Abstract
Background:
This study was designed to evaluate the comparison of insertion of levonorgestrel (LNG)-releasing intrauterine system versus oral medroxyprogesterone acetate on endometrial hyperplasia in a randomized controlled trial.
Materials and Methods:
A total of 60 women with the initial histopathological diagnosis of endometrial hyperplasia in two groups received LNG or medroxyprogesterone (10 mg/d orally) for 12 days a month for 3 months). Endometrial biopsy was obtained for all patients after 3 months of treatment. Response to treatment was defined based on the histopathology of the post treatment pipelle endometrial specimens in three categories of resolution, persistence and progression.
Results:
Treatment response rate in patients in the LNG group was 89.3% (25 of 28 patients), versus 70.4% (19 of 27 patients) in patients in the medroxyprogesterone group. The rate of persistence was 10.7% (3 of 28 patients) and 22.2% (6 of 27 patients) in LNG and medroxyprogesterone groups respectively. No progression of endometrial hyperplasia observed in any of the patients in LNG group, but progression of endometrial hyperplasia was observed in 7.4% (2 of 27 patients) in the medroxyprogesterone group. There was no statistically significant difference between groups regarding the response to treatment (P = 0.15). Side effects such as bloating, weight gain, fatigue and hair loss were comparable between the groups (P > 0.05). Hirsutism was significantly more in the medroxyprogesterone group than LNG group (P = 0.013).
Conclusion:
Results showed that the use of LNG for treating endometrial hyperplasia for 3 months was associated with high-treatment response rate and the low proportion of patients with progression compared to the use of medroxyprogesterone.
Keywords: Endometrial hyperplasia, levonorgestrel-releasing intrauterine system, medroxyprogesterone
INTRODUCTION
Endometrial cancer comprises about 4% of all cancers in women globally, and in young patients is a very serious social and medical concern. In the industrialized world, endometrial cancer is now considered the most frequent gynecologic malignancy and the incidence is still rising. Endometrial hyperplasia as the most common malignancy of the female genital tract can progress to adenocarcinoma.[1,2] To prevent the development of endometrial cancer, correct and accurate diagnosis, and optimal treatment of endometrial hyperplastic lesions are essential. The treatment is hysterectomy or hormone therapy with progesterone.[3]
For endometrial hyperplasia, hysterectomy can be expected to provide a complete cure but it can occasionally have serious side-effects including death and it requires time away from paid and unpaid work with follow on effects for employers, family and the community.[4,5,6] Hormone therapy with progesterone which antagonize the estrogen effect on the endometrium, can induce endometrial regression, and prevent progression to cancer may be appealed for many women as less invasive medical therapies.[7] However, complain of side effects such as breast tenderness, mood changes and weight gain find in some daily dose of oral progestin.[4] To treat endometrial hyperplasia the main progestational agents used are oral norethisterone acetate, megestrol acetate, and medroxyprogesterone acetate.[8,9,10] Medroxyprogesterone acetate has been the most frequently used progestagen with numerous side effects such as headache, nausea, and also long-term use results in metabolic changes and exposes the woman to a higher risk of thromboembolic events.[11]
In recent times, the levonorgestrel-releasing intrauterine system (LNG-IUS) is another mode of progestin administration which developed primarily as a contraceptive device is an alternative treatment option for endometrial hyperplasia and has been used successfully to treat endometrial hyperplasia.[4,7] LNG-IUS has been shown to have excellent tolerability with high-intrauterine and is used extensively for the treatment of heavy menstrual bleeding.[12,13] It is shown that LNG-IUS is effective for up to 5 years and has become a popular alternative to hysterectomy to manage menorrhagia.[14] This system has been proven to achieve higher concentrations of progestogens in the endometrium by almost 100-fold compared with oral administration.[13]
Despite the success in the treatment of proliferative endometrial processes, recurrence and resistance to hormonal therapy is one of the problems concerning therapeutic approach.[15,16] Furthermore, even though treatment of endometrial hyperplasia with the LNG-IUD option has become more common during the last decades, the data on effective application of LNG-IUS in atypical hyperplasia are limited and some small studies have also shown that the LNG-IUS causes regression of endometrial hyperplasia with no atypia. In Iran this therapy is not yet routinely recommended. Hence, the present study was aimed to assess the comparison of insertion of LNG-IUS versus oral medroxyprogesterone acetate on endometrial hyperplasia.
MATERIALS AND METHODS
This randomized, parallel-group, double blind clinical trail was conducted between July, and December, 2013, on 60 women with the initial histopathological diagnosis of endometrial hyperplasia who were referred to in Beheshti Hospital in Isfahan, Iran. The Ethics Committee of Isfahan University of Medical Sciences approved this study (No, 3994), and written informed consent was obtained from all studied patients.
Patients with endometrial hyperplasia (simple or complex) who had no desire for pregnancy in the coming 3 years, and did not receive hormonal treatment prior to therapy for endometrial hyperplasia were eligible. Furthermore, exclusion criteria included sleeping disorders, breastfeeding, congenital uterine abnormality, history of vascular or coagulation disorders, concomitant use of medication or presence of an underlying disease/condition known to affect the metabolism or pharmacokinetics of the study medications, allergy to progestin and family history of breast cancer.
A total of 60 eligible patients were randomly divided into two 30-member groups using random-maker software “Random Allocation”. Group LNG include patients with insertion of LNG-IUS (Bayer Schering Pharma, Berlin, Germany, with release rate of LNG 20 μg/day). Group medroxyprogesterone include patients who received 10 mg/daily oral medroxyprogesterone acetate (Aburaihan pharmaceutical company, Iran) for 12 days a month for 3 months.
Outpatient pipelle endometrial biopsy was obtained between the 20th and 24th day of the menstrual cycle for each patient after 3 months of treatment. The outcome was determined by comparing the diagnosis of the follow-up pipelle endometrial biopsy with the initial histologic diagnosis. The gynecologic pathologist was blinded to the modality of treatment. The patients in group medroxyprogesterone were advised to use condoms as a contraceptive method during the treatment period.
Collected data included age, body mass index (BMI), waist, triglyceride, cholesterol, history of diabetes, hypertension, menstrual status, treatment outcomes, and side effects which were recorded for each group.
Response to treatment as the main outcome was defined based on the histopathology of the post treatment pipelle endometrial specimens in three categories included resolution, persistence and progression. Resolution was defined as posttreatment diagnosis of secretory, proliferative, inactive or atrophic pattern endometrium. Persistence was defined as post treatment diagnosis of endometrial hyperplasia if the specimen showed simple endometrial hyperplasia. Progression was defined as post treatment diagnosis of endometrial hyperplasia if the specimen showed complex endometrial hyperplasia and/or atypia.
Statistical analyses
The sample size was calculated with two-sided log-rank test, α = 0.05, and 80% power. All statistical analyses were done using SPSS software for Windows, version 20 (SPSS Inc., Chicago, IL, USA). Descriptive data are reported as mean ± standard deviation or number (percent) as appropriate. Normality of data was assessed by Shapiro-Wilk normality test and all data were normally distributed (P > 0.05). Independent sample t-test and Chi-square test were used to comparing all studied variables between groups also if assumption for Chi-square were not established, Fisher exact test or exact method were used as appropriate. P < 0.05 were considered statistically significant.
RESULTS
Figure 1 shows the flowchart of the study, eight of 68 reviewed patients did not enter to the study (three did not eligible, and five refused informed consent). During follow-up 2 patients in LNG group and 3 patients in the medroxyprogesterone group were excluded from the study due to the adverse effect. Finally, 55 patients (27 in the medroxyprogesterone group and 28 in LNG group) completed the study and analyzed.
Figure 1.
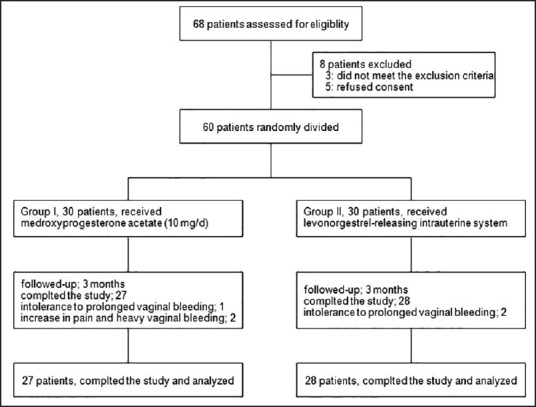
Patients who entered to the study, divided into the study groups and analyzed
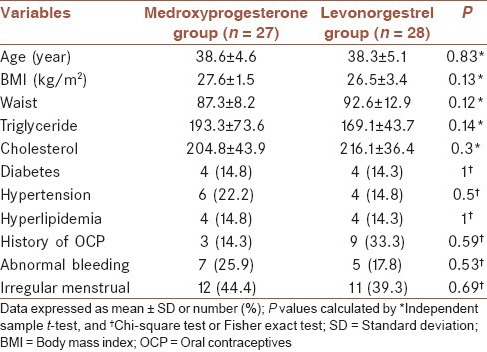
The mean age of the studied patients was 38.4 ± 4.8 years old. Table 1 shows baseline characteristics of patients between studied groups. No significant differences were noted between groups for mean of age, BMI, waist, triglyceride, and cholesterol, history of diabetes, hypertension and irregular menstrual (P > 0.05).
Table 1.
Baseline characteristics in 55 studies patients by groups
Patients in the LNG group showed a rate of resolution 89.3%, where the resolution rate in patients in the medroxyprogesterone group was 70.4%. A regression rate of 25% was showed in the patients in LNG group whereas patients receiving medroxyprogesterone showed a regression rate of 33.4%. The rate of persistence in LNG and medroxyprogesterone groups was 10.7% and 22.2%, respectively. No progression of endometrial hyperplasia occurred in any of the patients in LNG group, while progression of endometrial hyperplasia occurred in 2 patients in the medroxyprogesterone group. There was no statistically significant difference between groups regarding the response to treatment [Table 2].
Table 2.
Outcomes of different modalities of treatment after 3 months of therapy in 55 studies patients by groups
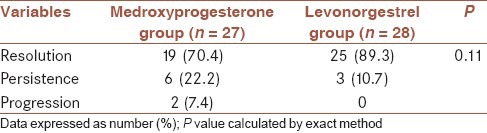
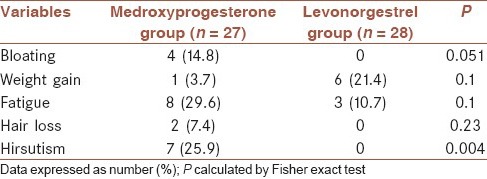
Table 3 shows the comparison of the side effects between the groups. The side effects were comparable between the groups. Bloating developed in 2 patients in the medroxyprogesterone group and no patients in the LNG group, weight gain was occurred in 1 patients in the medroxyprogesterone group and 6 patients in the LNG group, fatigue was occurred in eight of patients in the medroxyprogesterone group and 3 patients in the LNG group and also hair loss was reported in 2 patients in the medroxyprogesterone group, where no patients in the LNG group reported hair loss. There was no statistically significant difference between groups regarding the presence of bloating, weight gain, fatigue and hair loss (P > 0.05). Hirsutism was significantly more occurred in patients in the medroxyprogesterone group compare to LNG group (P = 0.004).
Table 3.
The side effects in 55 studies patients by groups
DISCUSSION
Endometrial hyperplasia is a common disease. After oral low-dose progestin therapy, in up to 50% of patients, therapy failure has been reported.[8,17] Several clinical studies have demonstrated during the last decades that in the treatment of endometrial hyperplasia the LNG-IUS have been a safe and effective therapy with complete response, and also it is shown to represent a sufficient alternative to hysterectomy.[12,18]
In this randomized study, LNG-IUS was compare with oral medroxyprogesterone acetate in the treatment of endometrial hyperplasia and our findings show that in patients in the LNG group the rate of resolution was more than patients in the medroxyprogesterone group, but persistence and progression rate in the patients in LNG group were lower than in patients who received medroxyprogesterone. However, the differences between the groups were not statistically significant. Bloating, weight gain, fatigue and hair loss as side effects were similar in both studied groups. Hirsutism was significantly more occurred in the medroxyprogesterone group than LNG group.
In a systematic review and metaanalysis,[7] a total of 24 primary studies, including 1001 women with endometrial hyperplasia were review. Results in this study showed that response to treatment of endometrial hyperplasia in women treated with oral progestogens was 66% for complex hyperplasia and 69% for atypical hyperplasia which was lower compared with women treated with LNG-IUS, 92%, for complex hyperplasia and 90% for atypical hyperplasia, which were statistically significant between the two treatments. For simple hyperplasia response to treatment was not significantly difference between the two treatments (89% in LNG group vs. 96% in the medroxyprogesterone group). In a study by Buttini et al. study[4] among 10 women who used oral progestin treatment (10-20 mg daily), treatment response rate was 90%. Furthermore, they showed that in all 21 women, treated with LNG-IUS treatment response rate was 100%. In similar to these studies, our results showed that response to treatment rate in the medroxyprogesterone group was lower than in LNG group (89.3% in LNG group vs. 70.4% in the medroxyprogesterone group), but was not statistically significant between the two treatments. Response to treatment in both groups in systematic review and Buttini et al. studies[4,7] was more than response to treatment rate in our study, the difference between these results may be is due to difference in the design of studies whereas the present study is a randomized, parallel-group study, but all studies in systematic review and Buttini et al. study were observational studies that are tense with potential biases and confounders. Furthermore, the difference could be explained by the longer duration of follow-up in systematic review compare to 3 months follow-up in our study.
In a study by Ismail et al. LNG-IUS and medroxyprogesterone (10 mg daily) were compared in 60 patients and authors reported that the rate of resolution in the LNG-IUS group was 66.67% and in the medroxyprogesterone group was 36.66%.[18] This was higher than our results with resolution rate of 89.3% in LNG group and 70.4% in the medroxyprogesterone group. The higher rate of response to treatment in our study compare to results of Ismail et al. study could be explained by the difference in studied patients whereas our study included patients with all types of endometrial hyperplasia but in Ismail et al.[18] study patients included endometrial hyperplasia patients without atypia.
Our results are in accordance with those obtained by Vereide et al. reported that, after 3 months, all patients treated with LNG-IUS showed regression of hyperplasia, whereas 55% of patients in the medroxyprogesterone (10 mg daily) group had a response to treatment.[14] Furthermore, in the results of Ørbo et al. response to treatment was reported 100% with the LNG-IUS, and they conclude that the LNG-IUD treatment was significantly superior to oral medroxyprogesterone (10 mg daily) treatment.[19] The higher rate of response to treatment in Vereide et al. and Ørbo et al. studies[17,19] compare to our results could be explained by the difference in the design of the studies and the difference in the duration of follow-up.
Our results are in accordance with those obtained by Ismail et al.[18] who reported a regression rate of 33.3% and 60%, for LNG-IUD and medroxyprogesterone treatment respectively. The regression rate in our study in LNG and medroxyprogesterone groups was 25 and 33.4% respectively. The rate of persistence in our results was 10.7% and 22.2% for LNG and medroxyprogesterone groups, respectively. However, in Ismail et al. study[18] persistence rate was 3.3% in the medroxyprogesterone group whereas in LNG-IUD group persistence was no observed in any of studied patients. The differences in results could be explained by differences in studied patients, our study included patients with all types of endometrial hyperplasia but Ismail et al. study included endometrial hyperplasia patients without atypia.
CONCLUSION
According to the results of the present study, even though relatively small in sample size, showed that between LNG and medroxyprogesterone in the treatment of endometrial hyperplasia were not different but the use of LNG for treating endometrial hyperplasia for 3 months was associated with high-treatment response rate and low proportion of patients with progression compared to the use of medroxyprogesterone. Furthermore, side effects in LNG group were lower than the medroxyprogesterone group. Thus, LNG appears to represent an effective, convenient treatment option for endometrial hyperplasia; however, future studies with appropriate sample size, different does of LNG and longer follow-up are warranted.
AUTHOR'S CONTRIBUTION
FB and AGH carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. MT provided assistance in the design of the study, coordinated and carried out all the experiments and manuscript preparation.
ACKNOWLEDGMENT
This study was supported by a grant from Isfahan University of medical sciences, Isfahan, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Karimi-Zarchi M, Dehghani-Firoozabadi R, Tabatabaie A, Dehghani-Firoozabadi Z, Teimoori S, Chiti Z, et al. A comparison of the effect of levonorgestrel IUD with oral medroxyprogesterone acetate on abnormal uterine bleeding with simple endometrial hyperplasia and fertility preservation. Clin Exp Obstet Gynecol. 2013;40:421–4. [PubMed] [Google Scholar]
- 2.Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: Clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004;14:348–53. doi: 10.1111/j.1048-891x.2004.014220.x. [DOI] [PubMed] [Google Scholar]
- 3.Orbo A, Arnes M, Pettersen I, Larsen K, Hanssen K, Moe B. Down-regulated progesterone receptor A and B coinciding with successful treatment of endometrial hyperplasia by the levonorgestrel impregnated intrauterine system. Acta Obstet Gynecol Scand. 2010;89:1438–46. doi: 10.3109/00016349.2010.512068. [DOI] [PubMed] [Google Scholar]
- 4.Buttini MJ, Jordan SJ, Webb PM. The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: An Australian study and systematic review. Aust N Z J Obstet Gynaecol. 2009;49:316–22. doi: 10.1111/j.1479-828X.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 5.Wildemeersch D, Dhont M. Treatment of nonatypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol. 2003;188:1297–8. doi: 10.1067/mob.2003.346. [DOI] [PubMed] [Google Scholar]
- 6.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–7. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 7.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: A systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:547.e1–10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 9.Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21:248–56. doi: 10.1093/humrep/dei290. [DOI] [PubMed] [Google Scholar]
- 10.Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85:314–25. doi: 10.1016/j.fertnstert.2005.07.1315. [DOI] [PubMed] [Google Scholar]
- 11.Lähteenmäki P, Rauramo I, Backman T. The levonorgestrel intrauterine system in contraception. Steroids. 2000;65:693–7. doi: 10.1016/s0039-128x(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf) 1982;17:529–36. doi: 10.1111/j.1365-2265.1982.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 13.Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivelä A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: Randomized trial 5-year follow-up. JAMA. 2004;291:1456–63. doi: 10.1001/jama.291.12.1456. [DOI] [PubMed] [Google Scholar]
- 14.Pashov AI, Diduk OV. Obstetrics and Gynecology. Krasnoyarsk: Pressing Questions: The Collection of Proceedings; 2009. The nature of a cancer of a body of a uterus in Krasnoyarsk edge; pp. 93–8. [Google Scholar]
- 15.Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: Long-term follow-up. Obstet Gynecol. 2002;99:709–19. doi: 10.1016/s0029-7844(02)01945-2. [DOI] [PubMed] [Google Scholar]
- 16.Lindahl B, Alm P, Fernö M, Norgren A. Endometrial hyperplasia: A prospective randomized study of histopathology, tissue steroid receptors and plasma steroids after abrasio, with or without high dose gestagen treatment. Anticancer Res. 1990;10:725–30. [PubMed] [Google Scholar]
- 17.Vereide AB, Arnes M, Straume B, Maltau JM, Ørbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: A study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2003;91:526–33. doi: 10.1016/j.ygyno.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Ismail MT, Fahmy DM, Elshmaa NS. Efficacy of levonorgestrel-releasing intrauterine system versus oral progestins in treatment of simple endometrial hyperplasia without atypia. Reprod Sci. 2013;20:45–50. doi: 10.1177/1933719112459243. [DOI] [PubMed] [Google Scholar]
- 19.Ørbo A, Arnes M, Hancke C, Vereide AB, Pettersen I, Larsen K. Treatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation only. Gynecol Oncol. 2008;111:68–73. doi: 10.1016/j.ygyno.2008.06.014. [DOI] [PubMed] [Google Scholar]


