Abstract
BACKGROUND
Most small renal tumors are amenable to partial nephrectomy (PN). Studies have documented the association of radical nephrectomy (RN) with an increased risk of comorbid conditions, such as chronic kidney disease. Despite evidence of equivalent oncologic outcomes, PN remains under used within the United States. In this study, the authors identified the most recent trends in kidney surgery for small renal tumors and determined which factors were associated with the use of PN versus RN within the United States.
METHODS
A population-based patient cohort was analyzed using the Surveillance, Epidemiology and End Results cancer registry (SEER 1999-2006). The authors identified 18,330 patients ages 40 to 90 years who underwent surgery for kidney tumors ≤4 cm in the United States between 1999 and 2006.
RESULTS
In total, 11,870 patients (65%) underwent RN, and 6460 patients (35%) underwent PN. The ratio of PN to RN increased yearly (P < .001), representing 45% of kidney surgeries in 2006 for small tumors. There were significant differences in the cohort of patients who underwent PN versus RN, including age, sex, tumor location, marital status, year of treatment, and tumor size. When adjusting for these variables, being a man, age ≤70 years, urban residence, smaller tumor size, and more recent treatment year were predictors of PN.
CONCLUSIONS
Although the total numbers of PN procedures increased in the United States between 1999 and 2006, there remains a significant under use of PN, particularly among women, the elderly, and those living in rural locations. Further investigation will be required to determine the reasons for these disparities, and strategies to optimize access to PN need to be developed.
Keywords: partial nephrectomy, radical nephrectomy, renal cell carcinoma, Surveillance, Epidemiology, End Results Program, health disparity, sex, age
Health disparities are differences in the incidence, prevalence, mortality, and burden of disease and other adverse health conditions that exist among specific population groups in the United States. National Institutes of Health, 2002.1
Kidney cancer is the third most common genitourinary malignancy in the United States and ranks seventh among the leading causes of cancer in men and ninth among the leading cause of cancer in women.2 Surgical resection, primarily by radical nephrectomy (RN), has been the standard treatment for localized kidney tumors for over 40 years. During the past decade, there has been a change in the management of localized kidney tumors with an emphasis on avoiding removal of the entire affected kidney.3,4 This change has been driven by a downward size and stage migration of newly diagnosed renal cortical tumors, a better understanding of the biology of the disease, and an appreciation for the impact of surgical treatment on both oncologic and nononcologic outcomes.5,6 Partial nephrectomy (PN), which once was reserved for patients with essential indications (such as preexisting kidney disease, tumor in a solitary kidney, and bilateral renal masses) now is used routinely at tertiary care centers for the management of localized kidney tumors. It has been demonstrated that PN achieves oncologic outcomes equivalent to those produced by RN in tumors that measure <4 cm and in select tumors up to 7 cm.7-10 Furthermore, PN reportedly reduced the risk of chronic kidney disease compared with RN and, subsequently, also may reduce the risk of adverse cardiovascular events and premature death in these patients.11-13
Currently, at academic centers of excellence, up to 90% of patients with T1a tumors (≤4 cm) undergo PN.14,15 Despite this, an examination of national practice patterns does not appear to reflect a similar trend, and there remains a disparity in the use of PN among certain targeted groups (elderly patients and women).16 An analysis of the Surveillance, Epidemiology and End Results (SEER) cancer registry up to 2001 indicates that only 20% of patients who had renal tumors that ranged in size from 2 cm to 4 cm underwent PN17 and also reflected a disparity in the use of PN among women and the elderly.16 Therefore, the objectives of the current study were to examine data from the most current SEER cancer registry (1999-2006), to evaluate contemporary national practice pattern trends in renal surgery, and to elucidate whether or not disparities in the use of PN continue to occur in the United States.
MATERIALS AND METHODS
Data
Our patient sample was obtained from the most recent SEER 17 registries database established by the National Cancer Institute, which was released in April 2009. SEER is an authoritative source of information on population-based cancer registries and represents approximately 26% of the US population. The SEER registry collects information on cancer incidence and survival in the United States as well as information regarding the site and extent of disease, the first course of treatment, sociodemographic characteristics with active follow-up, and the date and cause of death.
Patient Selection
The SEER database was analyzed for renal cortical tumors diagnosed between 1999 and 2006 that were coded as primary site “kidney, not otherwise specified” (International Classification of Diseases for Oncology, 2nd Revision topography code C64.9). Excluded were nonparenchymal tumors, such as transitional cell carcinoma, and other nonrenal cortical tumors on the basis of available histo-logic data. We included only patients who underwent a surgical procedure (RN, PN, or local tumor destruction with ablative techniques, including percutaneous methods) for their renal tumor. Because of limitations in coding, several patients who underwent ablative procedures could not be differentiated from those who underwent PN (code 30). Therefore, these patients were categorized as undergoing PN, although this represented less than 5% of all classified kidney procedures. We excluded all patients who underwent observation or “other” treatment of their renal mass. Patient demographic variables included age, sex, race (white, black, or other), urban-rural location (patients listed as living in “counties in metropolitan areas” were considered urban), and marital status. The cohort included patients ages 40 to 90 years who had tumors that measured ≤4 cm in greatest dimension, and this resulted in a final study group of 18,330 patients.
Statistics
Summary statistics were constructed appropriately, depending on whether variables were continuous or categorical. Unadjusted associations between the type of renal surgery and patient characteristics were examined using either chi-square or Fisher exact tests. Multivariable logistic regression was used to estimate the likelihood of undergoing PN versus RN. The odds ratios (ORs), 95% confidence intervals, and P values were calculated for each predictor. Significance was defined as a P value ≤.05. All analyses were performed using STATA 10 software (Stata Inc., College Station, Tex).
RESULTS
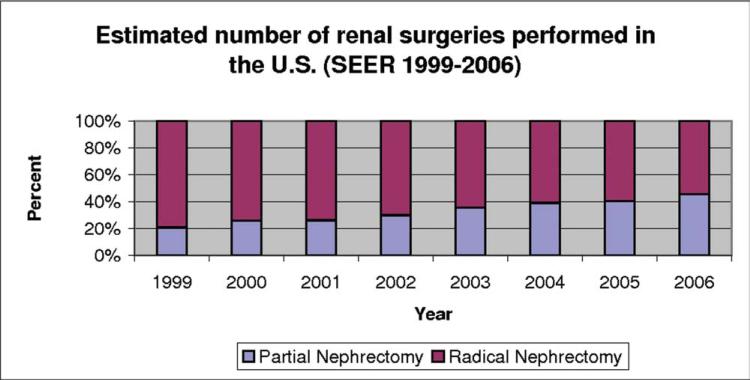
The study cohort included 18,330 patients who underwent surgical intervention for a renal tumor ≤4 cm that was diagnosed between 1999 and 2006. Of these, 6460 patients underwent PN, and 11,870 patients underwent RN. There were statistically significant differences in nearly all patient characteristics except for race (Table 1). Between 1999 and 2006, the total number of renal surgeries performed in the United States increased yearly along with the percentage of PNs performed (Fig. 1). During the same period, the PN-to-RN ratio also increased (P < .001).
Table 1.
Comparison of Demographic Characteristics Among Patients Who Underwent Partial Nephrectomy Versus Radical Nephrectomy: Study Cohort Characteristics, Surveillance, Epidemiology, and End Results Database 1999 to 2006
| Characteristic | No. of Patients (%) | Total | P | |
|---|---|---|---|---|
| PN | RN | |||
| No. of Patients | 6460 (35.2) | 11,870 (64.8) | 18,330 | |
| Age, y | <.001a | |||
| 40-50 | 1153 (37.7) | 1906 (62.3) | 3059 | |
| 51-60 | 1807 (37.6) | 3005 (62.4) | 4812 | |
| 61-70 | 1896 (36.3) | 3322 (63.7) | 5218 | |
| 71-80 | 1304 (31.1) | 2890 (68.9) | 4194 | |
| 81-90 | 300 (28.7) | 747 (71.3) | 1047 | |
| Sex | <.001a | |||
| Men | 4108 (36.8) | 7067 (63.2) | 11,175 | |
| Women | 2352 (32.9) | 4803 (67.1) | 7155 | |
| Race | .24 | |||
| White | 5471 (35.2) | 10,088 (64.8) | 15,559 | |
| Black | 599 (34.2) | 1155 (65.8) | 1754 | |
| Other | 348 (37.4) | 582 (62.6) | 930 | |
| Unknown | 87 | |||
| Residence | <.001a | |||
| Urban | 5820 (35.7) | 10,469 (64.3) | 16,289 | |
| Rural | 632 (31.3) | 1386 (68.7) | 2018 | |
| Unknown | 23 | |||
| Year of surgery | <.001a | |||
| 1999 | 170 (20.8) | 646 (79.2) | 816 | |
| 2000 | 471 (26) | 1343 (74) | 1814 | |
| 2001 | 544 (26.3) | 1526 (73.7) | 2070 | |
| 2002 | 673 (30) | 1570 (70) | 2243 | |
| 2003 | 879 (35.7) | 1585 (64.3) | 2464 | |
| 2004 | 1072 (39) | 1677 (61) | 2749 | |
| 2005 | 1165 (40.3) | 1723 (59.7) | 2888 | |
| 2006 | 1486 (45.2) | 1800 (54.8) | 3286 | |
| Marital status | <.001a | |||
| Married | 4422 (36.3) | 7757 (63.7) | 12,179 | |
| Not married | 1826 (33.2) | 3666 (66.8) | 5492 | |
| Unknown | 659 | |||
| Size of lesion, cm | <.001a | |||
| 0.1-1.0 | 251 (40.7) | 365 (59.3) | 616 | |
| 1.1-2.0 | 2158 (55.1) | 1757 (44.9) | 3915 | |
| 2.1-3.0 | 2603 (37.3) | 4383 (62.7) | 6986 | |
| 3.1-4.0 | 1448 (21.3) | 5365 (78.7) | 6813 | |
PN indicates partial nephrectomy; RN, radical nephrectomy.
Significant P value.
Figure 1.
This chart illustrates trends in the surgical treatment of small (≤4 cm) renal masses. SEER indicates Surveillance, Epidemiology, and End Results.
Adjusting for age, sex, year of surgery, residence (rural vs urban), marital status, and tumor size (per 1 cm), men were more likely than women to undergo PN (OR, 1.22; P < .001) (Table 2). Additional predictors of PN included a more recent year of surgery (OR, 1.30-2.94; P < .05) and smaller tumor size (P < .001). No differences were noted based on race. Patients aged >70 years (OR, 0.77-0.85; P < .01) and patients who lived in a rural location had significantly lower odds of undergoing PN than their urban counterparts. Although married status was predictive of PN, it was not significant in multivariate analysis (P = .53).
Table 2.
Adjusted Model Demonstrating Independent Patient Factors Associated With the Use of Partial Nephrectomy
| Characteristic | Predictors of Partial Nephrectomy: Adjusted OR (95% CI) | P |
|---|---|---|
| Age, y | ||
| 40-50 | Referent | |
| 51-60 | 1.04 (0.94-1.15) | .48 |
| 61-70 | 0.99 (0.90-1.1) | .94 |
| 71-80 | 0.85 (0.76-0.94) | <.01a |
| 81-09 | 0.77 (0.65-0.91) | <.01a |
| Sex | ||
| Women | Referent | |
| Men | 1.22 (1.14-1.30) | <.001a |
| Race | ||
| White | Referent | |
| Black | 0.94 (0.84-1.05) | .24 |
| Other | 1.09 (0.94-1.26) | .27 |
| Residence | ||
| Rural | Referent | |
| Urban | 1.25 (1.12-1.39) | <.001a |
| Year of surgery | ||
| 1999 | Referent | |
| 2000 | 1.30 (1.06-1.61) | <.05a |
| 2001 | 1.30 (1.05-1.59) | <.05a |
| 2002 | 1.55 (1.27-1.90) | <.001a |
| 2003 | 1.96 (1.60-2.38) | <.001a |
| 2004 | 2.33 (1.92-2.83) | <.001a |
| 2005 | 2.45 (2.02-2.97) | <.001a |
| 2006 | 2.94 (2.42-3.56) | <.001a |
| Marital status | ||
| Not married | Referent | |
| Married | 1.07 (0.99-1.16) | .053 |
| Size of lesion per 1 cm | 0.53 (0.51-0.55) | <.001a |
OR indicates odds ratio; CI, confidence interval.
Significant P value.
DISCUSSION
Over the past decade, there has been increasing awareness of the safety, efficacy, and benefits of PN over RN for small kidney tumors. PN reportedly provides oncologic efficacy equivalent to that of RN and demonstrates benefits over RN, including the prevention of chronic kidney disease as well as nononcologic morbidity and mortality16,18,19 The use of PN has risen dramatically at tertiary care centers of excellence in the past decade, and up to 90% of patients with newly diagnosed T1a tumors currently undergo PN.14,15 This increase is because of a downward stage and size migration of newly diagnosed tumors, a better understanding of the biology of the disease, and a better appreciation of the impact of treatment in these patients.3,4
However, this trend has adopted been poorly in the general practice patterns in the United States. On the basis of SEER data up to 2001, it was noted that only 20% of patients with small renal tumors (from 2 cm to 4 cm in size) underwent PN.17 In our current analysis, only 45% of patients with small renal masses underwent PN in 2006. The increased use of PN over the last several years is encouraging, and it represents a trend that hopefully will continue in the future. We also noted that, for each 1-cm increase in tumor size, there was a 47% lower odds of undergoing PN. Although the number of patients undergoing PN has risen over time, our results demonstrate that RN remains the most commonly performed surgery for small renal tumors. Many investigators have speculated about why there has been such a slow dissemination of PN. The reasons probably are multifactorial and include an under appreciation of the impact of surgery on kidney function, the inherent technical challenges associated with PN, and the greater potential for surgical complications. In addition, not many practicing urologists in the United States have received training in nephron-sparing approaches. Furthermore, as laparoscopy becomes an increasingly popular tool in a surgeon's armamentarium, the skill set required to safely and effectively perform laparoscopic PN over laparoscopic RN has kept laparoscopic PN in the hands of only the most experienced surgeons.20
In addition to a general under use of PN, we identified several disparities in the use of PN for patients with small kidney tumors. One such disparity is the use of PN in women. This disparity was noted previously by Huang et al in their analysis of a previous SEER dataset (1995-2002) linked to Medicare claims.16 In our study, we observed that men had 22% higher odds of undergoing PN over women. In an analysis of the National Cancer Database by Woldrich et al, women reportedly had a significantly higher percentage of stage I tumors than men, suggesting that women should have a greater use of PN.21 In a separate logistic regression model, we included an interaction term between age (as binary variable: <65 years or ≥65 years) and sex. The interaction term was not statistically significant (P = .3) and, thus, indicated that the sex bias was not limited only to a specific age category. The reason for the disturbing disparity in the use of PN among women remains unclear. It is possible that physicians underestimate the risk of chronic kidney disease based on women's lower baseline serum creatinine and a perception that women have fewer comorbid conditions and, thus, are less susceptible to the deleterious effects of RN. Alternatively, the disparity also may be patient-driven, because women may prefer more conservative and “less risky” procedures, although this clearly remains unproven. Finally, the discrepancy also may be related to the finding that women are 2 to 4 times more likely to have benign or complex cystic renal tumors compared with men; thus, they may elect to undergo active surveil-lance for their renal lesions instead of surgical extirpation.22,23 This unexplained sex difference has been noted in other areas of medicine, including access to coronary revascularization, renal transplantation, and hip-and-knee arthroplasty.24-26 Further investigation of this sex bias is warranted, and increased education for patients and healthcare providers may alleviate the discrepancy of care between men and women in the near future.
In our analysis, we also demonstrated a significant disparity in the use of PN among the elderly (aged >70 years). Although >37% of patients ages 40 to 60 years underwent PN, only 29% of patients aged >80 years underwent PN for a kidney tumor <4 cm in size. The reason for this disparity most likely is physician-related, because it is perceived that RN incurs less perioperative complications and morbidity than PN.27,28 Furthermore, it also may be perceived that the potential benefits of PN do not extend to the elderly population. On the basis of emerging data, however, this cohort of patients may benefit most from PN or even no treatment at all.13,29 Elderly patients have a lower baseline glomerular filtration rate and a greater number of comorbid conditions, which increase their risk of mortality after any treatment. In addition, it has been demonstrated that both RN and a sudden decrease in the glomerular filtration rate independently are predictors of premature death in the elderly.12,16 Therefore, the use of RN in this cohort may have a substantial impact on nononcologic outcomes. Given the indolent nature of many small renal tumors and the finding that up to 25% have benign histology, the use of RN (and, possibly, any surgery) may be viewed as “over-treatment” in elderly patients.30 With the increasing interest in surveillance and minimally invasive ablative therapies, this discrepancy may be corrected over time in these patients. However, until long-term outcomes validate these treatment options, PN should be extended to this elderly population.
Other significant disparities that were identified our series were year of surgery, residence (urban vs rural), and lesion size. In 1999, nearly 21% of patients with renal tumors underwent PN. This increased to >45% by 2006. This probably is a reflection of increased surgeon education and greater surgeon comfort with PN. Thus, as more and more surgeons understand the benefits of PN and acquire the skills necessary to perform it, the number and size of renal tumors treated with PN likely will continue to increase. Because tertiary care centers or “centers of excellence” typically are located in more metropolitan areas, it is not surprising that there was a 25% increase in the use of PN in urban locations. We hope that, through continued physician and patient education, this disparity will dissolve over time.
There are several limitations of this study worth mentioning. Although SEER is the largest, most comprehensive, and highest quality cancer registry in the United States that has been widely studied and validated,31 the disparities in the use of PN and RN may depend heavily on other variables that were not examined in our study, such as comorbid conditions, patient preferences, and tumor location/indications for surgery. There also are inherent difficulties in accurately determining the rates of PN based on ambiguity in coding. Despite these limitations, we believe that our study represents the most comprehensive and contemporary analysis of the use of kidney surgery to date in the United States.
Since the initiation of Healthy People 2010, the Department of Health and Human Services has committed the nation to achieving the goal of eliminating health disparities in the United States. Analyses of population-based databases like the SEER cancer registry have demonstrated uniformly the under use of PN and disparities in the care for patients with small renal tumors. Our analysis of the most recent SEER database indicates that discrepancies remain in the treatment practice patterns for older patients, women, and patients in rural settings. It is imperative that, as healthcare providers, we recognize these disparities to eliminate these biases and ensure the equal delivery of quality healthcare to all patient populations in the United States.
Acknowledgments
We thank Elena B. Elkin, Department of Epidemiology and Biostatistics and Division of Urology, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY for her expertise in statistical analysis and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in creating the SEER database.
Drs. Lowrance and Russo are indebted to the Stephen Hanson Family Fellowship for their support. This project was also supported by NIH T32CA82088 and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Footnotes
Author contributions include, but are not limited to the following: The last and senior author contributed to the study concept and design, drafting and revising the article, and supervision of the study; he had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The first author took part in the study design and was responsible for interpretation of the data and for drafting and revision of the article, the second author was responsible for acquisition of the data, analysis of the data, and revision of the article, and the third author contributed to study concept and provided critical revision of the article for important intellectual content.
CONFLICT OF INTEREST DISCLOSURES
Drs. Dulabon and Huang made no financial disclosures.
REFERENCES
- 1.National Institutes of Health (NIH) Fiscal Years 2002-2006. Vol. 1. National Institutes of Health; Bethesda, Md: 2002. NIH Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 4.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year follow-up. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 5.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 6.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113:78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 7.Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000;163:730–736. [PubMed] [Google Scholar]
- 8.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int. 2006;97:939–945. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 9.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 10.Patard JJ, Shvarts O, Lam JS, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171(6 pt 1):2181–2185. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 12.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RH, Kaag M, Vickers A, et al. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol. 2009;181:993–997. doi: 10.1016/j.juro.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zini L, Patard JJ, Capitanio U, et al. The use of partial nephrectomy in European tertiary care centers. Eur J Surg Oncol. 2009;35:636–642. doi: 10.1016/j.ejso.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. discussion 61-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853–857. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. discussion 472-473. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511–520. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 20.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 21.Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ. Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993-2004. J Urol. 2008;179:1709–1713. doi: 10.1016/j.juro.2008.01.024. discussion 1713. [DOI] [PubMed] [Google Scholar]
- 22.Murphy AM, Buck AM, Benson MC, McKiernan JM. Increasing detection rate of benign renal tumors: evaluation of factors predicting for benign tumor histologic features during past 2 decades. Urology. 2009;73:1293–1297. doi: 10.1016/j.urology.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 23.Eggener SE, Rubenstein JN, Smith ND, et al. Renal tumors in young adults. J Urol. 2004;171:106–110. doi: 10.1097/01.ju.0000099028.95679.52. [DOI] [PubMed] [Google Scholar]
- 24.Hawker GA, Wright JG, Coyte PC, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. 2000;342:1016–1022. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 25.Ghali WA, Faris PD, Galbraith PD, et al. Sex differences in access to coronary revascularization after cardiac catheterization: importance of detailed clinical data. Ann Intern Med. 2002;136:723–732. doi: 10.7326/0003-4819-136-10-200205210-00007. [DOI] [PubMed] [Google Scholar]
- 26.Segev DL, Kucirka LM, Oberai PC, et al. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol. 2009;20:621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC Intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2007;51:1606–1615. doi: 10.1016/j.eururo.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130–134. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 29.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180:505–508. doi: 10.1016/j.juro.2008.04.033. discussion 508-509. [DOI] [PubMed] [Google Scholar]
- 30.Schlomer B, Figenshau RS, Yan Y, Venkatesh R, Bhayani SB. Pathological features of renal neoplasms classified by size and symptomatology. J Urol. 2006;176(4 pt 1):1317–1320. doi: 10.1016/j.juro.2006.06.005. discussion 1320. [DOI] [PubMed] [Google Scholar]
- 31.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries [serial online]. BMC Health Serv Res. 2009;9:92. doi: 10.1186/1472-6963-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]