Abstract
Background:
We aimed to compare clinical effects of sugammadex versus combination of anticholinergic-anticholinesterase agents for reversing of nondepolarizing neuromuscular block in pediatric patients.
Materials and Methods:
A total of 60 pediatric patients whom should be performed general anesthesia in the supine position were enrolled to this randomized double-blinded clinical trial. Fentanyl 1 μg/kg, propofol 2 mg/kg, rocuronium 0.6 mg/kg were used in induction and sevofluran, 50% O2-50% N2O in maintenance of anesthesia. Neuromuscular conductions were assessed by train of four (TOF)-Watch SX (Organon, Schering-Plough, Ireland) acceleromyograph. Patients were intubated at the moment of TOF 0. At the end of the operation emergence of T2 point was replied by 2 mg/kg sugammadex administration in group 1 and 0.06 mg/kg neostigmine +0.02 mg/kg atropine in group 2. At the moment of T0.9 inhalation, gases were ceased, and patients were extubated. Hemodynamic alterations, access to T0.9, extubation time, recovery parameters, drug consumptions and adverse effects were recorded.
Results:
Train of four scores showed a lesser increase in group 2 than group 1 from 15th s to 30th min during post reverse period (from 6.9 ± 6.4 to 91.7 ± 7.2 in group 2 vs. from 35.4 ± 21.4 to 99.5 ± 1.0 in group 1) (p < 0.0004). Group 1 patients exhibited much more complete muscle strength rates than group 2 (P < 0.001). T0.9 and extubation times were significantly longer in group 2 than group 1 (P < 0.001). Comparison of adverse effects yielded no difference.
Conclusion:
Sugammadex can be considered as a safe agent in order to reverse neuromuscular block in pediatric patients.
Keywords: Atropine, gamma cyclodextrins, neostigmine, neuromuscular nondepolarizing agents
INTRODUCTION
Current advancements have provided highly detailed data about effects of neuromuscular blocker (NMB) agents and their modes of action. However, the high incidence of residual paralyze is still a problem despite the introduction of newer intermediate-acting nondepolarizing NMBs and better monitoring techniques.[1] Today many agents are available for reversing postoperative effects of nondepolarizing blockade. The anticholinesterase agents’ effects include direct stimulation of motor end terminal, increasing acetylcholine (Ach) secretion, removing muscle relaxants from receptors and reversible inactivation of Ach esterase enzyme.[2] In addition to blocking reversing effects, cholinesterase inhibitors have some adverse muscarinic effects over cardiovascular, gastrointestinal and pulmonary systems.[2,3,4] Atropine sulfate and glycopyrrolate can subside these adverse muscarinic effects with some unfavorable anticholinergic actions.[2,5]
Sugammadex that is a modified gamma cyclodextrin has introduced to anesthesia practice in recent years. It removes NMB in a quick and safe manner.[6] Sugammadex encapsulates steroid NMB agents (rocuronium, vecuronium) and detach them from nicotinic acetylcholin receptors such like a synthetic receptor.[6,7] Sugammadex is a biologically inactive, well-tolerable and safe agent, which has not revealed any significant cardiovascular or hemodynamic adverse effect in preclinical studies up to known.[6,8,9]
Train of four (TOF) Watch acceleromyography device is used for recording evoked muscle responses because of its accuracy, simplicity and suitability for routine usage during surgery and in the post anesthesia care unit. In acceleromyography, a small piezoelectric transducer converts measured accelerations into electric signals then they are presented as clear neuromuscular transmissions. Four stimulation patterns is currently used in acceleromyographs; single twitch, TOF, tetanic stimulation and post tetanic count, double burst stimulation.
In this study, we compared the clinical effects of sugammadex versus classical combination of anticholinergic-anticholinesterase agents.
MATERIALS AND METHODS
Ethical Committee approval and informed consents of parents’ were obtained before the study (Research Project Number 0428/10.08.2011). Sixty pediatric patients age between 2 and 12 and American Society of Anesthesiologist 1-2 whom should be performed general anesthesia in the supine position for ear nose and throat surgery were enrolled in this randomized double-blinded clinical trial. Patients with renal disease, liver disease, neuromuscular disease, known malign hyperthermia in the family history, drug uptake that can cause interaction with rocuronium, have predictors for intubation limitation didn’t included to the study. The patients randomized with opaque closed envelope technique into two groups; as sugammadex receiving (n: 30) group 1 and neostigmine/atropine (n: 30) receiving group 2. After arrival to the operation room, monitoring of; noninvasive arterial blood pressure, peripheral oxygen saturation, electrocardiograms and cutaneous temperature were applied. Upon the operation peripheral, cutaneous temperatures were kept above 32°C by using blankets. Neuromuscular conduction were assessed by TOF-Watch SX (Organon, Schering-Plough, Ireland) acceleromyograph device. In order to decrease surface tension, the cutaneous sites were wiped by alcoholed cotton. Soon after a couple of electrodes were placed 3-4 cm apart from each other through ulnar nerve trace of left wrist while transducer was placed on pulp of left thumb. 1 μg/kg fentanyl and 2 mg/kg propofol administrated to patients by intravenous (iv) route. Basal activities, which occurred after supramaximal stimulation of ulnar nerve via TOF device were recorded by intervals of 15 s. This preceding stabilization accelomylography phase ended 3 min later and followed by administration of 0.6 mg/kg rocuronium (Esmeron 50 mg/5 ml Schering-Plough) to complete the induction. All the patients were intubated at the moment of TOF 0. Maintenance anesthesia provided by 50% O2-50% N2O and sevofluran (MAC 1.3-1.5) combination. We evaluated the need for additional muscle relaxant by using TOF Watch acceleromyograph during the operation. When we observed 2 twitches present in TOF stimulation it is time to administered additional muscle relaxant, and rocuronium (0.1-0.2 mg/kg) were applied for this purpose. Measurements were carried out before intubation, at 2nd, 5th, 10th, 30th min after entubation as well as 15th, 30th, 60th, 90th s and 2nd, 5th, 10th, 15th, 20th, 25th, 30th min after reversing of blockade period. Emergence of T2 point (two contractions) on TOF device was replied by iv 2 mg/kg sugammadex (Bridion 200 mg/ml Merck Sharp Dohme) administration to group 1 patients and 0.06 mg/kg neostigmine + 0.02 mg/kg atropine administration to group 2 patients. The test drug was preparing by a researcher and giving to the researcher who administered the drug and was blind to the drug. The person who evaluates the patients’ situation and adverse effects was also blind to the drug. Some parameters, including hemodynamic alterations, access to T0.9 and extubation time were recorded for each patient. Inhalation agents were sustained until T0.9 point in order to avoid any interaction with muscle relaxation. At the moment of T0.9 inhalation, gases were ceased, and patients were extubated properly after spontaneous breathing. In the post extubation, phase patients were monitored, and all observations were recorded and scored at 5th, 10th, 15th, 20th, 25th, 30th min of this phase. The scored observations involved; respiration status (able to breath and coughing: 2, dyspnea or limited respiration: 1), O2 saturation (In room atmosphere >92%: 2, reaches SpO2 >90% by O2 support: 1, <90% despite O2 therapy: 0), head elevation (>5 s yes: 1, no: 2), exhibiting tongue (yes: 1, no: 2), muscle strength (total paralyze: 0, no loss of muscle strength: 10) and state of consciousness (fully awake: 2, awake by stimulus: 1, no response: 0). Total rocuronium and fentanyl consumption and related adverse effects including vomiting, spasm and desaturation for each patient were recorded also.
Statistical analysis
In order to provide an analytic power of 95% and a probability value of 5%, the postreverse T0.9 reaching durations of groups were evaluated and at least a difference of 15.2 units in terms of geometrical means between two groups, considered to be significant in the analysis, we needed at least 17 patients for each group. All the data were analyzed by SPSS (SPSS Inc, Chicago, Il, USA) program. For constant variants descriptive analysis involved mean ± standard deviation or median (minimum-maximum) values and for the categorical variants involved values of a number of cases and percentages (%). Significance of differences was tested by Student's t-test and Mann-Whitney U-test. Categorical variants analyzed by Pearson's Chi-square or Fischer's Chi-square test. Repeated measurements were evaluated by variant analysis of repeated measurements. Greenhouse-Geisser method was performed in order to detect differences among groups. Since P < 0.05 was accepted as an error value, Bonferroni correction was performed to avoid type 1 mistakes in all multiple comparisons.
RESULTS
Comparison of demographic data revealed no significance between groups (P > 0.05) [Table 1].
Table 1.
Demographic characteristics of the groups
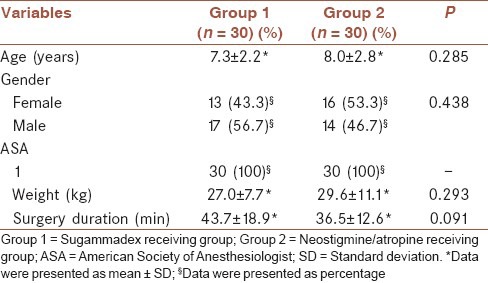
A significant difference about heart rate alterations was found among groups (F = 7.237 and P < 0.001). When compared to prereverse period at 15th, 30th and 60th s of the postreverse period a heart rate decrease occurred in group 1 and an increase occurred in group 2 (P < 0.0004) [Table 2].
Table 2.
Heart rate and blood pressure data of the groups during follow-ups
No significant difference was found in terms of mean arterial blood pressure alterations between two groups (F = 0.899 and P = 0.492) [Table 2]. Since a significant difference detected about saturation alterations (F = 2.592 and P = 0.037), performing a Bonferroni correction converted it to an insignificant (P > 0.0004) level.
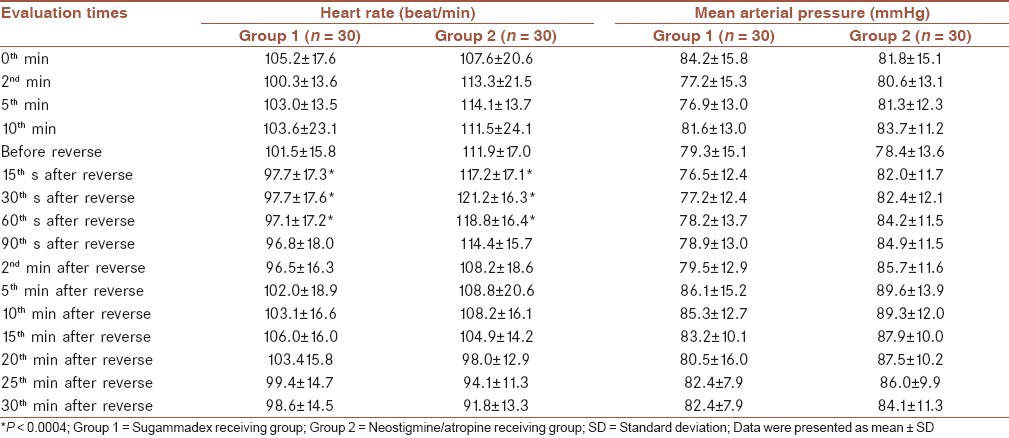
Comparison of prereverse and postreverse TOF score alterations revealed a significant difference between two groups (F = 61.333 and P < 0.001). TOF scores showed a lesser increase in group 2 than group 1 between 15 s and 30 min during postreverse period [Table 3].
Table 3.
TOF levels according to follow-up times within groups
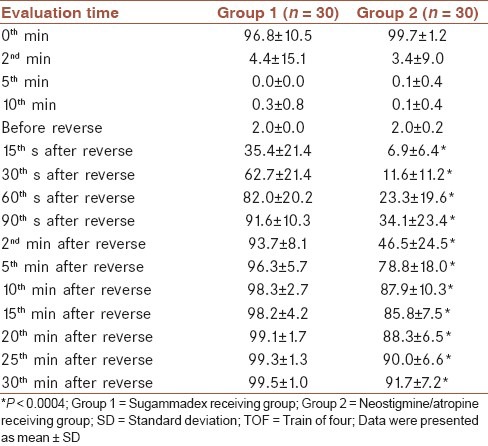
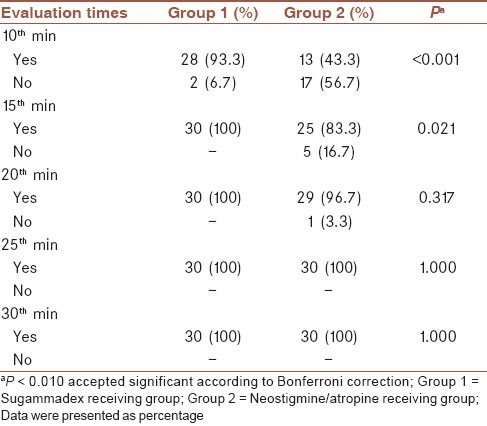
No significant difference was found regarding respiration parameters and tongue exposing examinations between two groups (P > 0.01). Head elevation scores revealed a significant difference (P < 0.001) only at 10th min between groups while they were not significant at the rest of follow-up (P > 0.01) [Table 4].
Table 4.
Head elevation status of cases in each group during follow-up
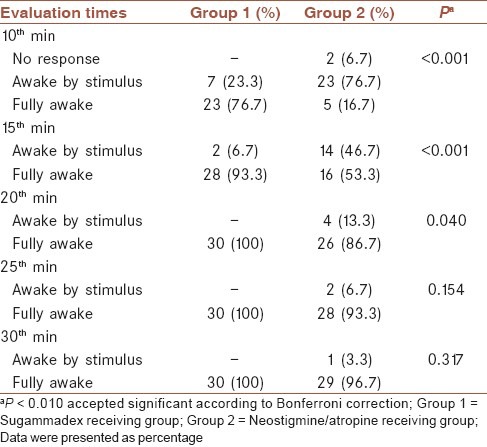
State of consciousness revealed a significant difference (P < 0.001) only at 10th and 15th min between groups while they were not significant at the rest of follow-up (P > 0.01) [Table 5].
Table 5.
State of consciousness of cases in each group during follow-up
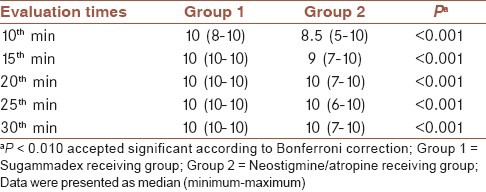
A significant difference regarding muscle strength was found between groups, covering entire follow-up. Group 1 patients exhibited much more complete muscle strength rates than group 2 (P < 0.001) [Table 6].
Table 6.
Muscle strength evaluations of each group during follow-up
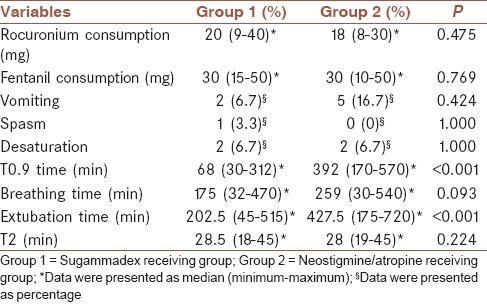
TO9 and extubation times were significantly longer in group 2 than group 1 (P < 0.001). No significant difference was found for the remaining observations, including rocuronium consuming, fentanil consuming, vomiting, spasm occurrence, desaturation, respiration rate and T2 scores (P > 0.05) [Table 7]. Two patients (6.7%) in each group, experienced desaturation after extubation, and they responded to O2 support quickly (P = 1.000). These patients were at the level of T0.9 that points out sufficient muscle strength and lack of anticholinesterase effect. Hence, the occurrence of desaturation is likely related to the nature of the operation of our study group.
Table 7.
Other clinical observations of groups
DISCUSSION
Neuromuscular blocker agents are widely preferred because of their potent muscle relaxant actions during surgery and enable tracheal intubation during anesthesia.[10,11] Administration of bolus NMBs by iv route facilitates surgery, and it is considerable for major operations. However, late recovery, increased costs and adverse effects, due to these NMBs are still subjects of concerns.[12] To the best of our knowledge, there are numerous studies subjected sugammadex in the literature, but only one of them had involved pediatric patients.
Jones et al., had formed a deep blockade with rocuronium and compared sugammadex versus neostigmin + glycopyrrolate in adult patients.[13] They found only, an increase of heart rate at 2, 5, and 10 min after administration of neostigmin + glycopyrrolate, which was insignificant both clinically and statistically. Khuenl-Brady et al., had compared sugammadex versus neostigmin + glycopyrrolate during reverse of vecuronium blockade and reported an insignificant heart rate increase at 2nd and 5th min for neostigmin group.[14] Plaud et al. had evaluated effects of sugammadex (0.5, 1, 2, 4 mg/kg) and placebo, in a study involving infant, pediatric and adult patients. They could not find any significant clinical difference regarding hemodynamic alterations, except a bradicardia incident seen in an infant of placebo group.[15] In our study, we detected a significant difference about heart rate alterations; when compared to prereverse period, at 15th, 30th and 60th s of the postreverse period, the heart rate decrease occurred in group 1 and an increase occurred in group 2 (P < 0.0004). But when we tailor, our findings to the criteria in the study of Jones et al. they seem to be insignificant clinically.[13] Khuenl-Brady et al. could not demonstrate a significant difference regarding diastolic and systolic blood pressures, except an increase of diastolic pressure at second minute of neostigmin + glycopyrrolate receivers.[14] However, Jones et al. failed to report any significant alterations regarding hemodynamic responses.[13] Flockton et al. had observed sugammadex's effect on reverse of rocuronium mediated neuromuscular block as well as neostigmin's, on reverse of cisatracurium mediated. They could not detect a significant difference regarding hemodynamic alterations.[16] Sorgenfrei et al., had reported the hypotension with the use of sugammadex in a study subjected adult patients.[17] Woo et al. did not find any clinically relevant difference between their groups in mean systolic or diastolic blood pressure or heart rate.[18]
Complying with most of the studies above, our study also could not show any significant hemodynamic alterations such as mean arterial pressures, during pre and post reverse periods. We think, the prominent results of Sorgenfrei et al.'s study are likely due to few number of patients involved and administration of different dosages to subgroups.
Sacan et al. had compared sugammadex, neostigmin + glycopyrrolate and edrofonium + atropin groups during reverse of rocuronium mediated blockade in adult patients. In this study access to TOF 0.9 was reported to be 107 ± 61 s for sugammadex receivers, 331 ± 27 s for edrofonium group and 1044 ± 590 s for neostigmin received patients.[18] Plaud et al., had reported durations access to TOF 0.9, 1.5 min for pediatric patients and 1.1 min for adolescents with sugammadex (2 mg/kg).[15] Blobner et al. studied reverse of NMB mediated by rocuronium and had defined durations access to TOF 0.9, 1.5 min in sugammadex (2 mg/kg) group and 18.6 min in neostigmine group.[19]
In our study, we demonstrated a significant difference regarding TOF alterations between groups (F = 61.333 and P < 0.001). TOF scores exhibited a lesser increase between 15th s and 30th min in group 2 when compared with group 1, during postreverse period (P < 0.004). Access to TOF 0.9 was defined as, 68 s (30-312) for group 1 and 392 s (175-720) for group 2 (P < 0.01). Similarly, extubation durations were 202.5 s (45-515) for group 1 and 427.5 (175-720) for group 2 (P < 0.01). Our findings regarding TOF 0.9 durations, were complying with studies above except, a shortening in neostigmin receiving groups. This result is probably related with our patients’ age group since elimination and biotransformation of drugs are more rapid in pediatric populations.
There were no significant difference regarding respiration status and saturations during postextubation phase in our study (P > 0.01).
Sacan et al. could not show any significance, regarding head elevation up to 5 s, among groups.[20] Jones et al. had evaluated co-operation and general muscle strength status, that yielded no significance among groups.[13] When we performed a comparison regarding head elevation scores up to 5 s, there were a significant difference (P < 0.001) only at 10th min. At this moment, rate of patients who could not accomplish head elevation up to 5 s, were n: 2/30 (6.7%) in group 1 and n: 17/30 (56.7%) in group 2. There was not any significant difference at the rest of follow-up regarding this parameter.
Plaud et al. had monitored, tongue exposing as a marker of neuromuscular status and they failed to show any significance.[15] In our study when we compared tongue exposing capabilities at 10 min, the rates occurred as; yes: 29 (96.7%), no: 1 (3.3%) in group 1 and yes: 23 (76.7%), no: 7 (23.3%) in group 2 (P = 0.024). These findings were transformed to a significant level when we performed Bonferrini correction statistically (P < 0.010).
In their study Jones et al. had reported rates of awake and orientated patients during postextubation phase as, n = 26/37 (70%) in the sugammadex group and n = 20/34 (59%) in neostigmin group.[13] When we looked at our study, rates of fully awake patients occurred as n = 23 (76.7%) at 10th min and n = 28 (93.3%) at 15th min in group 1 and n = 5 (16.7%) at 10th min and n = 16 (53.3%) at 15th min in group 2 (P < 0.001). Patients involved in the sugammadex group exhibited a faster recovery in terms of muscle strength and consciousness during postextubation phase prior to (Post Anesthesia Care Unit) PACU. PACU monitoring of patients in the neostigmine group, detected moderate muscle strength within first 10 min (5 point). Recovery of motor functions was slower when compared to group 1 and this finding was statistically significant (P < 0.001).
Sacan et al. could not show any significance, in vomiting rates and they related it to few numbers of patients.[20] Woo et al. reported nausea and vomiting in 3 patients in the sugammadex group and 6 patients in the neostigmine group and all patients from both treatment groups who experienced nausea and vomiting had at least two baseline risk factors for postoperative nausea and vomiting.[18] Abrishami et al. had focused, to adverse effects in their study and reported no significance between sugammadex and neostigmin receiving groups.[21] Plaud et al. had conducted a study involving pediatric patients and reported some adverse effects with sugammadex. With a dose of 4 mg/kg sugammadex, 2 patients exhibited urinary retention, dysuria and one exhibited fever, weight loss, hematuria and diarrhea during a patient experienced vomiting by 2 mg/kg sugammadex.[15]
In our study, we could not demonstrate a significance involving vomiting-nausea, and rates of vomiting-nausea were detected as n = 2 (6.7%) for group 1 and n = 5 (16.7%) for group 2 (P = 0.424).
Ledowski et al. compared reversal strategies in their study and found that those reversed with sugammadex showed fewer episodes of postoperative oxygen desaturation (15% vs. 33%; P < 0.05). TOF ratios of <0.7 and also <0.9 were more likely associated with X-ray results consistent with postoperative atelectasis or pneumonia.[22] In current study 2 patients (6.7%) in each groups, experienced desaturation after extubation, and they responded to O2 support quickly (P = 1.000). These patients were at the level of T0.9 that points out sufficient muscle strength and lack of anticholinesterase effect. Hence, the occurrence of desaturation is likely related to the nature of the operation of our study group. Our study has some limitations; we included pediatric patients whom ear, nose and throat surgery were performed, and the nature of the operation caused complications like edema or laryngeal spasm due to the manuplations. Complication like desaturation was our evaluation criteria, and we confused about the nature of it; inadequate reversal of muscle relaxant or spasm or edema of the involved area. Did not select the operation, which the muscle relaxant effect is more necessary and easily observed, like abdominal surgery, was our second limitation. If we select abdominal surgery group, we may evaluate necessity of additional muscle relaxant usage or the reversal effect more appropriately.
CONCLUSION
As a conclusion, pediatric patients who received sugammadex, demonstrated significantly a shorter duration of TOF 0.9 and extubation than neostigmin + atropin receivers. Other recovery parameters including, head elevation, muscle strength and state of consciousness were also regained more rapidly in the sugammadex administrated group than neostigmine group. Comparison of adverse effects yielded no difference as a highlighted finding. In the light of all these findings sugammadex can be considered as a safe agent in order to reverse neuromuscular block in a safe and proper manner with few adverse effects.
AUTHORS’ CONTRIBUTION
ÇÖ contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. TÇ contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. BB contributed in the design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. HB contributed in the conception and design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Shorten GD. Postoperative residual curarisation: Incidence, aetiology and associated morbidity. Anaesth Intensive Care. 1993;21:782–9. doi: 10.1177/0310057X9302100606. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DM. Clinical pharmacology of neuromuscular blocking agents. Am J Health Syst Pharm. 1999;56:S4–9. doi: 10.1093/ajhp/56.S4. [DOI] [PubMed] [Google Scholar]
- 3.FDA Anesthetic and Life Support Advisory Committee Meeting. Sugammadex Sodium Injection (NDA 22-225) March 11 2008. Briefing Document (Background Package) Organon USA, A part of Schering –Plough Corporation. http://www.fda.gov/ohrms/dockets/ac/08/brifing/2008-4346b1-02- organon.pdf .
- 4.Fernández Meré LA, Alvarez-Blanco M. Sugammadex, a novel drug for neuromuscular blockade reversal. Rev Esp Anestesiol Reanim. 2010;57:95–102. doi: 10.1016/s0034-9356(10)70171-7. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A, Hunter JM. Reversal of neuromuscular block. Br J Anaesth. 2009;103:115–29. doi: 10.1093/bja/aep093. [DOI] [PubMed] [Google Scholar]
- 6.Yang LP, Keam SJ. Sugammadex: A review of its use in anaesthetic practice. Drugs. 2009;69:919–42. doi: 10.2165/00003495-200969070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ, et al. Cyclodextrin-derived host molecules as reversal agents for the neuromuscular blocker rocuronium bromide: Synthesis and structure-activity relationships. J Med Chem. 2002;45:1806–16. doi: 10.1021/jm011107f. [DOI] [PubMed] [Google Scholar]
- 8.Hogg RM, Mirakhur RK. Sugammadex. A selective relaxant binding agent for reversal of neuromuscular block. Expert Rev Neurother. 2009;9:599–608. doi: 10.1586/ern.09.22. [DOI] [PubMed] [Google Scholar]
- 9.Cameron KS, Clark JK, Cooper A, Fielding L, Palin R, Rutherford SJ, et al. Modified gamma-cyclodextrins and their rocuronium complexes. Org Lett. 2002;4:3403–6. doi: 10.1021/ol020126w. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen T, Viby-Mogensen J, Ringsted C. Anaesthetic practice and postoperative pulmonary complications. Acta Anaesthesiol Scand. 1992;36:812–8. doi: 10.1111/j.1399-6576.1992.tb03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 12.Caldwell JE. The continuing search for a succinylcholine replacement. Anesthesiology. 2004;100:763–4. doi: 10.1097/00000542-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: A randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 14.Khuenl-Brady KS, Wattwil M, Vanacker BF, Lora-Tamayo JI, Rietbergen H, Alvarez-Gómez JA. Sugammadex provides faster reversal of vecuronium-induced neuromuscular blockade compared with neostigmine: A multicenter, randomized, controlled trial. Anesth Analg. 2010;110:64–73. doi: 10.1213/ane.0b013e3181ac53c3. [DOI] [PubMed] [Google Scholar]
- 15.Plaud B, Meretoja O, Hofmockel R, Raft J, Stoddart PA, van Kuijk JH, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110:284–94. doi: 10.1097/ALN.0b013e318194caaa. [DOI] [PubMed] [Google Scholar]
- 16.Flockton EA, Mastronardi P, Hunter JM, Gomar C, Mirakhur RK, Aguilera L, et al. Reversal of rocuronium-induced neuromuscular block with sugammadex is faster than reversal of cisatracurium-induced block with neostigmine. Br J Anaesth. 2008;100:622–30. doi: 10.1093/bja/aen037. [DOI] [PubMed] [Google Scholar]
- 17.Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: A dose-finding and safety study. Anesthesiology. 2006;104:667–74. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Woo T, Kim KS, Shim YH, Kim MK, Yoon SM, Lim YJ, et al. Sugammadex versus neostigmine reversal of moderate rocuronium-induced neuromuscular blockade in Korean patients. Korean J Anesthesiol. 2013;65:501–7. doi: 10.4097/kjae.2013.65.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: Results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27:874–81. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 20.Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: A comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569–74. doi: 10.1213/01.ane.0000248224.42707.48. [DOI] [PubMed] [Google Scholar]
- 21.Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009:CD007362. doi: 10.1002/14651858.CD007362.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Ledowski T, Hillyard S, O’Dea B, Archer R, Vilas-Boas F, Kyle B. Introduction of sugammadex as standard reversal agent: Impact on the incidence of residual neuromuscular blockade and postoperative patient outcome. Indian J Anaesth. 2013;57:46–51. doi: 10.4103/0019-5049.108562. [DOI] [PMC free article] [PubMed] [Google Scholar]


