-006-0173-5. [DOI] [Google Scholar]
- Lange R, Beninde J, Reichel V, Werminghausen J, Gerlach T, Anthes N. Copulation duration does not predict sperm transfer in a marine hermaphrodite. Anim Behav. 2012;83:469–472. doi: 10.1016/j.anbehav.2011.11.021. [DOI] [Google Scholar]
- Kimura K, Shibuya K, Chiba S. The mucus of a land snail love-dart suppresses subsequent matings in darted individuals. Anim Behav. 2013;85:631–635. doi: 10.1016/j.anbehav.2012.12.026. [DOI] [Google Scholar]
- Chase R, Darbyson E. Differential survival of allosperm by location within the female storage organ of the snail Cornu aspersum. Can J Zool. 2008;86:1244–1251. doi: 10.1139/Z08-109. [DOI] [Google Scholar]
- Baur B. Multiple paternity and individual variation in sperm precedence in the simultaneously hermaphroditic land snail Arianta arbustorum. Behav Ecol Sociobiol. 1994;35:413–421. doi: 10.1007/BF00165844. [DOI] [Google Scholar]
- Rogers D, Chase R. Determinants of paternity in the garden snail Helix aspersa. Behav Ecol Sociobiol. 2002;52:289–295. doi: 10.1007/s00265-002-0519-6. [DOI] [Google Scholar]
- Evanno G, Madec L, Arnaud JF. Multiple paternity and postcopulatory sexual selection in a hermaphrodite: what influences sperm precedence in the garden snail Helix aspersa? Mol Ecol. 2005;14:805–812. doi: 10.1111/j.1365-294X.2005.02449.x. [DOI] [PubMed] [Google Scholar]
- Garefalaki ME, Triantafyllidis A, Abatzopoulos TJ, Staikou A. The outcome of sperm competition is affected by behavioural and anatomical reproductive traits in a simultaneously hermaphroditic land snail. J Evol Biol. 2010;23:966–976. doi: 10.1111/j.1420-9101.2010.01964.x. [DOI] [PubMed] [Google Scholar]
- Rogers D, Chase R. Dart receipt promotes sperm storage in the garden snail Helix aspersa. Behav Ecol Sociobiol. 2001;50:122–127. doi: 10.1007/s002650100345. [DOI] [Google Scholar]
- Jordaens K, Pinceel J, Backeljau T. Mate choice in the hermaphroditic land snail Succinea putris (Stylommatophora: succineidae) Anim Behav. 2005;70:329–337. doi: 10.1016/j.anbehav.2004.10.021. [DOI] [Google Scholar]
- Frank SA. Sperm competition and female avoidance of polyspermy mediated by sperm-egg biochemistry. Evol Ecol Res. 2000;2:613–625. [Google Scholar]
- Levitan DR, Terhorst CP, Fogarty ND. The risk of polyspermy in three congeneric sea urchins and its implications for gametic incompatibility and reproduction isolation. Evolution. 2007;61:2007–2014. doi: 10.1111/j.1558-5646.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- Koene JM, Chase R. Changes in the reproductive tract of the snail Helix aspersa caused by mucus from the love dart. J Exp Biol. 1998;201:2313–2319. doi: 10.1242/jeb.201.15.2313. [DOI] [PubMed] [Google Scholar]
- Takeda N, Tsuruoka H. A sex pheromone secreting gland in the terrestrial snail, Euhadra peliomphala. J Exp Zool. 1979;207:17–25. doi: 10.1002/jez.1402070103. [DOI] [Google Scholar]
- Nagasawa T. Notes on the copulation and spawning of Euhadra quaesita. Newsl Malacol Soc Jpn. 1991;22:65–68. [Google Scholar]
- Bojat N, Sauder U, Haase M. Functional anatomy of the sperm storage organs in Pulmonata: the simple spermatheca of Bradybaena fruticum (Gastropoda, Stylommatophora) Zoomorphology. 2002;121:243–255. doi: 10.1007/s00435-002-0062-z. [DOI] [Google Scholar]
- Jordaens K, Dillen L, Backeljau T. Effects of mating, breeding system and parasites on reproduction in hermaphrodites: pulmonate gastropods (Mollusca) Anim Biol. 2007;57:137–195. doi: 10.1163/157075607780377965. [DOI] [Google Scholar]
- Martin OY, Hosken DJ. Strategic ejaculation in the common dung fly Sepsis cynipsea. Anim Behav. 2002;63:541–546. doi: 10.1006/anbe.2001.1929. [DOI] [Google Scholar]
- Jones TM, Featherston R, Paris DBBP, Elgar MA. Age-related sperm transfer and sperm competitive ability in the male hide beetle. Behav Ecol. 2006;18:251–258. doi: 10.1093/beheco/arl077. [DOI] [Google Scholar]
- Dreijers E, Reise H, Hutchinson JMC. Mating of the slugs Arion lusitanicus auct. non mabille and A. Rufus (L.): different genitalia and mating behaviours are incomplete barriers to interspecific sperm exchange. J Mollusc Stud. 2013;79:51–63. doi: 10.1093/mollus/eys033. [DOI] [Google Scholar]
- Kimura K, Chiba S. Delayed spermatophore removal in the land snail Euhadra peliomphala. Biol J Linnean Soc. 2013;108:806–811. doi: 10.1111/bij.12008. [DOI] [Google Scholar]
- Barker GM. In: The biology of terrestrial molluscs. Barker GM, editor. Wallingfrd: CABI Publishing; 2001. Gatropods on land: phylogeny, diversity and adaptive morphology; pp. 1–139. [Google Scholar]
- Gómez BJ. In: The biology of terrestrial molluscs. Barker GM, editor. Wallingfrd: CABI Publishing; 2001. Structure and function of the reproductive system; pp. 307–331. [Google Scholar]
- Koemtzopoulos E, Staikou A. Variation in spermathecal morphology is independent of sperm competition intensity in populations of the simultaneously hermaphroditic land snail Cornu aspersum. Zoology. 2007;110:139–146. doi: 10.1016/j.zool.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Locher R, Baur B. Effects of intermating interval on spermatophore size and sperm number in the simultaneously hermaphroditic land snail Arianta arbustorum. Ethology. 1999;105:839–849. doi: 10.1046/j.1439-0310.1999.00452.x. [DOI] [Google Scholar]
- Tan GN, Govedich FR, Burd M. Social group size, potential sperm competition and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: glossiphoniidae) J Evol Biol. 2004;17:575–580. doi: 10.1111/j.1420-9101.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Hänggi C, Locher R, Baur B. Intermating interval and number of sperm delivered in the simultaneously hermaphroditic land snail Arianta arbustorum (Pulmonata: helicidae) Veliger. 2002;45:224–230. [Google Scholar]
- Adamo SA, Chase R. Dissociation of sexual arousal and sexual proclivity in the garden snail Helix aspersa. Behav Neural Biol. 1990;54:115–130. doi: 10.1016/0163-1047(90)91310-8. [DOI] [PubMed] [Google Scholar]
- De Boer PACM, Jansen RF, Koene JM, Ter Maat A. Nervous control of male sexual drive in the hermaphroditic snail Lymnaea stagnalis. J Exp Biol. 1997;200:941–951. doi: 10.1242/jeb.200.5.941. [DOI] [PubMed] [Google Scholar]
- Locher R, Baur B. A new technique to assess the number of spermatozoa in spermatophores of stylommatophoran gastropods. J Mollus Stud. 1997;63:555–556. doi: 10.1093/mollus/63.4.555. [DOI] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GS. Mixed effects models and extensions in ecology with R. NY: Springer; 2009. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sperm transfer number of focal snails.


