Abstract
Background:
Preterm labor is the most common complication of the pregnancy in the second trimester and has been suggested as the cause of two-thirds of neonatal mortality. Antenatal corticosteroid is used for fetal lung maturity in preterm labor and makes a significant reduction in the incidence of respiratory distress syndrome (RDS). The aim of this study was to compare the prenatal administration of single and multiple courses of betamethasone and neonatal outcomes, effectiveness and safety of its weekly administration.
Materials and Methods:
A randomized, double-blind placebo-control clinical trial study conducted in pregnant women at risk for preterm birth by gestational age between 28 and 35 weeks. The women received a course of betamethasone at first, and then divided into a single course and multiple betamethasone courses. They evaluated for the incidence of RDS, need for oxygen, surfactant administration, the need for ventilation, duration of hospitalization and neonatal mortality. Data were analyzed using SPSS-version 16 and Chi-square test and t-test.
Results:
The need for O2, the incidence of RDS, the need for hospitalization, days of hospitalization, the need for continuous positive airway pressure, ventilation and surfactant and the mortality significantly lower in the multiple course groups and betamethasone had a clear positive effect in this regard. Mean weight, height and head circumferences were significantly lower in the multiple course group.
Conclusion:
Despite a positive impact of multiple betamethasone usage on mortality and morbidity in neonates, it is recommended to avoid routinely using of betamethasone multiple courses until the adequate data of studies prove the safety of reduction in weight, height, and head circumference in a long period.
Keywords: Betamethasone, neonate, preterm
INTRODUCTION
Preterm labor (before 37 weeks) is the most common complication lead to the death in the second half of pregnancy in seven to 10% of deliveries. One of the main reasons of preterm labor is neonatal respiratory distress syndrome (RDS).[1] Since 30 years ago glucocorticoids was used for preterm labor to mature fetal lung and make a significant reduction in the incidence of RDS, ventricular hemorrhage and neonatal morbidity and mortality up to 50%.[2] Stimulation of the alveolar surfactant release through the gene expression is the most important effect of glucocorticoids. Production of antioxidant enzymes, Lung liquid absorption and alveolar maturation (different effects of glucocorticoids) decrease the incidence of respiratory distress, and neonatal mortality provided that the delivery is delayed for 24 h after injection.[3] Specialized National Institutes of Health (NIH) recommended corticosteroids prescription for fetal lung maturation in cases of threatened preterm labor in 1955. These recommendations continued universally. NIH recommend a single course of prenatal betamethasone in the pregnant women with gestational age between 34 and 24 weeks that are at risk of premature delivery.[4,5] It is believed that corticosteroids have maximum effects when 24 h to 7 days after receiving corticosteroid courses the neonate has been delivered. Weekly injections of corticosteroids have been designed on the same basis.[6,7,8] It has been reported that administration of repeated dose betamethasone versus single dose caused a significant reduction in neonatal morbidity and improved the neonatal prognosis but reduces weight. However, another study found no change in weight, height and head circumference. Due to infant mortality due to RDS is a common cause of mortality, interventions to reduce the RDS lead to lower infant mortality.[9,10] The aim of this study was to compare the prenatal administration of single and multiple courses of betamethasone, neonatal outcomes and the efficacy and safety of repeated administration of betamethasone.
PATIENTS AND METHODS
Method and samples
A randomized, double-blind placebo-control clinical trial study was conducted. The subjects included 1348 women with 28-35 gestational ages who referred to five medical centers in North of Iran.
This study approved as a project in the Ethics Committee of Mazandaran University of medical sciences, and also the study registered in IRCT (Iran) with the following registration code: IRCT138711261689N1.
The inclusion and exclusion criteria were as follow: Inclusion criteria included all patients with a risk of spontaneous preterm labor or preterm delivery history, placenta previa, chronic detachment and cerclage history. Patients at risk for preterm labor was defined as patients with a gestational age of 28-35 weeks, painful or painless uterine contractions, symptoms such as lower abdomen pain and cervical dilatation <3 cm. Exclusion criteria included premature rupture of fetal membranes before entering the study, major fetal anomalies, chorioamnionitis, taking systemic corticosteroids during pregnancy, insulin-dependent diabetes and intrauterine growth restriction. All study procedures were explained to the patients, and they were enrolled after obtaining written informed consent.
Materials and study process
Maternal gestational age was determined based on last menstrual period (LMP) and ultrasounds performed at weeks 11-20 of gestation. In cases which mothers LMP was not adapted to the ultrasounds, gestational age were calculated based on the first ultrasonography which was conducted weeks 11-20. In this study, betamethasone that had been made by the Iran Hormone Pharmaceutical Corporation was used. Placebo packages were similar to betamethasone ones and had been made by the same company. Betamethasone courses prescribed by intramuscular injection of 12 mg (3 cc) and repeated 24 h after the first injection. 3 cc placebos were injected similarly. The 1348 women received the first course of betamethasone. The patients were then randomly divided into two groups, placebo (single course of betamethasone) and case group (multiple courses of betamethasone) by the table of random numbers. Injections of placebo in single course group and betamethasone in multiple course groups repeated each 10 days up to two courses. These were conducted in the research center by a trained nurse. Both the patients and nurses were blinded to the injected material. Characteristics of maternal age and gravidity and number of receiving courses were recorded in a questionnaire. Weekly contact with the mother until delivery and in the event of preterm birth, height, weight, and head circumference were measured by trained nurses 72 h of birth. Infants were evaluated in regard to the incidence of RDS, need for oxygen, surfactant administration, the need for neonatal ventilation, duration of hospital stay and mortality until discharge from hospital. Infants were matched based on gestational age. Neonatal outcomes, infant weight, Height, and head circumference, the incidence of RDS, need for oxygen, require an auxiliary positive-pressure ventilation continuous positive airway pressure (CPAP), receiving surfactant and mortality in pregnancy in a single course of betamethasone and repeated courses were compared.
Statistical analysis
Data entered to Excel 2007 software and reviewed via software package used for statistical analysis version 18.0 (SPSS Inc., Chicago). Descriptive analysis of quantitative and qualitative data presented as mean ± standard deviation and frequency tables respectively. Furthermore, nominal data analyzed using Chi-square test and t-test used for examining the difference between sample means in two groups. Significance level determined ≤0.05.
RESULTS
There were 674 women in each group. The mean age of patients in the placebo group (single course) was 23/54 years, and multiple courses group was 23/4 years (P > 0.05). 768 participants were nulligravid, and 580 were multi-gravid. Gravidity distribution was similar in both groups. Of the 674 women in the placebo group, 48 patients were excluded from the study. 310 patients, who had received betamethasone, were delivered after being term, and 316 patients had preterm labor. In the case group, 56 patients were excluded. 347 patients had term deliveries, and 271 were cases of preterm labor. Thus, a total of 587 patients were analyzed as preterm labor, of whom 316 (53/8%) were in the placebo group and 271 patients (46/2%) were in the multiple course group [Table 1]. 138 patients of the case group received two courses, and 149 patients received three courses of betamethasone. The whole gestational ages of the neonates is shown in Figure 1.
Table 1.
Demographic features of patients
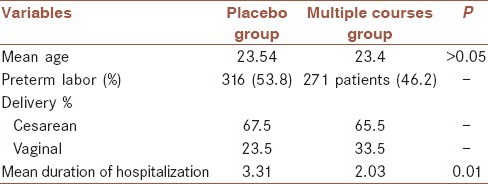
Figure 1.
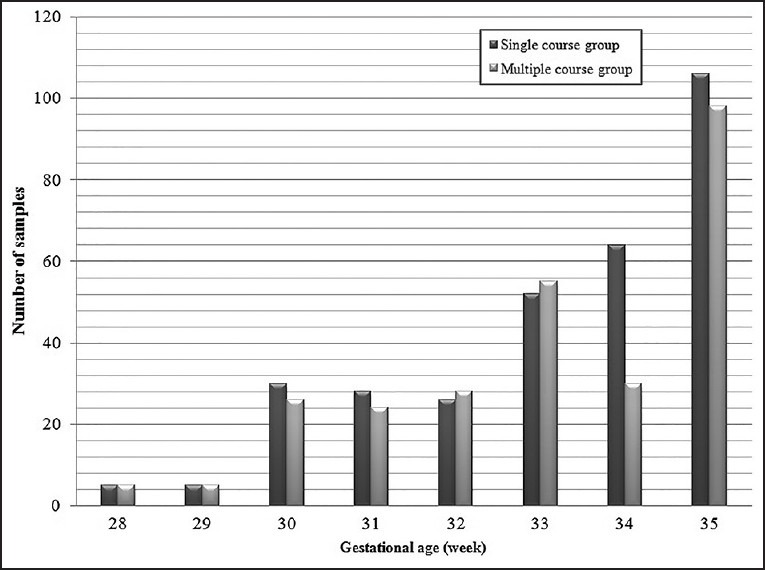
Gestational ages in a single course and multiple course group
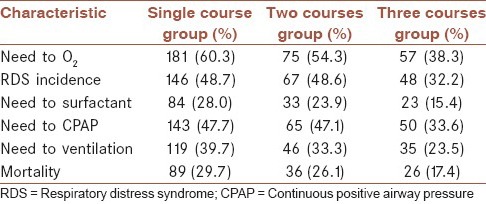
A 52.3% of newborns were female, and 41.7% were male. In the placebo group, 67.5% of deliveries were cesarean, and 23.5% were vaginal deliveries. In the case group 65.5% had cesarean and 33.5% of mothers delivered vaginally. A total of 42 women had twin pregnancies in which 31 of them had preterm delivery. 15 individuals out of these 31 cases were in a single course and 16 patients in multiple courses. The mean duration of hospitalization in the placebo group was 3/31 days and 2/03 days in the case group. The difference between them was statistically significant (P = 0.01). 193 infants of the placebo group (61/1%) required O2, whereas that was 120 (44/3%) in the case group which difference was statistically significant (P = 0.01). RDS occurred in the placebo group in 49/4% and 38/7% in the case group (P = 0.01). 49/4% in the placebo group required CPAP, although that was 38/7% in the case group. Surfactant need in the placebo group and the multiple course group were 27/7% and 19/2% respectively (P = 0.01). The difference was significant in both cases. The need for ventilation in the placebo group and the case group were 39/6% and 27/7% respectively (P = 0.002). While the death rate was observed 29/4% and 21/4% in the placebo group and case group respectively (P = 0/002). The 1st and 5th min Apgar were no showing significant difference between the two groups (P > 0.05). Mean height in Placebo group was 46/88 ± 5/22 cm and about 46 ± 5/32 cm in the case group. The difference was significant (P = 0.02). In the placebo group, the mean weight was 2015/86 ± 421/7 g and in the case group was 1938/01 ± 428/826 g (P = 0.027). The mean head circumference was seen 31/294 cm in the placebo group and 30/688 cm in the multiple course groups. The difference between the two groups was considerable (P = 0.001). The infants were divided into three categories according to gestational age to analyze the effects of betamethasone several factors in these gestational ages: First group with the age 28-30 weeks, group II 31-32 weeks, and group III 33-35 weeks [Table 2].
Table 2.
The frequency of samples based on the needs to oxygen, ventilation, surfactant, RDS incidence and mortality in a single course, two courses and three courses of betamethasone
In the analysis of twins, the difference of mean height, weight, and head circumference in two groups was not significant (P > 0.05). The needs for hospitalization, ventilation, oxygen, incidence of RDS and mortality in the multiple course groups was significantly lower. While no significant difference was shown in two groups as the need to surfactant and amount of hospitalization days.
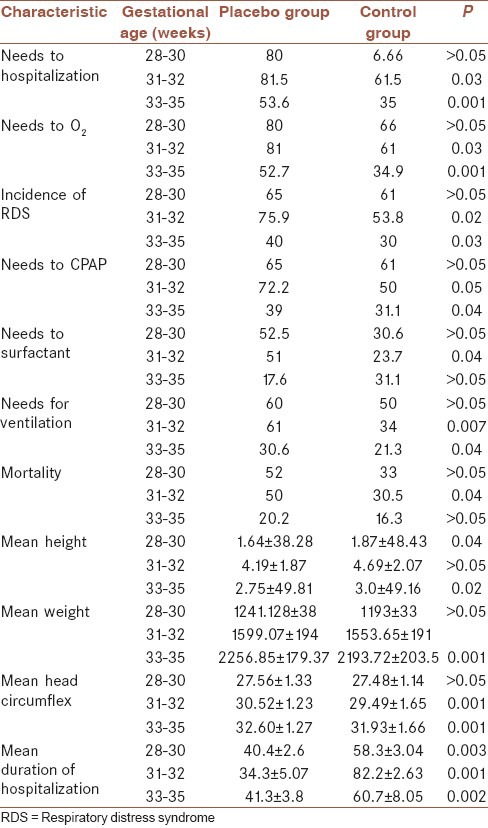
As far as the patients of the case group had received different courses of betamethasone, therefore for better understanding of the effects of these courses and compared them with the placebo group, statistical analysis was performed and here the results are shown in the Table 3.
Table 3.
The frequency of samples based on three periods of gestational age in placebo (single course betamethasone) and control (multicourse betamethasone) group
DISCUSSION
Preterm labor is one of the most important factors of prenatal mortality with the high prevalence even in the developed countries. RDS is one of the most common causes of death in premature infants that interventions such as using corticosteroids have reduced its incidence.[11] Since the maximum effect of corticosteroid is 4-7 days after injection, some bodies argue that the replication of corticosteroid improves infants respiratory function. Many studies have been conducted with contradictory results in this regard.[12,13,14] The results of the present study showed regardless of gestational age the need for O2, the incidence of RDS, requiring hospitalization, duration of hospitalization, and need for CPAP, ventilation and surfactant and mortality were significantly lower in the recipients of multiple course betamethasone than the single course one. It clearly indicated betamethasone was effective in this area, but in addition to these beneficial effects, weight, height and head circumference were significantly decreased in the multiple course group. Effect of reduction in infants’ anthropometric indexes necessitates evaluation of these infants in the coming years. Much research such as those conducted by Crowther et al. and Wapner et al. significantly shows differences in the incidence of RDS, the need for surfactant and CPAP, ventilation, and mortality rates in repeated courses of betamethasone.[15,16] While Peltoniemi et al. that had been prescribed A single dose booster of betamethasone just before labor in patients who were not delivered until 7 days after betamethasone came to the conclusion that the re-injection of betamethasone cause neonatal respiratory adaptation to impaired.[17] Betamethasone has beneficial effects on neonatal respiratory condition when is administered as a full course of two intramuscular injections of 12 mg within 24 h.[18] As a result may be incomplete courses and a single dose of betamethasone cause respiratory status of infants in the study performed by Peltoniemi et al.[17] In the Mazumder et al. study the incidence of RDS was similar in the both groups, but the mortality due to RDS was reported in the control group less frequently.[19] Of course, perhaps small study population justifies the different results. About anthropometric indexes Wapner et al. and Mazumder et al. reported reducing weight and head circumference in the multiple course groups like our study.[16,19] Wapner et al., via long-term follow-up showed no difference between the two groups about the neural and physical development of infants 3 years after receiving the betamethasone.[16] In the present study, some mothers received two courses, and some of them took three courses of betamethasone. However, in statistical analysis there was not seen any different about height, weight and head circumference between them. However, in infants receiving three courses of height, weight and head circumference were significantly reduced compared to placebo was observed. This division has not been done in the other studies. Results of Wapner et al. study indicated that receiving multiple courses of betamethasone in those aged between 28 and 30 weeks had no positive effect on mortality and incidence of RDS, although it had no effect on the newborns height.[16] But in the group of 31 and 32 weeks requiring hospitalization and O2, CPAP, surfactant and ventilation, the incidence of RDS, the mean duration of hospital stay and mortality was significantly lower in the control group and also the height and weight have not decreased. Although head circumference was significantly lower. At ages 33-35 weeks, days of staying in hospital, need for O2, CPAP, ventilation and RDS was statistically significantly less frequent in the control group but the need for surfactant and mortality rates were not significantly different in the two groups. In the Mazumder et al. study all infants who were <32 had been studied.[19] We found different results in the two groups when we divided the children into two age groups. In the Crowther et al. study infants were divided into two groups: 32 weeks and 32-36 weeks. The need for surfactant, O2 and the rate of RDS was lower in the control group, while these variables did not differ between the two groups in the ages 32-36 weeks.[15] Wapner et al., in their study also reported multiple betamethasone injection reduces the need for ventilation, surfactant and CPAP especially in infants <32 weeks.[16] In our study, the mortality rate in the both placebo and case group had a significant difference compared with other studies. Murphy in his study mentioned neonatal mortality was 4% in both placebo and case, though in Mazumder et al. study it was 18% and 11% in the placebo group and case group respectively.[19,20] Peltoniemi et al. reported that the mortality rate in the placebo group was 2% and in the case group was 5%.[17] This difference in mortality rates may be due to differences in neonatal Intensive Care Unit equipment and neonatal care. In our study, for twin pregnancies despite the considerable decreasing in the need for hospitalization, ventilation, oxygen and risk of mortality in the control group we were not seen a significant difference between groups about the mean height, weight and head circumference. However, in regard to the low number of the twins this finding is not applicable and need further studies.
CONCLUSION
According to the results of this study, in spite of positive effects of multiple courses betamethasone in reducing infant mortality and morbidity rate until the adequate studies have not proven the safety of decreasing weight, height and head circumference due to betamethasone utilization, multiple courses of it, should not be recommended routinely.
AUTHOR'S CONTRIBUTION
ZA: Developed the concept and designed the study.
MT: Prepared the manuscript, analyzed output data and supervised the study designing process.
HR: Edited the manuscript and interpreted the data.
SF: Assembled input data and helped in a clinical study.
MT: Interpreted the data and helped in a clinical study.
ACKNOWLEDGMENT
This manuscript is the result of the research project approved by the research council of the Mazandaran University of Medical Sciences (MAZUMS). In this way, we thank the president, deputies and all staff of painstaking research and technology deputy of the Mazandaran University of Medical Sciences, for their assistance in all process of ratifying and implementing a research project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Grgic G, Fatusic Z, Bogdanovic G. Stimulation of fetal lung maturation with dexamethasone in unexpected premature labor. Med Arh. 2003;57:291–4. [PubMed] [Google Scholar]
- 2.Danesh A, Janghorbani M, Khalatbari S. Effects of antenatal corticosteroids on maternal serum indicators of infection in women at risk for preterm delivery: A randomized trial comparing betamethasone and dexamethasone. J Res Med Sci. 2012;17:911–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Grier DG. Treat Respiratory Medicine. North Irland, UK: Oueenos University Belfost; 2004. Effect of glucocorticoids of fetal and natal lung development; pp. 295–306. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, 3rd, Wenstrom KD. 22nd ed. New York, USA: McGraw-Hill; 2005. Williams Obstetrics. [Google Scholar]
- 5.Aghajafari F, Murphy K, Ohlsson A, Amankwah K, Matthews S, Hannah ME. Multiple versus single courses of antenatal corticosteroids for preterm birth: A pilot study. J Obstet Gynaecol Can. 2002;24:321–9. doi: 10.1016/s1701-2163(16)30625-9. [DOI] [PubMed] [Google Scholar]
- 6.Vural M, Yilmaz I, Öztunç F, Ilikkan B, Erginöz E, Perk Y. Cardiac effects of a single course of antenatal betamethasone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F118–22. doi: 10.1136/adc.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bontis N, Vavilis D, Tsolakidis D, Goulis DG, Tzevelekis P, Kellartzis D, et al. Comparison of single versus multiple courses of antenatal betamethasone in patients with threatened preterm labor. Clin Exp Obstet Gynecol. 2011;38:165–7. [PubMed] [Google Scholar]
- 8.Bonanno C, Wapner RJ. Antenatal corticosteroids in the management of preterm birth: Are we back where we started? Obstet Gynecol Clin North Am. 2012;39:47–63. doi: 10.1016/j.ogc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stutchfield P, Whitaker R, Russell I Antenatal Steroids for Term Elective Caesarean Section (ASTECS) Research Team. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: Pragmatic randomised trial. BMJ. 2005;331:662. doi: 10.1136/bmj.38547.416493.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermillion ST, Soper DE, Bland ML, Newman RB. Effectiveness of antenatal corticosteroid administration after preterm premature rupture of the membranes. Am J Obstet Gynecol. 2000;183:925–9. doi: 10.1067/mob.2000.108845. [DOI] [PubMed] [Google Scholar]
- 11.Peltoniemi OM, Kari MA, Halmesmäki E, Raudaskoski T, Tammela O, Uotila J, et al. The Effect of Second Course of Antenatal Betamethasone (BM) on Neonatal Morbidity in Preterm Infants: A Randomized Trial. Pediatri Res. 2005;58:404. [Google Scholar]
- 12.Surbek D, Drack G, Irion O, Nelle M, Huang D, Hoesli I. Antenatal corticosteroids for fetal lung maturation in threatened preterm delivery: Indications and administration. Arch Gynecol Obstet. 2012;286:277–81. doi: 10.1007/s00404-012-2339-x. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca L, Ramin SM, Mele L, Wapner RJ, Johnson F, Peaceman AM, et al. Bone metabolism in fetuses of pregnant women exposed to single and multiple courses of corticosteroids. Obstet Gynecol. 2009;114:38–44. doi: 10.1097/AOG.0b013e3181a82b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatelais F, Berthelot J, Beringue F, Descamps P, Bonneau D, Limal JM, et al. Effect of single and multiple courses of prenatal corticosteroids on 17-hydroxyprogesterone levels: Implication for neonatal screening of congenital adrenal hyperplasia. Pediatr Res. 2004;56:701–5. doi: 10.1203/01.PDR.0000142733.50918.6E. [DOI] [PubMed] [Google Scholar]
- 15.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: A randomized controlled trial. Lancet. 2006;367:1913–9. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 16.Wapner RJ, Sorokin Y, Mele L, Johnson F, Dudley DJ, Spong CY, et al. Long term outcome after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1190–8. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 17.Peltoniemi OM, Kari MA, Tammela O, Lehtonen L, Marttila R, Halmesmäki E, et al. Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics. 2007;119:290–8. doi: 10.1542/peds.2006-1549. [DOI] [PubMed] [Google Scholar]
- 18.Spinillo A, Chiara A, Bergante C, Biancheri D, Fabiana D, Fazzi E. Obstetric risk factors and persistent increases in brain parenchymal echogenicity in preterm infants. BJOG. 2004;111:913–8. doi: 10.1111/j.1471-0528.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Mazumder P, Dutta S, Kaur J, Narana A. single versus multiple courses of antenatal betamethasone and neonatal outcome: A randomized controlled trial. Indian Pediatr. 2008;45:661–7. [PubMed] [Google Scholar]
- 20.Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, et al. Multiple courses of antenatal corticosteroids for preterm birth study collaborative group. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstet Gynecol. 2012;119:917–23. doi: 10.1097/AOG.0b013e31825189dc. [DOI] [PubMed] [Google Scholar]


