Abstract
Fibrin formation and turnover are intimately associated with inflammation and wound healing. To explore whether fibrin(ogen)-derived peptides exert direct effects upon cells involved in inflammation and tissue repair we examined the capacity of human fibrinopeptide B (hFpB), a thrombin-derived proteolytic cleavage product of the fibrinogen B beta-chain, to stimulate neutrophils (PMN), monocytes, and fibroblasts. hFpB caused directed cell migration of PMN and fibroblasts that was optimal at approximately 10(-8) M. This chemotactic activity was blocked by preincubating hFpB with antiserum to hFpB. hFpB was not chemotactic for monocytes. The chemotactic potency of hFpB for PMN was equivalent to that of anaphylatoxin from the fifth component of human complement (C5a), leukotriene B4 (LTB4), and formyl-methionyl-leucyl-phenylalanine (fMLP), and for fibroblasts its chemotactic activity was comparable to that of platelet-derived growth factor. hFpB did not interact with PMN receptors for C5a, LTB4, or fMLP as (a) desensitization with 10(-7) M hFpB abolished chemotaxis to hFpB but had no effect upon chemotaxis to C5a, LTB4, or fMLP and (b) induction of chemotactic responses to fMLP and LTB4 in neutrophilic leukemic cells (HL-60 cells) by incubation with dimethylsulfoxide did not extend to hFpB. Like fMLP, hFpB caused a rapid, dose-dependent increase in PMN cytoskeletal associated actin, but unlike fMLP, hFpB did not cause PMN aggregation, release of lysosomal enzymes (lysozyme and beta-glucuronidase), or the production of superoxide anion. These results suggest that hFpB may have a role in recruiting PMN and fibroblasts at sites of fibrin deposition and turnover. The capacity of hFpB to cause PMN chemotaxis without causing concurrent release of lysosomal enzymes or the production of superoxide anion is further evidence for the complexity of PMN responses to chemotactic agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983 Jan;96(1):282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J. G., Van Epps D. E. Stimulus interactions in release of superoxide anion (O2-) from human neutrophils. Further evidence for multiple pathways of activation. Inflammation. 1985 Mar;9(1):67–79. doi: 10.1007/BF00915413. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. B., Mosesson M. W., Dvorak H. F. Delayed-type hypersensitivity skin reactions in congenital afibrinogenemia lack fibrin deposition and induration. J Clin Invest. 1979 Jun;63(6):1302–1306. doi: 10.1172/JCI109425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Bell W. R., Kaiser D., Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 1985 Mar 22;227(4693):1487–1490. doi: 10.1126/science.4038818. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Curtiss L. K., Plow E. F. A linkage between the hemostatic and immune systems embodied in the fibrinolytic release of lymphocyte suppressive peptides. J Immunol. 1985 Jan;134(1):471–477. [PubMed] [Google Scholar]
- Francis C. W., Markham R. E., Jr, Barlow G. H., Florack T. M., Dobrzynski D. M., Marder V. J. Thrombin activity of fibrin thrombi and soluble plasmic derivatives. J Lab Clin Med. 1983 Aug;102(2):220–230. [PubMed] [Google Scholar]
- Kaminski M., McDonagh J. Studies on the mechanism of thrombin. Interaction with fibrin. J Biol Chem. 1983 Sep 10;258(17):10530–10535. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. Activation and control mechanisms of Hageman factor-dependent pathways of coagulation, fibrinolysis, and kinin generation and their contribution to the inflammatory response. J Allergy Clin Immunol. 1975 Dec;56(6):491–506. doi: 10.1016/0091-6749(75)90067-6. [DOI] [PubMed] [Google Scholar]
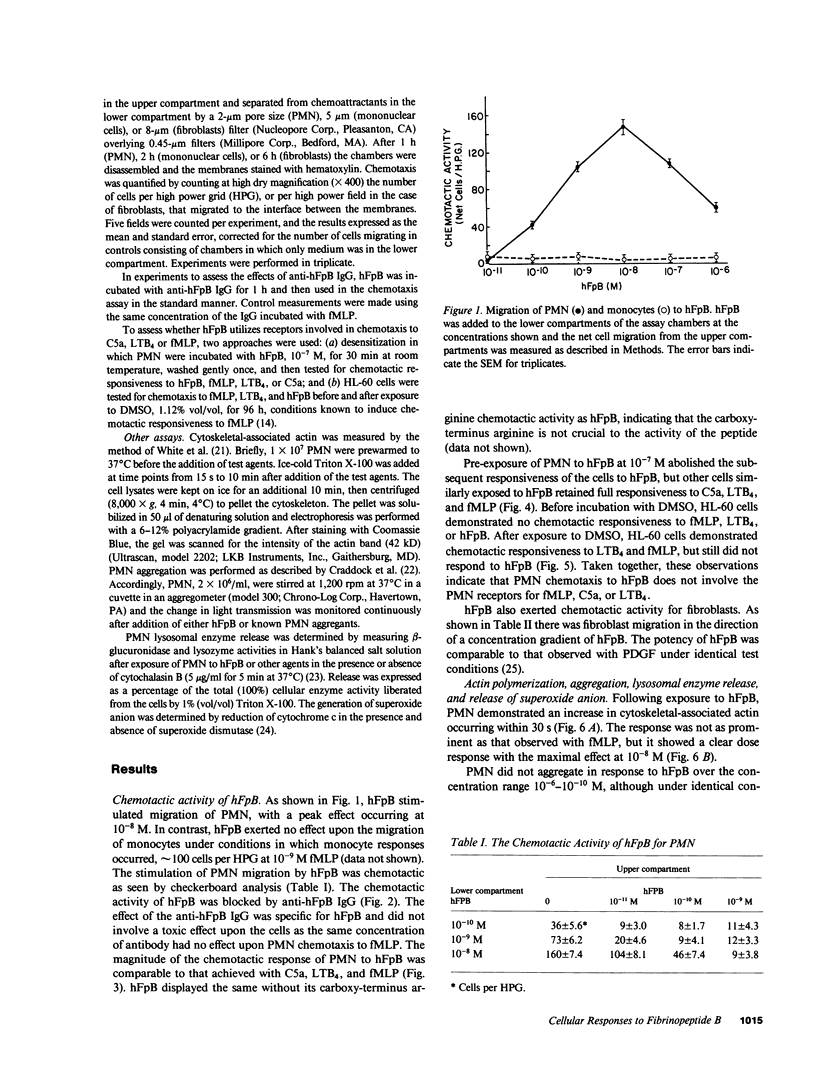
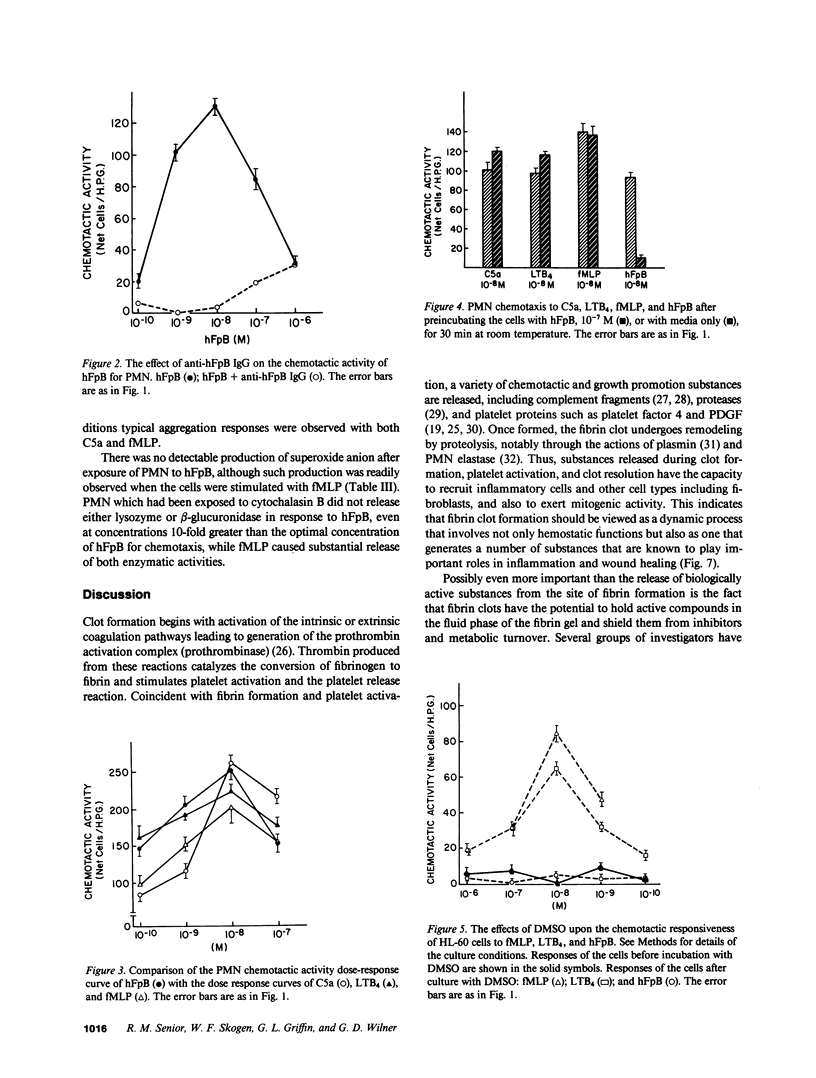
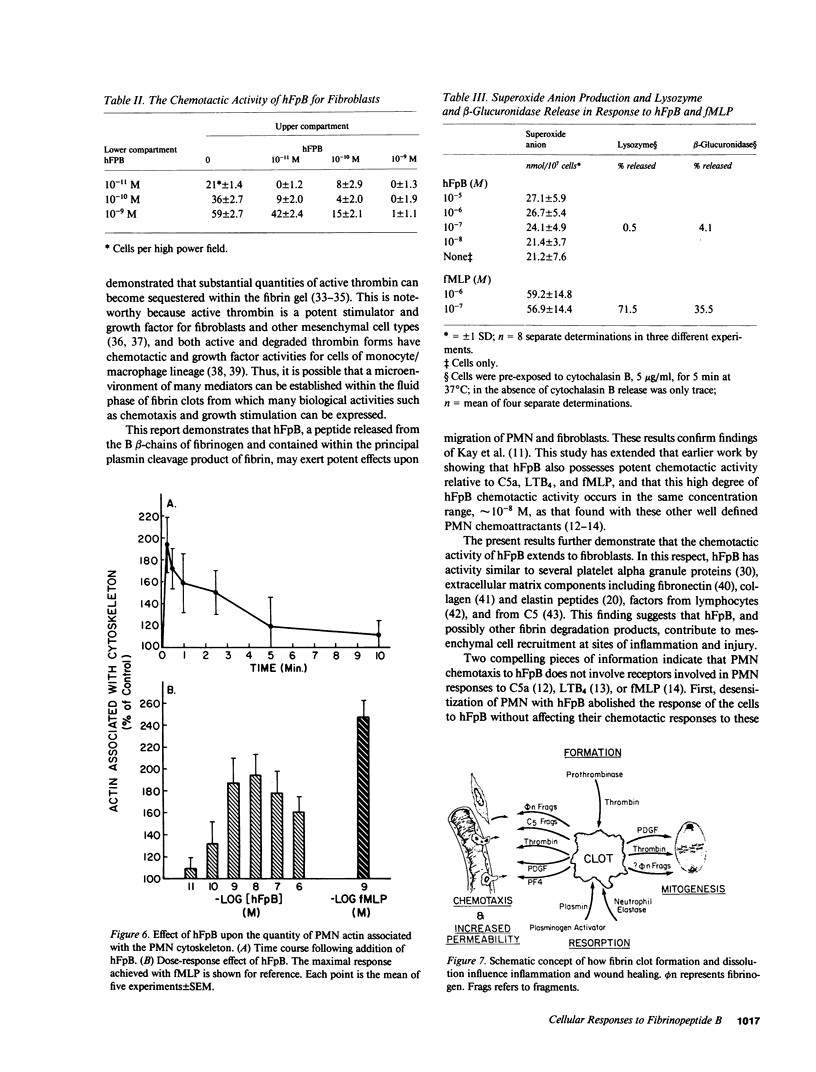
- Kay A. B., Pepper D. S., McKenzie R. The identification of fibrinopeptide B as a chemotactic agent derived from human fibrinogen. Br J Haematol. 1974 Aug;27(4):669–677. doi: 10.1111/j.1365-2141.1974.tb06633.x. [DOI] [PubMed] [Google Scholar]
- Kreisle R. A., Parker C. W. Specific binding of leukotriene B4 to a receptor on human polymorphonuclear leukocytes. J Exp Med. 1983 Feb 1;157(2):628–641. doi: 10.1084/jem.157.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G. Membrane-bound enzyme complexes in blood coagulation. Prog Hemost Thromb. 1984;7:1–23. [PubMed] [Google Scholar]
- Marder S. R., Chenoweth D. E., Goldstein I. M., Perez H. D. Chemotactic responses of human peripheral blood monocytes to the complement-derived peptides C5a and C5a des Arg. J Immunol. 1985 May;134(5):3325–3331. [PubMed] [Google Scholar]
- McKenzie R., Pepper D. S., Kay A. B. The generation of chemotactic activity for human leukocytes by the action of plasmin on human fibrinogen. Thromb Res. 1975 Jan;6(1):1–8. doi: 10.1016/0049-3848(75)90145-0. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Lange G., Madaras J., Starcher B. Elastin synthesis by ligamentum nuchae fibroblasts: effects of culture conditions and extracellular matrix on elastin production. J Cell Biol. 1981 Aug;90(2):332–338. doi: 10.1083/jcb.90.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossel H. L. Relative proteolysis of the fibrinogen B beta chain by thrombin and plasmin as a determinant of thrombosis. Nature. 1981 May 14;291(5811):165–167. doi: 10.1038/291165a0. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Vitkauskas G., Becker E. L., Ward P. A. Selective neutrophil desensitization to chemotactic factors. J Cell Biol. 1979 Mar;80(3):564–572. doi: 10.1083/jcb.80.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue J. F., Lubenskyi W., Kivity E., Sonder S. A., Fenton J. W., 2nd Protease mitogenic response of chick embryo fibroblasts and receptor binding/processing of human alpha-thrombin. J Biol Chem. 1981 Mar 25;256(6):2767–2776. [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. An alternative pathway for fibrinolysis. I. The cleavage of fibrinogen by leukocyte proteases at physiologic pH. J Clin Invest. 1975 Jul;56(1):30–38. doi: 10.1172/JCI108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F. Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. J Clin Invest. 1982 Mar;69(3):564–572. doi: 10.1172/JCI110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Seyer J. M., Kang A. H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978 Feb;75(2):871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. Generation of a fibroblast chemotactic factor in serum by activation of complement. J Clin Invest. 1979 Nov;64(5):1379–1385. doi: 10.1172/JCI109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. L., Pepper D. S., Kay A. B. Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol. 1976 Apr;32(4):507–513. doi: 10.1111/j.1365-2141.1976.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Rowland F. N., Donovan M. J., Picciano P. T., Wilner G. D., Kreutzer D. L. Fibrin-mediated vascular injury. Identification of fibrin peptides that mediate endothelial cell retraction. Am J Pathol. 1984 Dec;117(3):418–428. [PMC free article] [PubMed] [Google Scholar]
- Saldeen K., Christie N., Nelson W. R., Movat H. Z. Effect of a fibrin(ogen)-derived vasoactive peptide on polymorphonuclear leukocyte emigration. Thromb Res. 1985 Jan 1;37(1):85–89. doi: 10.1016/0049-3848(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest. 1982 Sep;70(3):614–618. doi: 10.1172/JCI110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H. E., Yamada K. M., Seppä S. T., Silver M. H., Kleinman H. K., Schiffmann E. The cell binding fragment of fibronectin is chemotactic for fibroblasts. Cell Biol Int Rep. 1981 Aug;5(8):813–819. doi: 10.1016/0309-1651(81)90253-8. [DOI] [PubMed] [Google Scholar]
- Shefcyk J., Yassin R., Volpi M., Molski T. F., Naccache P. H., Munoz J. J., Becker E. L., Feinstein M. B., Sha'afi R. I. Pertussis but not cholera toxin inhibits the stimulated increase in actin association with the cytoskeleton in rabbit neutrophils: role of the "G proteins" in stimulus-response coupling. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1174–1181. doi: 10.1016/0006-291x(85)90309-2. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. N-Formylmethionyl peptide receptors on equine leukocytes initiate secretion but not chemotaxis. Science. 1980 Jul 25;209(4455):493–495. doi: 10.1126/science.6248959. [DOI] [PubMed] [Google Scholar]
- Snyderman R. Regulatory mechanisms of a chemoattractant receptor on leukocytes. Fed Proc. 1984 Sep;43(12):2743–2748. [PubMed] [Google Scholar]
- Spilberg I., Mandell B., Mehta J., Sullivan T., Simchowitz L. Dissociation of the neutrophill functions of exocytosis and chemotaxis. J Lab Clin Med. 1978 Aug;92(2):297–302. [PubMed] [Google Scholar]
- Stecher V. J., Sorkin E. The chemotactic activity of fibrin lysis products. Int Arch Allergy Appl Immunol. 1972;43(6):879–886. doi: 10.1159/000230905. [DOI] [PubMed] [Google Scholar]
- Sueishi K., Nanno S., Tanaka K. Permeability enhancing and chemotactic activities of lower molecular weight degradation products of human fibrinogen. Thromb Haemost. 1981 Feb 23;45(1):90–94. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Sulavik M. C., Johnson K. J. Activated rat neutrophils. Correlation of arachidonate products with enzyme secretion but not with O(2)- generation. Am J Pathol. 1985 Jul;120(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Sha'afi R. I. Stimulation by chemotactic factor of actin association with the cytoskeleton in rabbit neutrophils. Effects of calcium and cytochalasin B. J Biol Chem. 1983 Nov 25;258(22):14041–14047. [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]
- Wilner G. D., Thomas D. W., Nossel H. L., Robbins P. F., Mudd M. S. Immunochemical analysis of rabbit antihuman fibrinopeptide B antibodies. Biochemistry. 1979 Nov 13;18(23):5078–5082. doi: 10.1021/bi00590a009. [DOI] [PubMed] [Google Scholar]