Abstract
Advancements in the field and rising interest among pharmaceutical researchers have led to the development of new molecules with enhanced therapeutic activity. Design of new drugs which can target a particular pathway and/or explore novel targets is of immense interest to ocular pharmacologists worldwide. Delivery of suitable pharmacologically active agents at proper dose (within the therapeutic window) to the target tissues without any toxicity to the healthy ocular tissues still remain an elusive task. Moreover, the presence of static and dynamic barriers to drug absorption including the corneal epithelium (lipophilic), corneal and scleral stroma (hydrophilic), conjunctival lymphatics, choroidal vasculature and the blood-ocular barriers also pose a significant challenge for achieving therapeutic drug concentrations at the target site. Although many agents are currently available, new compounds are being introduced for treating various ocular diseases. Deeper understanding of the etiology and complex mechanisms associated with the disease condition would aid in the development of potential therapeutic candidates. Novel small molecules as well as complex biotechnology derived macromolecules with superior efficacy, safety and tolerability are being developed. Therefore, this review article provides an overview of existing drugs, treatment options, advances in emerging therapeutics and related recent patents for the treatment of ocular disorders such as glaucoma, age related macular degeneration (AMD) and uveitis.
Keywords: Glaucoma, age related macular degeneration (AMD), uveitis, treatment, novel therapeutics, patents
INTRODUCTION
Drug delivery to the eye is still a challenging task to the ocular pharmacologists and pharmaceutical scientists. The unique anatomy of the eye limits the entry of therapeutics at the desired site of action. The complex anatomy and physiology of the eye renders it a highly protected organ. The globe is made up of highly specialized and complex groups of tissues which are essential for vision. The presence of highly different and special structures with specific physiological functions makes it a very distinct organ [1-3]. Human eye can be generally divided into the anterior and the posterior segments. The anterior segment includes the cornea, conjunctiva, iris, ciliary body, aqueous humor and lens while the posterior segment comprises sclera, choroid, retina and vitreous humor Fig. (1). Cornea, the outermost transparent multilayered membrane of the eye, is devoid of blood supply and acquires its nourishment from aqueous humor and limbal blood capillaries [4]. The human cornea is comprised of five layers i.e. corneal epithelium, Bowman’s membrane, stroma, Descemet’s membrane, and endothelium. The aqueous humor is a fluid present in the anterior segment of the eye. It is the major source of nutrition to the crystalline lens and cornea which are devoid of blood vessels. Iris is the colored portion of the eye comprising pigmented epithelial cells and circular muscles (constrictor iridial sphincter muscles). The opening in the middle of the iris is called pupil. The iris sphincter and dilator muscles help in adjusting the pupil size which regulates the amount of light entering the eye. The ciliary body, a ring-shaped muscle attached to the iris is pro-duced by ciliary muscles. Contraction and relaxation of the ciliary muscle controls the shape of the lens. The lens is a crystalline and flexible unit consisting of layers of tissue enclosed in a capsule. It is suspended from the ciliary muscles by very thin fibers called the zonules. Conjunctiva is a clear mucous membrane that lines the inside of eyelids and spreads from the anterior surface of sclera upto the limbus. It facilitates lubrication in the eye by generating mucus and helps adherence to tear film. The sclera is a white sheath surrounding the eye-ball and is called “white of the eye”. It acts as a principal shield to protect the internal organs. Sclera is juxtaposed by a highly vascularized tissue known as the choroid which is sandwiched between the retina and the sclera. It constitutes a vessel layer (composed of arteries and veins), choriocapillaris (dense network of capillaries), and Bruch’s membrane. Choroid provides nourishment to the photoreceptor cells in the retina. The retina is a multi-layered sensory, light sensitive tissue that lines the back of the eye. It contains millions of photoreceptors/photosensitive elements (rods and cones) that capture light rays and convert them into electrical impulses. These impulses travel all along the optic nerve to the brain where they are converted into images. The vitreous humor is also a jelly-like substance or considered a hydrogel matrix, distributed between retina and lens. This matrix is composed of hyaluronic acid and collagen fibrils. It is alienated from the anterior chamber by hyaloids membrane and is connected through ligaments to the retina.
Fig. (1).
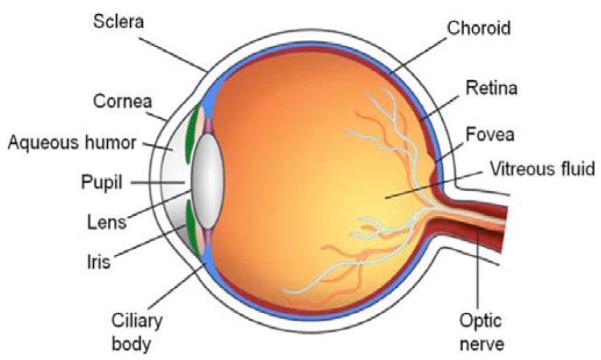
Anatomy of the human eye.
The eye is susceptible to various diseases and effective treatment depends on the efficacy of the drug and the mode of delivery. To deliver pharmacologically active agents, circumvention of the unique anatomical barriers of the eye is necessary. Physicochemical and biological barriers often seem to be responsible for alteration in drug permeation and reduced bioavailability. Physicochemical properties including lipophilicity, molecular weight, solubility and charge play a significant role in drug transport. Also, biological barriers such as epithelial tight junctions, blood-ocular barriers, efflux proteins and enzymatic degradation restrict ocular drug entry [1,5-6]. Despite many efforts by scientists, several potential drug candidates are often dropped from the initial screening portfolio due to failure in overcoming these physiological barriers. The current literature showcases increasing number of patent applications being filed and issued in the field of ophthalmic drug delivery. These patents either disclose a new molecule or a derivative of an existing therapeutic molecule for the treatment of various ocular diseases. Presented is a brief background on disease condition, review of existing drugs, focus on emerging therapeutics and related recent patents for the treatment of glaucoma, age related macular degeneration (AMD) and uveitis.
GLAUCOMA
Glaucoma is a common multifactorial ocular complication characterized by progressive loss of retinal ganglionic cells potentially leading to optic nerve damage and visual field loss [7-8]. This complication usually progresses from the periphery to the center Fig. (2). Excessive inflow or obstructed aqueous humor outflow from the eye at iridocorneal angle is considered to be the major factor responsible for this common cause of irreversible blindness worldwide. Recent statistics estimated 60.5 million people as glaucoma victims which is predicted to increase upto 79.6 million by the year 2020 [9]. In US, more than 2.5 million individuals are suffering from glaucoma, of which > 120,000 are observed to be legally blind [10]. Glaucoma is broadly categorized into open-angle and closed-angle (angle-closure) depending on the appearance of anterior chamber angle. Open-angle glaucoma is characterized by an unobstructed and normal iridocorneal angle whereas the closed-angle glaucoma is characterized by occlusion of the anterior chamber angle by the peripheral iris. The normal intraocular pressure (IOP) in open angle glaucomas typically range from normal tension glaucoma (10-21 mm Hg) to primary (or chronic) open-angle glaucoma (POAG) with an elevation in IOP [11, 12]. A balance between the aqueous humor inflow and the outflow determines the IOP levels. Aqueous humor is formed from the secretory epithelium of ciliary body processes which leaves the anterior chamber via a conventional trabecular meshwork pathway and an unconventional uveoscleral pathway. Flow in the trabecular pathway is through the trabecular meshwork into Schlemm’s canal and aqueous veins, whereas the uveoscleral pathway is through the ciliary muscle and downstream choroid, sclera, and episcleral tissues [13-15]. The exact relationship between elevation of IOP and glaucoma is not fully understood. However, glaucoma treatment is often directed towards controlling the IOP, since it is considered to be the major causative risk factor for initiation and development of POAG and normal tension glaucoma [16]. This is borne out by the fact that drugs which could lower IOP in the anterior chamber through one or more mechanisms of action could treat glaucoma. Pilocarpine was the first drug indicated for the reduction of ocular hypertension. Subsequent introduction of newer ocular hypotensives reduced the rate of glaucoma drainage surgeries. Currently used anti-glaucomatous drugs include α-adrenergic antago-nists, carbonic anhydrase inhibitors and α2-adrenergic ago-nists which reduce aqueous inflow and prostaglandin analogs which increase aqueous outflow via the uveoscleral pathway.
Fig. (2).
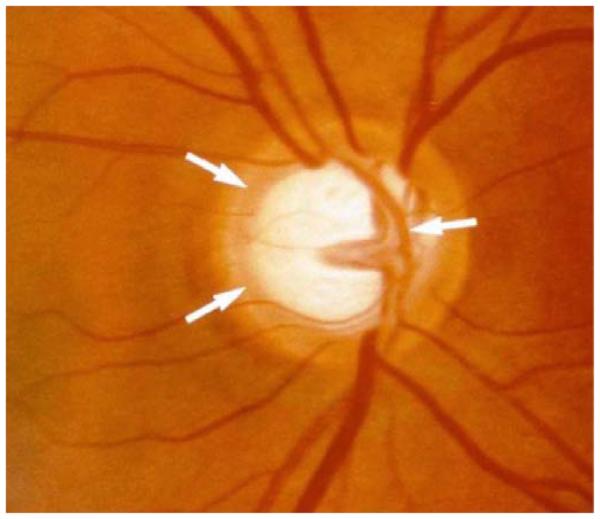
Characteristic nerve appearance with damage from glaucoma. The center of the nerve has an excavated or "scooped out" appearance.
Existing Ocular Hypotensive Agents
β-Adrenergic Antagonists
β-adrenergic antagonists were introduced into clinic in 1970s. These agents compete with adrenoceptor agonists for β-adrenoceptors and are also termed as β-blockers. These compounds lower IOP by diminishing aqueous humor production at ciliary processes level [17]. β-blockers include timolol, carteolol, levobunolol and metipranolol, of which timolol served as the gold standard for glaucoma treatment prior to the introduction of prostaglandin analogs. Timolol possesses β1- and β2-adrenoceptor binding ability, but current treatment is restricted to combination therapy. Levobunolol and metipranolol are non-selective β-adrenoceptor blockers. Levobunolol undergoes enzymatic hydrolysis to generate dihydrolevobunolol, a potent β-adrenoceptor blocker in the cornea, aqueous humor and iris-ciliary body. Both these molecules exhibit comparable activity in reducing IOP [18, 19]. Carteolol, a hydrophilic non-selective partial β-adrenoceptor antagonist reduces IOP and is more preferable in patients with bradycardia, heart failure and pulmonary complications. Though β-blockers are effective in glaucoma therapy, their use is limited due to systemic adverse effects such as bradycardia and bronchial hyper-reactivity in individuals compromised with pulmonary and cardiovascular functions [20, 21].
Parasympathomimetic Drugs
Parasympathomimetics such as pilocarpine, echothiophate iodide and carbachol enhance aqueous outflow by opening trabecular meshwork lamellae through induced contraction of iris sphincter and the ciliary muscle. Pilocarpine and carbachol directly stimulate the muscarinic receptors of the ciliary muscle. Echothiophate iodide acts indirectly by cholinesterase inhibition [22]. Frequent usage of these cholinergic drugs cause miosis, increased intestinal motility, enhanced bronchial and urinary smooth muscle tone, risk of retinal detachment and an unexpected hypo- or hypertension [23, 24].
Carbonic Anhydrase Inhibitors
Carbonic anhydrase isoenzyme II is primarily responsible for fluid transport throughout the body. In particular, inhibition of this enzyme results in reduced sodium transport into the posterior segment and diminishes aqueous humor production [25]. Dorzolamide was the first topical carbonic anhydrase inhibitor whose magnitude of efficacy was equal either alone or in combination with timolol [26, 27]. Topical dorzolamide was marginally less effective compared to its systemic use owing to minor ocular hypotensive efficacy of systemic acidosis. Brinzolamide was introduced later. An ophthalmic suspension (1.0%) is significantly more comfortable than dorzolamide (2.0%) and equally effective in lowering IOP to timolol [28, 29]. In a retrospective study, adjunctive therapy with dorzolamide provided a statistically significant lowering in IOP at 1 year in eyes that were inadequately controlled with latanoprost alone [30]. Common ocular side effects of carbonic anhydrase inhibitors include stinging, blurred vision, headache, skin rashes and tearing.
α2-Adrenoceptor Agonists
α-Adrenoceptor agonists possess varying degrees of α1 and α2−adrenoceptor specificities. Apraclonidine, a para amino derivative of clonidine, is a selective second generation α2− adrenergic agonist. It was initially indicated for the prophylaxis of IOP spikes caused by anterior segment laser procedures. Brimonidine, a third generation α2-adrenergic agonist possesses a quinoxaline ring, which enhances α2-receptor selectivity [23]. These molecules decrease sympathetic tone at ciliary processes and subsequently lower aqueous humor production [31]. Apraclonidine show signs of tachyphylaxis and prevents post-trabeculoplasty pressure spikes. Brimonidine is associated with fewer adverse effects than apraclonidine. Side effects include mydriasis, chemosis, eyelid retraction, and ciliary vasoconstriction [23]. However, ocular allergic reactions such as blepharoconjunctivitis, follicular conjunctivitis and conjunctival hyperemia are some of the concerns with long term therapy [32].
Prostaglandin Analogs
Prostaglandin F2α (PGF2α) analogs are frequently indicated now-a-days for the treatment of glaucoma. Isopropyl unoprostone was the first topical prostaglandin analog (PGA) which was commercially available in 1994, followed by latanoprost (1996), bimatoprost and travoprost (2001). Latanoprost and travoprost are ester prodrugs of naturally occurring PGF2α and are highly selective prostaglandin F (PGF) receptor (FP receptor) agonists [33-35]. Bimatoprost is a PGF2α analog synthesized by replacement of carboxylic acid group with a neutral ethyl amide substituent. Its mechanism of action is initially reported to be mediated via a prostamide receptor, a novel class of naturally occurring substances with inherent IOP lowering properties biosynthesized from endocannabinoid anandamide by the enzyme COX-2 [36,37]. However, recent reports suggest that bimatoprost, an ethyl amide derivative of 17-phenyl-trinor PGF2α is a potent prostaglandin FP receptor agonist [38] and is a prodrug. Sufficient evidence implies that human and bovine corneal tissues metabolize bimatoprost to its free acid which may account for the observed IOP reduction through FP receptor agonist activity [39-41]. Ciliary muscle relaxation via FP receptors, matrix metalloproteinase upregulation and tissue inhibitory effect may alter extracellular matrix and reduce trabecular meshwork resistance contributing to IOP reduction [42-44]. PGAs appear to be more potent than timolol maleate, the previous gold standard [45]. Although PGAs are quite effective in lowering IOP, such therapies are often associated with side effects such as conjunctival hyperemia, irreversible iris darkening probably due to PGA-stimulated melanogenesis [46-48]. In one of the clinical trials, the incidence of reversible periocular skin pigmentation was found to be 1.5 % for latanoprost and 2.9% for bimatoprost and travoprost [45]. Although not serious but other common side effects include iris cysts, anterior uveitis, cystoid macular edema and herpes simplex reactivation [49].
Several fixed combinations of two anti-glaucoma drugs have been evaluated for their synergistic activity to reduce ocular hypertension. Most combinations include timolol (0.5%) along with another anti-glaucoma drug such as pilocarpine 2 or 4% (Timpilo™), dorzolamide 2% (Cosopt™), brinzolamide 1% (Azarga™), brimonidine 0.2% (Combi-gan™), latanoprost 0.005% (Xalacom™), travoprost 0.004% (DuoTrav™) and bimatoprost 0.03% (Ganfort™).
Emerging Ocular Antihypertensive Drugs
Actin-Disrupting Agents
The actin-myosin system consists of actin microfilaments and related proteins. It is greatly structured in trabecular meshwork and Schlemm canal cells and provides cells with mechanical structure, mobility, contractility and adhesion [50]. Trabecular outflow resistance may possibly be affected due to alteration in the flow pathway as well as the amount and composition of the extracellular matrix. Latrunculins (LAT) are such potent compounds which disrupt actin cytoskeleton and increase trabecular outflow [51-53]. INS-115644, a LAT-B compound lowered IOP only at 0.02% and 0.05% doses, producing a maximal 12 hour decrease of 4 mm Hg and was well tolerated. Topical administration of LAT-B 0.005% or 0.01%, twice-daily for 4.5 days, reduced IOP in cynomolgus monkeys without any systemic or ocular adverse effects [54]. A single higher topical dose of 0.02% reduced IOP of ocular normotensive cynomolgus monkeys with a small change in corneal thickness, increase in corneal endothelial permeability, and anterior chamber flare [55]. However, such effective IOP lowering compounds may be delivered by novel drug delivery methods if suitably formulated to reduce adverse events.
Rho-Kinase Inhibitors
Rho guanosine triphosphatases (GTPases) play an important role in cellular processes, predominantly those involving actin cytoskeleton assembly, actin-myosin mediated cell contraction and motility [56, 57]. ROCK (Rho-associated protein kinase) is a downstream effector of Rho in the Rhodependent signal transduction pathway [57, 58]. ROCK1 and ROCK2 selectively interact with different Rho GTPases and activate Rho GTPase [59]. Therefore, ROCK specific inhibitors which can alter actin cytoskeleton and cellular motility of the trabecular meshwork, Schlemm’s canal, and ciliary muscle may encompass a potential new class of ocular anti-hypertensives that can enhance aqueous outflow [60-62]. Inhibitors of ROCK and Rho GTPase were found to reduce IOP in animal models. These compounds act by inducing relaxation of ciliary muscle and trabecular meshwork by decreasing myosin light chain kinase phosphorylation. Y-27632 is the first identified specific ROCK inhibitor. However, it is difficult to find selective ROCK inhibitors because of structurally similar active binding sites [63, 64] in various protein kinases. Y-27632 and H-1152 exhibited a rapid and prolonged IOP decrease by competitive inhibition of ROCK with ATP. These compounds are not specific inhibitors of ROCK because of non-selective inhibition of other protein kinases such as protein kinase C, protein kinase A and myosin light chain kinases [65].
Several ROCK inhibitors currently in clinical trials include AR-12286 and K-115. ROCK inhibitors such as Y-39983/SNJ-1656/RKI-983 and INS-117548 are still under development, but have encountered efficacy and tolerability issues. Topically applied Y-39983 (0.05%) showed dissimilarities in IOP responses when studied in ocular normotensive cynomolgus monkeys and rabbits. These differences may be due to anatomical, physiological, pharmacokinetic differences, or varying expression levels of ROCK and ROCK substrates in various ocular tissues. Also, punctate subconjunctival hemorrhage and conjunctival hyperemia were commonly observed in both the species [66]. Although, INS-117548 could produce mild reductions in IOP, it suffers from dose-related adverse effects, particularly ocular burning and stinging. AR-12286, a 6-aminoisoquinoline amide derivative exhibited very high potency against ROCK. Topical administration of AR-12286 (0.6%) reduced IOP in ocular normotensive non-human primates primarily by augmenting trabecular outflow [67, 68]. It was reported to cause moderate conjunctival hyperemia. However, its long-term tolerability profile and IOP-lowering efficacy in humans are yet to be studied. K-115 is an isoquinolinesulfonamide compound with potent selectivity against Rho-kinase (IC50 = 31 nM). Topical administration of K-115 (0.1%, 0.2%, 0.4%) produced a greater decrease in IOP in monkeys relative to latanoprost 0.005% [69].
Adenosine Receptor Agonists
Adenosine is a purine nucleoside which plays an important role in biochemical, physiological and pathophysiological processes through activation of G protein-coupled adenosine receptors. Four receptor subtypes A1, A2A, A2B, and A3 have been identified and cloned till now. Identification of a specific receptor that is associated with IOP modulation is very difficult because of non-specific selectivity for a subtype. Adenosine receptor agonists act by reducing cell volume and remodeling of the extracellular matrix following secretion of matrix metalloproteinases in human trabecular meshwork cells, thereby increasing conventional aqueous outflow [70, 71]. R-PIA, an adenosine agonist, possess binding affinities (Ki) at human adenosine receptor subtypes of 2 nM (A1), 16 nM (A3), 860 nM (A2A), and 3,800–33,700 nM (A2B) [72, 73]. Interestingly, A3 antagonists could potentially control aqueous humor formation by avoiding adenosine-induced activation of chloride channels in human non-pigmented ciliary epithelial cells and act as IOP lowering agents. Novel adenosine analogs currently in clinical trials include INO-8875/PJ-875 (A1 agonist), OPA-6566 (A2A agonist), ATL-313 (A2A agonist) and CF-101 (A3 agonist). Preclinical studies showed a significant IOP reduction (20-25%) in ocular normotensive cynomolgus monkeys and pigmented rabbits when administered topically. This compound is currently in Phase I/II dose-escalation clinical trial for assessing the tolerability, safety and efficacy of twicedaily topical INO-8875 administration in patients with ocular hypertension or POAG. It lowers IOP by increasing trabecular outflow facility through the conventional outflow pathway [74].
OPA-6566 and ATL-313 are other two novel adenosine A2A receptor agonists recently progressed to Phase I clinical trials. Both compounds activate G proteins and stimulate adenylyl cyclase, mediate vasodilation, and may perhaps be downregulated after constant exposure to agonist [75]. CF-101, an A3 agonist is currently in Phase II safety and efficacy clinical trial in patients with elevated IOP or POAG. It produced an unexpected reduction in IOP at week 12 in a Phase II dry eye trial [76]. Another A3 antagonist OT-7999 targeting A3 receptors is identified as a potent and selective antagonist. A single topical 1% dose of OT-7999 was well tolerated and reduced IOP by 1.6 mm Hg in conscious male cynomolgus monkeys [77, 78]. These interesting drug candidates (antagonists) may provide a new potential therapy for the treatment of glaucoma.
Serotonin Modulators
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter responsible for the activation of large family of G-protein coupled receptors and ligand gated ion channels [79, 80]. G-protein coupled family involves 5-HT1A, 1B, 1D, 1E, 1F, 5-T2A, 2B, 2C, 5-HT4, 5-HT5A, 5B, 5-HT6, and 5-HT7. Interesting results from preclinical studies demonstrated an effective IOP reduction in ocular normotensive and hypertensive cynomolgus monkeys by topical application of 5-HT2 agonists [81, 82]. R-DOI (R-2,5-dimethoxy-4-iodoamphetamine) is a 5-HT2A,2B,2C receptor partial agonist which reduced IOP in monkeys by raising uveoscleral out-flow. Also, this compound has been reported to cause a slight increase in aqueous humor formation [81,83]. Since several scaffolding proteins and kinases regulate 5-HT2A receptor function and both agonists and antagonists induce down-regulation of receptors, 5-HT2 receptor agonists and antagonists have been proposed as IOP lowering agents [84, 85]. One of such compounds is BVT.28949, a 5-HT2A receptor antagonist which showed 1–3 mm Hg reduction in IOP of ocular normotensive cynomolgus monkeys and in glaucomatous or ocular hypertensive patients upon topical administration. However, further progress in the development of this compound is not evident [86].
Cannabinoid Receptor Agonists
Endocannabinoids and tetrahydrocannabinol derived from Cannabis sativa activate G protein-coupled cannabinoid (CB) receptors. Two subtypes (CB1 and CB2) have been cloned and characterized [79,87]. WIN 55212–2, a potent synthetic cannabinoid CB1 and CB2 receptor agonist has been studied to understand the local mechanisms in IOP reduction. Topical administration of WIN 55212–2 reduced aqueous humor flow without altering the tonographic out-flow facility [88]. SAD-448 (0.02%), a CB1 and CB2 receptor agonist reduced IOP only by 2.3–2.4 mm Hg, while latanoprost (0.005%) reduced IOP by 3.5 mm Hg [13]. Palmitoylethanolamide (PEA), an endogenous fatty acid ethanolamide appears to act at cannabinoid-like receptors [89]. This compound was evaluated as an adjunctive oral therapy in POAG patients and the observed IOP reduction was more than 2.4–3.4 mm Hg. However, the exact role of cannabinoids and its receptor agonists in modulating IOP still remains unclear.
Recent Patents for the Treatment of Glaucoma
A recent patent application by Park et al. discusses the utilization of naphthoquinone based compounds or its prodrugs, solvate or isomer for the treatment of glaucoma. The applicants have reported that glaucoma induced rats are susceptible to oxidative stress which is the key factor for glaucoma onset, optic nerve damage and augmentation of reactive oxygen species (ROS). Elevated ROS levels are toxic, degenerates retinal ganglionic cells (RGC) and axons in the optic nerve. The inventors reported that the compounds of this invention could reduce ROS induced oxidative stress and prevent degeneration of RGC and its axons. These compounds may be formulated to be suitably absorbed into the target site and may also exert desired pharmacological effect to treat glaucoma related complications [90].
In another invention the usage of Complement C1q inhibitors for the treatment of glaucoma and ocular hypertension is reported. Complement C1q, a member of classical complement pathway is involved in initial binding to the antigen. Subsequent protease activation (C1r, C1s, and C2-C4) amplifies the complement process and forms classical C3 convertase, central mediator of the complement cascade, primarily leading to the formation of membrane attack complex. The inventors mentioned that the administration of these inhibitors may provide neuroprotection, prevent RGC loss and either reduce/prevent ocular damage resulting from glaucoma. Inhibitors of this invention include small molecule inhibitors, inhibitory antibodies, non-antibody proteins, small interfering RNAs, short hair pin RNA, ribozymes, deoxyribozymes and antisense RNAs. The applicants state that these compounds or derivatives thereof can be formulated and administered in combination with other IOP lowering agents such as rho kinase inhibitors, prostaglandin analogs, carbonic anhydrase inhibitors, miotics and neuroprotectants [89].
A more recently issued patent by Clark et al. discloses RNA interference (RNAi) for the treatment of glaucoma. RNAi is a process where double stranded DNA is applied to silence gene expression. Since glaucoma is associated with cellular changes resulting from the expression of Frizzled Related Protein-1 (FRP-1) mRNA that leads directly or indirectly to glaucoma or glaucoma related conditions, use of RNAi for the glaucoma treatment has been discussed. Silencing FRP-1 mRNA expression interferes with the Wnt signaling pathway and prevents a cascade of upcoming glaucomatous conditions. RNAi may consist of a sense nucleotide strand, an antisense nucleotide strand and a region of atleast near perfect contiguous complimentarity of atleast 19 nucleotides. The inventors claim that these pharmaceutically acceptable carriers may be administered via topical, transscleral, intravitreal, periocular, subconjunctival, subtenon, intracameral, subretinal, retrobulbar, intracanalicular or suprachoroidal routes [91].
A patent application by Hellberg et al. describes the application of 3-hydroxy-3-methylglutaryl conenzyme A (HMG-CoA) reductase inhibitors in regulating IOP and treating glaucoma. The preferred compounds with a retention index of 0.2-0.7 were reported to be suitable for topical application to the eye. HMG-CoA reductase inhibitors (i.e. statins) act by delayed loss of cellularity as observed in glaucoma condition. Furthermore, anti-oxidative and free radical scavenging effects of statins can aid in overcoming oxidative damage or stress in the trabecular meshwork and retina. Treatment with statins preserves trabecular meshwork cellularity, improves aqueous humor outflow and reduces IOP. Statins offer neuroprotection by reducing ischemic damage to neural tissue. Moreover, these compounds appeared to prevent over accumulation of extracellular matrix, suppress CTGF expression and fibronectin secretion thereby reducing the resistance to aqueous outflow and finally lowering IOP [92].
A series of patents by Dantanarayana et al. disclosed pyrazolo[3,4-e]benzoxazoles [93], substituted 1-alkylamino-1H-indazoles [94], substituted furo[2,3-g]indazoles [95] and their analogs to lower IOP and treat glaucoma. All the above disclosed compounds are 5-HT2 agonists which do not enter central nervous system, but possess enhanced chemical stability and produce sufficient therapeutic activity to lower or regulate elevated IOP. The inventors stated that these compounds can be preferably formulated as topical suspension or solution, with a pH of 5-8. All these compounds can be formulated either alone or in combination with other ocular antihypertensive agents such as β-blockers, carbonic anhydrase inhibitors, α1-antagonists, α2-agonists and prostaglandin analogs.
AGE RELATED MACULAR DEGENERATION
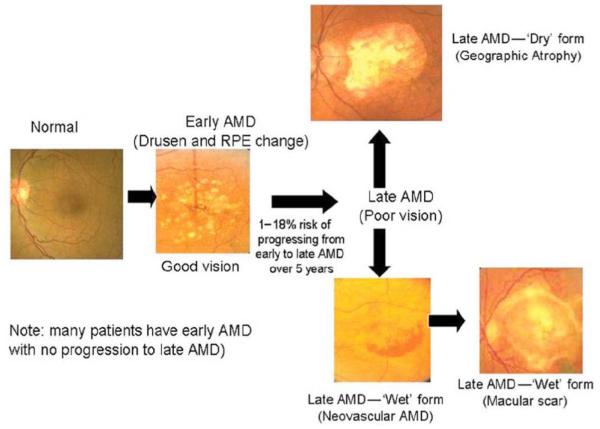
Age-related macular degeneration (AMD) is another leading cause of vision loss and blindness in patients over the age of 65 particularly in Europe and North America. At present, approximately 13 to 14 million Americans are suffering from AMD and it is expected that the number of AMD cases will double by 2020 [96, 97]. Two distinct clinical forms of AMD are known: Non-exudative AMD and Exudative AMD Fig. (3) [98]. Non-exudative AMD or Dry AMD, accounts for almost 85 to 90% of cases, however it is responsible for only 10% of vision loss [99]. It is characterized by focal deposition of acellular, polymorphous debris between the basal lamina of the retinal pigment epithelium (RPE) and inner collagenous layers of Bruch’s membrane. These focal yellow deposits known as ‘drusen’ are categorized depending upon the size as ‘small’ (< 63μm in diameter), ‘medium’ (63-124μm in diameter) or ‘large’ (> 124μm in diameter). Patients suffering from early dry AMD exhibit either several small drusen or a few medium size drusens with a minimal alteration in vision. Intermediate dry AMD involves formation of several medium sized drusens or few large drusens which may cause blurring of vision. Both early and intermediate stages can advance to late form of dry AMD which is characterized by 'geographical atrophy’ involving photoreceptors and other tissues of the central retina. Gradual vision loss could occur in patients with late dry AMD; however it takes years to develop. On the other hand, exudative AMD represents only 10 to 15% of overall incidence but causes approximately 90% of significant vision loss. Exudative AMD or Wet AMD is characterized by choroidal neovascularization (CNV) which refers to the proliferation of abnormal blood vessels from choroid to retina. These abnormal blood vessels leak fluid and blood into the subretinal spaces which lead to blurring of vision and sudden vision loss. Moreover, there is also a scar formation due to retinal detachment and fibrosis which causes irreversible central vision loss [100, 101]. The primary purpose of AMD management is to minimize vision loss and improve quality of patient’s life. A clear understanding of basic pathological mechanisms may lead to development of novel therapeutics for treating AMD. Current treatment options for AMD range from thermal laser photocoagulation, photodynamic therapy to anti-VEGF therapy.
Fig. (3).
Stages of age related macular degeneration (AMD). Reproduced with permission from Ref [98].
Existing Treatment
Laser Photocoagulation
Thermal laser therapy destroys choroidal neovascular membranes by photocoagulation and eventually halts their progression. Macular Photocoagulation Study (MPS) trial has appraised the efficacy of laser photocoagulation in the treatment of extra-foveal and sub-foveal CNV. Patients with extra-foveal CNV have shown significant reduction in relative risk of severe vision loss following laser photocoagulation treatment up to 2 years compared to untreated eyes. However, high recurrence of neovascularization (in approximately 54% of laser treated eyes) has been observed at 3 year follow up period [102]. In patients with juxta-foveal and sub-foveal CNV, the laser photocoagulation therapy has resulted in a limited benefit scale. Higher rates of recurrence with poor vision outcomes and evolution of better contemporary therapeutic options have limited the use of laser photocoagulation for the treatment of AMD [103-105].
Photodynamic Therapy [PDT] Using Verteporfin [Visudyne®]
This treatment evolved as an alternative to laser photocoagulation for the treatment of wet AMD. In this procedure, verteporfin, a photosensitive dye, is administered by IV followed by activation of the dye with a laser light of 689nm wavelength. Laser excitation of verteporfin generates ROS which damages endothelial cells and induces occlusion of CNV [106]. Unlike laser photocoagulation, it specifically destroys the CNV without damaging the retina and underlying choroid. Verteporfin in Photodynamic therapy (VIP) and treatment of AMD with Photodynamic Therapy (TAP) trials have proven the effectiveness of PDT in the treatment of sub-foveal CNV [107, 108]. Results of both trials have suggested approximately 50% reduction in the risk of vision loss. However with PDT, improvement in visual acuity is rare. Additionally, it induces inflammatory response and elevates vascular endothelial growth factor (VEGF) levels which contribute to recurrence of disease.
Anti-VEGF Therapy
VEGF is an angiogenic factor which binds to its receptor and induces the development of new blood vessels with increased vascular permeability. Numerous studies have revealed the involvement of VEGF upregulation in CNV. This led to the development of several anti-VEGF drugs for the treatment of AMD. Currently, three potential anti-VEGF agents-pegaptanib (Macugen®), ranibizumab (Lucentis®) and bevacizumab (Avastin®) are indicated in the treatment of CNV [109, 110]. Macugen®, a pegylated aptamer, is the first marketed anti-VEGF agent. It binds with heparin binding domain of the VEGF165 isoform and selectively inhibits angiogenesis. The effectiveness of Macugen® in patients with predominantly classic, minimally classic, and occult with no classic CNV has been assessed in multicenter VEGF Inhibition Study in Ocular Neovascularization (VISION) trials [111]. Clinical beneficiary effects have been observed in all groups treated with intravitreal pegaptanib injection. In pegaptanib treated group, 70% of subjects lost fewer than 15 letters of visual acuity (VA), relative to 55% of subjects in the sham injection group. In addition, the risk of severe visual loss was reduced from 22% in the control group to 10% in the pegaptanib treated group. Route of administration related adverse effects such as endophthalmitis, retinal detachment and cataract have been observed in less than 1.3% of patients after pegaptanib intravitreal injection with no signs of systemic side effects.
Ranibizumab (Lucentis®) a recombinant humanized anti-body fragment displays very high binding affinity towards all VEGF isoforms. Following intravitreal injection, because of small molecular weight it easily penetrates the retina and reaches the sub-retinal space where it inhibits VEGF. Two randomized, multi-center clinical trials MARINA and AN-CHOR were performed to evaluate effectiveness of monthly intravitreal ranibizumab. In both the clinical trials approximately 95-96% of the subjects treated with ranibizumab (0.5 mg) maintained stable vision within 3 lines at 1 year in comparison to 62-64% in case of sham or PDT group. Furthermore, almost in 40% of patients treated with ranibizumab, visual acuity was improved by at least three lines which were very high compared to PDT group (6%) and sham group effects. However, high cost is a limiting factor for wide-spread use of ranibizumab [112, 113].
Recently, ‘off label’ therapy with intravitreal bevacizumab (1.25mg) has been initiated for AMD. Bevacizumab (Avastin®) is a full length recombinant humanized monoclonal antibody which binds to all VEGF isoforms. Currently, it is approved by Medicare but not by FDA for treating patients with exudative AMD. In a small study involving 17 subjects, intravitreal injection at 2.5mg dosage showed significant improvements in vision acuity from baseline at four and eight weeks. However, no significant improvement in visual acuity was observed beyond that period [114]. Currently, a Comparative AMD Treatments Trial (CATT) is ongoing to assess the relative efficacy and safety of bevacizumab and ranibizumab for treating subfoveal neovascular AMD.
Corticosteroids
Corticosteroids are the first anti-inflammatory agents evaluated for the treatment of AMD [115]. Anti-inflammatory effect of steroids is contributed by their antiangiogenic, anti-fibrotic and antipermeability activity. Triamcinolone acetonide and dexamethasone are most commonly employed steroids usually combined with anti-VEGF or PDT for the treatment of AMD. Several clinical trials are ongoing for evaluating the benefit of steroids in combination therapy.
Combination Therapy
Many treatment options are emerging for AMD treatment, however no single treatment offers better cure for disease with improvement in vision acuity. AMD is another multifactorial disease and hence combination of two or more therapeutics may have synergistic effect. In FOCUS (FhuFab V2 Ocular Treatment Combining the Use of Visudyne to Evaluate Safety) trial, 91% of patients treated with combination therapy of ranibizumab with visudyne photodynamic therapy (V-PDT) showed stable vision compared to 68% with V-PDT monotherapy [116]. In addition, 31% of the combination therapy treated patients improved vision acuity by 3 lines compared to 15% in case of V-PDT alone. Augustin’s triple therapy regimen including intravitreal dexamethasone, bevacizumab with V-PDT emerged as an ideal and attractive approach for treating AMD. In a pilot study with triple therapy regimen, vision acuity was improved by 8.9 letters at 40 weeks follow up as well as both structural and functional improvement in the retina was observed [117]. So far promising results have been obtained with combination therapy and other clinical trials-the PROTECT study (ranibizumab plus PDT with verteporfin) and the VI-SION Phase IV study (pegaptanib plus PDT) are currently ongoing.
Emerging Drugs for AMD
VEGF Trap
Aflibercept or VEGF Trap is a recombinant protein comprising of portion of VEGF receptor 1 and 2 (VEGFR1 and 2) fused to Fc region of human IgG. It demonstrated very high binding affinity towards all VEGF isoforms than ranibizumab and bevacizumab. In Phase I study with VEGF trap, mean gain in visual acuity was 4.8 letters at 6 weeks and the mean gain was even higher in patients receiving higher dose. Currently, Phase II VEGF trap trial is enrolling patients [118, 119].
Small Interfering RNA [siRNA]
Bevasiranib is the first siRNA developed in an effort to diminish expression of VEGF. It is a double stranded RNA molecule and once inside the cell it gets converted to small interference RNA by an enzyme called dicer. The formed siRNA interacts with RNA-induced silencing Complex (RISC) and destroys the mRNA that encodes for VEGF thereby suppressing VEGF expression [120]. To evaluate efficacy of intravitreal bevasiranib in treating neovascular AMD, a randomized Phase II trial CARE (Cand5 Anti-VEGF RNA Evaluation) involving 127 subjects has been conducted [121]. At the end of 12 weeks, approximately 78% of the subjects lost visual acuity less than 3 lines. However, the improvement in visual acuity was not as promising as other anti-VEGF agents and also a delay in action was observed. This delayed action might be due to the ability of bevasiranib to target a relatively upstream component of VEGF pathway [122]. Therefore, its clinical effect in the disease progression may not be observed until all the VEGF is cleared. Currently, combination therapy including bevasiranib and an anti-VEGF antibody such as ranibizumab and bevacizumab to immediately bind to existent VEGF is under consideration in a Phase III clinical trial. Another drug with RNA interference mechanism is Sirna-027, a chemically stabilized siRNA. It blocks the expression of VEGFR1 mRNA. Sirna-027 has shown promising results in Phase I study and at present it is under Phase II clinical trial [123].
Receptor Tyrosine Kinase Inhibitors
Another approach of suppressing VEGF activity is to block interaction with VEGFR1 and VEGFR2 and thus inhibit tyrosine kinase cascade. Vatalanib® (Novartis International AG, Basel, Switzerland) is a potent inhibitor of both VEGFR1 and VEGFR2. Preclinical studies have shown its efficacy in the treatment of ischemic retinopathies induced by VEGF [124]. The Phase I/II (ADVANCE) trial for evaluating efficacy of combination therapy of PDT/vatalanib over PDT monotherapy is currently enrolling patients.
Pigment Epithelial-Derived Factor [PEDF]
PEDF is an endogenous angiogenesis inhibitor which is normally produced in the eye. In addition to anti-angiogenesis properties, it also protects photoreceptors in the retina. Adenoviral vector mediated gene delivery of PEDF (AdPEDF.11, GenVec, Gaithersburg, MD, USA) into the retina by either intravitreal or periocular route has demonstrated inhibition of ocular neovascularization in animal models. A Phase I study revealed no dose limiting toxicity with long term therapeutic effectiveness [125, 126].
OT-551
OT-551 (Othera Pharmaceuticals) is a disubstituted hydroxylamine possessing antioxidant and anti-inflammatory properties. Following systemic administration, both OT-551 and its metabolite have shown protective effect on RPE against light induced damages. The efficacy and safety of topical eye drops of OT-551 has been evaluated in Phase II study involving patients with bilateral geographic atrophy. It was found to be effective in maintaining visual acuity with no serious side effects. However, limited beneficiary effect in treating geographic atrophy suggests the requirement of evaluating its efficacy at high doses [127].
Anecortave Acetate
Anecortave acetate (Retaane®; Alcon Research, Fort Worth, TX, USA), is a unique cortisene with angiostatic activity and is devoid of glucocorticoid activity. It imparts angiostatic activity by inhibiting the expression of urokinase plasminogen activator (uPA) and matrix metalloproteinases (MMPs). In some studies it is observed to reduce the expression of the proangiogenic insulin-like growth factor (IGF-1) and IGF-1 receptor. Clinical trials in patients with exudative AMD have displayed an excellent safety record both for anecortave acetate and the posterior juxtascleral depot (PJD) administration, with no clinically relevant adverse events [128].
Recent Patents for the Treatment of AMD
A recent US patent discloses application of mesozeaxanthin, an endogenous carotenoid, for the treatment of macular diseases particularly for AMD. It has been proposed that macular pigments have protective effect on sensitive tissues against the damaging effects of blue light. Also, it has been observed that total zeaxanthin content in AMD affected eyes is 30% less than that of normal eyes. Among all three isomers of zeaxanthin (3R,3’R-zeaxanthin, 3R,3’S-(meso)-zeaxanthin and 3S,3’S-zeaxanthin), loss of meso-zeaxanthin in AMD affected eyes is more prominent than the other isomers. Hence a supplement of meso-zeaxanthin alone or in combination with other carotenoids and vitamins may increase macular pigments and is expected to prevent/treat AMD. In this patent it was suggested that meso-zeaxanthin at dosages between 0.5 and 50mg/day, preferably 1-20mg/day, most preferably 5-15mg/day would be effective in the prevention or treatment of AMD [129].
A recent patent by Marcus et al. discloses design and synthesis of prodrugs comprising of therapeutic agents linked to a carotenoid for the treatment of macular and retinal diseases. Prodrugs include anecortave acetate, anti-VEGF aptamer or protein kinase C inhibitor linked to carotenoids (lutein or zeaxanthin) for treating exudative AMD. As xanthophyll carotenoids are more concentrated within the macula, these prodrugs are expected to accumulate more in the macula leading to improvement in drug efficacy and treatment of macular diseases [130].
An US patent by Inana et al. discusses about diverse genes related to AMD and/or phagocytosis by RPE cells. By means of a novel expression cloning strategy called CHANGE (Comparative Hybridization Analysis of Gene Expression), around 200 AMD related genes and 60 phagocytosis related genes have been isolated among which MT1-MMP (membrane type matrix metalloproteinase) is an exemplary. MT1-MMP gene encodes for proteases involved in extracellular remodeling and is upregulated in the RPE and photoreceptor of AMD affected eyes as well as in phagocytosis. A 5.5 fold increase in MT1-MMP mRNA levels was observed in the retina affected with AMD compared to control eyes. The patent also discloses methods for treating AMD by targeting either MT1-MMP nucleic acid or protein. A subretinal injection of anti-MT1-MMP antibody delayed retinal degeneration in rats [131].
A recent US patent entitled “Methods and composition for the treatment of ocular neovascularization” discloses methods for treating ocular neovascularization, particularly CNV. MCP-1 is a monocyte chemoattractant protein-1 which binds to CCR-2 receptor and initiates macrophage recruitment. It plays a key role in initiating angiogenesis and facilitating CNV. Also, upregulation of MCP-1 was demonstrated in laser induced CNV model. This patent discusses about inhibiting macrophage infiltration by MCP-1 or CCR-2 antagonist as a novel strategy for preventing CNV. Examples of MCP-1 and CCR-2 antagonists include small molecules, peptides, nucleic acids, anti-MCP-1 and anti-CCR-2 antibodies. In laser induced CNV model, anti-MCP-1 anti-body significantly inhibited macrophage recruitment and almost 77% reduction in CNV was observed [132].
In another recently published patent by Tamaki et al., the use of vaccine therapy for treating CNV has been disclosed. Exudative AMD, the most common vision threatening ocular condition, is caused by CNV. VEGF is an angiogenic factor and its upregulation is implicated in the development of CNV. VEGF signaling is mediated by tyrosine kinase receptors - VEGFR1 and VEGFR2. Of the two, VEGFR2 mediates almost all of the known cellular responses of VEGF including CNV development. This patent application discloses utilization of VEGFR2 derived peptides as an immunizing agent for the treatment and/or prevention of CNV. Various peptide sequences have been designed and evaluated for producing immune response against CNV. A peptide VIAMFFWLL was observed to suppress VEGF upregulation in mice model and subsequently inhibited CNV growth [133].
UVEITIS
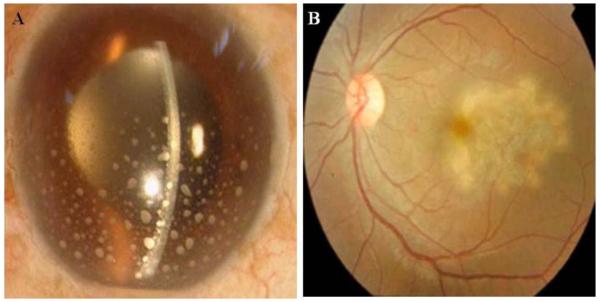
Uveitis is an autoimmune disease that affects the central region of the eye, known as the uvea. It is often associated with swelling, irritation and inflammation of the uvea. According to American uveitis society, this disorder accounts for 20% of the blindness in United States. The exact etiology of this disease is poorly understood. Several sources such as bacteria, virus, fungus, dust, etc were found to initiate inflammation/uveitis. Rachel R Caspi detailed the underlying mechanism for the autoimmune uveitis. It was observed that priming of the autoreactive T-cells occurs in the regional lymph node and on dendritic cells, in response to the exposure of retinal antigens or due to cross-reactive microbial mimic [134]. Based on the cause of inflammation, uveitis can be classified into two categories (i) infectious and (ii) non-infectious. According to recent disease guidelines, ocular inflammation may also be categorized based on the location of inflammation observed as anterior (iris), intermediate (front of retina and vitreous that includes pars plana and vitreous humor), and posterior (choroid, choroiditis, sometimes includes retina and optic nerve, retinitis) Fig. (4). Among the three inflammations, anterior uveitis is the most prevalent and a common idiopathic disease [135-138]. Posterior inflammation is considered as vision threatening because it may result in loss of vision by damaging the retinal cells, macula, and optic nerve and may also develop uveitic glaucoma [139, 140].
Fig. (4).
Swelling of the uvea. A) Eye with anterior uveitis, B) Eye with posterior uveitis.
Existing Anti-Uveitic Drugs
Steroidal Drugs
Steroids are considered as the standard gold therapy indicated for the treatment of ocular inflammations. Dexamethasone, prednisolone, triamcinolone acetonide, fluocinolone acetonide and loteprednol etabonate are commonly employed. These drugs act by various mechanisms which include inhibition of multiple inflammatory cytokines, fibrin deposition, and polymorphonuclear leukocyte migration. Anti-angiogenic activity by inhibition of nuclear factor-kappa-B (NF-kB) signal pathway by dexamethasone was reported recently [141].
Dexamethasone (9α-Fluoro-16α-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione) is a synthetic corti-costeroid synthesized from 16β-methylprednisolone acetate. It is more potent than prednisone, prednisolone and natural cortisol [142]. Oral and intravenous administrations are not recommended due to associated side effects. Maxidex® (0.1% dexamethasone suspension), dexamethasone sodium phosphate ophthalmic solution® (0.1%) are recommended for anterior ocular inflammations as topical eye drops. Other dexamethasone products available in combination with anti-microbials are Maxitrol® and Tobradex® eye ointment/suspensions. Adverse reactions developed with topical administration are elevated IOP and cataract development [143, 144]. For non-infectious posterior uveitis, dexamethasone is delivered via an Ozurdex® (0.7 mg dexamethasone) implant which was recently approved by FDA in 2009. A total of 153 patients participated in multi-center, double masked, ran-domized clinical studies for a period of 26 weeks. A statistical significant difference was observed for no inflammation in patients treated with Ozurdex® (47% vs 12%). Eight week study resulted in improved visual acuity of 3 lines from baseline in 20-30% subjects tested. Ocular adverse side effects such as IOP elevation and conjunctival hemorrhage were reported in 25% and 22% participants respectively. Conjunctival hyperemia, cataract, and vitreous detachment were reported in 7%, 5% and 2% of the patients respectively.
Prednisolone (11β, 17α, 21-Trihydroxy-1, 4-pregnadiene-3, 20-Dione) is currently employed for anterior and posterior uveitis by oral route. It elicits its pharmacological action within 1-2 hours of drug administration but causes side effects such as systemic immunosuppression, cardiovascular disturbance and other disorders [145]. To avoid these side effects local administration is preferred. Currently, prednisolone is available as Omnipred® and Pred Forte® (1%), Blephamide suspension® and in combination as Vasocidin® suspension (alcohol base) or topical drops (phosphate) for local administration. Also, ophthalmic drops of prednisolone sodium phosphate® and prednisolone sodium sulfacetamide phosphate® are available for topical drop administration. Adverse reactions are reported with chronic topical application which include elevation of IOP, development of glaucoma, optic nerve damage and posterior subcapsular cataract development, iritis and dense punctuate epithelial keratitis [146].
Triamcinolone (9α-Fluoro-11β, 16α, 17, 21-tetrahydroxy-1,4-pregnadiene-3,20-dione) was approved by FDA in 1957 for posterior ocular disease treatment as an intravitreal injection. Experimental studies in rabbits revealed triamcinolone to be more potent than other steroidal drugs such as cortisone, hydrocortisone, prednisone and prednisolone [147, 148]. The rationale to choose triamcinolone acetonide was its slow vitreous clearance with a half life of 1.6 days [149. 150]. Longer vitreous residence was observed for triamcinolone acetonide in comparison to dexamethasone [151, 152]. Currently, it is marketed as Triesence® (triamcinolone) and Trivaris® (triamcinolone acetonide). Efficacy evaluation of Triesence® revealed that intravitreal injection was safe on ocular tissues, inhibits edema, treat refractory uveitic cystoids macular edema and help visualizing the posterior segment structures [153-155]. Common steroidal side effects such as elevated IOP and cataract were noted. Also, route of administration based side effects such as vitreous floaters and conjunctival hemorrhage were reported with high dose (4mg) of triamcinolone.
Fluocinolone acetonide (6a, 9a-difluoro-16a,17a-isopropylidenedioxy-1,4-pregnadiene-3,20-dione) is another synthetic glucocorticoid and a derivative of hydrocortisone. Retisert®, an intravitreal implant, received FDA approval in 2005 for chronic posterior uveitis treatment. Release kinetics estimated that life span of the 2mg and 15 mg drug loaded devices could last for 2.7 and 18.6 years, respectively in the rabbit eye with no toxicity (confirmed with slit lamp examination, indirect ophthalmoscopy and histological examination) [156]. Early clinical trials were conducted with new implant by Jaffe et al. Safety and efficacy of the device was evaluated in a pilot study employing five patients with severe posterior uveitis. All the implant treated eyes demonstrated stable and improved vision with reduced corticosteroid requirement. Randomized clinical studies with Retisert® implant for the treatment of non-infectious posterior uveitis were conducted for 34 weeks. Visual acuity was improved in 87% individuals and the systemic corticosteroid requirement, periocular injections and topical steroid administrations were significantly reduced. Although with the development of new delivery devices the dose requirement of corticosteroid is reduced, but the steroid associated side effects such as elevated IOP and cataract still persists. Patients observed with cataract progression required cataract surgery [157]. Recurrence of non-infectious posterior uveitis was observed in patients treated with Retisert® implant due to drug depletion in the device. The mean time between first implantation of the device and the appearance of first episode of inflammation was 3.1 years. The drug depleted implants were either replaced with new implants or were placed in vitreous along with the existing device. The patients were followed for duration of 17 months. Recurrent iridocyclitis with hypopyon and vitreitis were observed. Other steroid based general complications such as cataract and elevated IOP were uncommon [158].
Lotiprednol etabonate (chloromethyl 17α-ethoxycarbonyloxy-11 β-hydroxy-3-oxoandrosta-1,4-diene 17 β-carboxylate) received FDA approval in 1998, for uveitis treatment. Lotemax®, Zylet® and Alrex® ophthalmic suspension/drops are commercially available. This drug has been shown to lack serious side effects in steroid responders [159, 160] and is used for the treatment of inflammation post cataract surgery. The formation of inactive metabolites following de-esterification reduces the likelihood of elevated IOP [161]. Two randomized, multicenter, double masked studies revealed that the patients treated with the 0.5% ophthalmic ointment had significant reduction in anterior chamber cell or flare (27.7% vs 12.5%) compared to control group (vehicle treated patients). Post ocular surgery, the drug was well tolerated with no pain and also visual acuity of patients was improved. Ocular side effects associated with 0.5% ointment were eye pain, iritis, anterior chamber inflammation, photophobia, corneal edema and conjunctival hyperemia [162] .
Under the conditions where inflammations requires high steroid dose or chronic dosing (>7.5 mg) for more than 1 month, side effects may arise which may result in steroid discontinuation. Patients are recommended for steroid sparing therapy and steroid therapy is slowly tapered off once the inflammation is stabilized. There are several classes of steroid sparing agents which are discussed below.
Antimetabolites
Antimetabolites are involved in blocking cell proliferation by inhibiting the nucleic acid synthesis [163]. Methotrexate, a folate analog, was initially identified as anti-neoplastic agent in 1948 [164]. Dihydro folate reductase is involved in thymidilate and purine nucleotide synthesis that results in rapid cell division. Methotrexate acts by inhibition of dihydrate folate reductase resulting in arrest of rapid cell division. Oral or intravenous administration of methotrexate is associated with adverse side effects such as hepatotoxicity, cytopenia and interstitial pneumonitis. Recently, a pilot study was conducted with an intravitreal injection of methotrexate (4mg/ml) to evaluate and treat uveitis and uveitic cystoid macular edema. Improvement in vision occurred at 3 and 6 months with 4 and 4.5 lines, respectively. No significant difference in visual acuity was observed (methotrexate vs high dose triamcinolone acetonide). Disease relapsed back at a median of four months and second dose administration showed similar improvement in vision [165].
Azathioprine is a prodrug of 6-mercaptopurine that finds application in autoimmune reactions such as organ transplantation, inflammatory bowel disease. Azathioprine actively interferes with DNA replication and RNA transcription, thereby inhibits the rapidly dividing immune cells. Oral administration of azathioprine shows rapid absorption with variability in metabolism. Azathioprine is metabolized by xanthine oxidase and thiopurine methyltransferase enzymes. Uveitis treatment dose ranges from 1-2.5 mg/kg/day. This drug causes gastrointestinal upset. Other adverse effects include hepatotoxicity and myelosuppression due to accumulation of toxic metabolites. A limited number of randomized clinical trials were conducted to evaluate its efficacy. Most of the studies established efficacy of this drug in the treatment of Behçet’s syndrome and anterior uveitis (chronic iridocyclitis) [166, 167].
Mycophenolate mofetil (MMF) is an immunosuppressive prodrug of mycophenolic acid. It is widely used in solid organ transplant and multiple autoimmune diseases [168]. Hydrolysis of MMF converts the prodrug into active parent drug, mycophenolic acid [169]. MMF reversibly inhibits the critical rate limiting enzyme called inosine-5-monophosphate dehydrogenase in the de novo synthesis of purines. Lymphocytes B and T require a fully functional de novo pathway for purine synthesis and proliferation. Thus, inhibition of the enzyme by MMF blocks the proliferation of both B and T lymphocytes. It was found to be effective in ocular inflammatory disease treatment alone or in combination with corticosteroids, or other immunosuppressive drugs. MMF was evaluated in chronic ocular inflammatory disease treatment in a total of 54 patients. In the study outcome, 54% of patients responded positively to corticosteroid-sparing effect of the drug and 75% success rate was achieved in controlling most difficult forms of uveitis. Adverse side effects were recorded in 44% of the patients under treatment which were resolved upon withdrawal of the medication. No morbidity or mortality was recorded with the use of this drug for inflammatory disease treatment [170].
Immunosuppressive Agents
Immunosuppressive agents are second line of treatment recommended for the steroid responsive patients. Mostly, cyclosporine and tacrolimus are recommended for the suppression of uveitis in steroid responders.
Cyclosporine A (CsA) is the drug of choice for non-infectious anterior and posterior uveitis and can be regarded as a replacement therapy for steroids [171, 172]. It is widely recommended in corneal transplant. Immunosuppressive properties of this drug were reported in 1976. Effect of the drug in suppressing uveitis was discovered with experimental autoimmune uveitis in animal model [173]. CsA is a calcineurin inhibitor which acts by abrogating the T-cell mediated signal transduction and down regulates interleukin-2 (IL-2) receptor expression [174]. The safety, tolerability and efficacy of CsA were evaluated in a randomized double masked study consisting of severe chronic idiopathic uveitis patients. The dose of CsA was tapered off from the initiated dose of 10 mg/kg/day. It was observed that the immunosup-pressive effect did not last with dose reduction. A combination of prednisolone and CsA produced synergistic immuno-suppressive effect and improved visual acuity. A low dose treatment (<5 mg/kg/day) was found to be effective in treating uveitis as observed by reduction in inflammation, improved vision and reduction in oral steroid dosage [175]. Side effects such as renal toxicity and hypertension were commonly observed [176]. But toxic effects of CsA limit its use, thus requiring a close supervision and prompt adjustments in drug dose.
Tacrolimus, an immunosuppressive agent, is 10-100 times more potent than CsA [177]. It is initially evaluated as immunosuppressive therapy in patients with solid organ transplant and found to be effective in treating non-infectious posterior uveitis. Currently, it is marketed as Prograf® for oral or IV administration. It also acts by abrogating T cell receptor signal transduction and down regulating the inter-leukin-2 (IL-2) receptor expression [174]. Efficacy studies for tacrolimus were compared with CsA in 72 patients. Results revealed a significant improvement in visual acuity and binocular indirect ophthalmoscopy scores were similar for both the drugs. Also, tacrolimus is less likely found to induce systemic hypertension and lipid abnormalities [178, 179]. No adverse effects were reported and better tolerability was observed in patients treated with tacrolimus. Long term efficacy and tolerability studies revealed improved visual acuity in patients with reduced side effects and increased tolerance [180].
Sirolimus (Rapamune®) is another immunosuppressive drug that finds application in organ transplant. It binds with FK binding protein-12 (FKBP) and forms a complex. This sirolimus bound FKBP-12 complex further binds with the mammalian target of rapamycin (mTOR) and suppresses the progression of cell cycle from G1 to S phase in T cells by IL-2 mediated signal transduction pathway blockage. In other words sirolimus blunts the responses of T- and B- cells to specific lymphocytes induced by both calcium dependent and calcium independent stimuli [181-183]. Dose of sirolimus for ocular inflammation treatment is not well established but current dose is similar to transplant dose. A pilot study with 8 patients was conducted to study the efficacy of sirolimus. The therapy was found to be effective in five of the eight patients, all of whom had their dose of corticosteroids reduced or discontinued. Adverse effects such as hyper-cholesterolemia, gastrointestinal, hematological and cutaneous disturbances have been reported [184].
Alkylating Agents
Alkylating agents were initially developed for cancer chemotherapy and subsequently their efficacy was recognized for uveitis treatment of unknown etiology [185]. Drugs such as chlorambucil and cyclophosphamide exert their cytotoxic effect by alkylating the 7-nitrogen position of guanine in DNA strands. Alkylation results in cross linking of DNA bases, formation of abnormal base pairing and breakage of DNA strands. Ultimately, this process results in impaired cell division [186, 187].
Cyclophosphamide, an alkylating agent is metabolized in liver and kidney to various metabolites. Acroline is one of the metabolites which is thought to cause bladder toxicity [188]. The oral dose for treating ocular inflammations is 1-3 mg/kg. A two month pilot study was conducted in 38 patients with severe ocular inflammation of diverse etiologies. Sixty eight percent of patients responded to the therapy, 55% of patients were devoid of ocular inflammation and 41% of the patients could terminate steroid therapy. The most common adverse effects observed with cyclophosphamide therapy include myelosuppression, bladder toxicity, and teratogenicity.
Chlorambucil is another alkylating agent whose mechanism of action is similar to that of cyclophosphamide. The drug is administered in two dosage regimens (i) short term and (ii) long term. In short term dosage regimen, high dose of 2 mg/day for 1 week followed by an increase of 2 mg/day each week until the inflammation subsides. In long term dosage regimen, 0.1 −0.2 mg/kg is recommended as a single dose for 1 year after inactivation of inflammatory disease. Sixty eight percent of the patients treated and followed for 46 months with long term low dose therapy exhibited positive response and 50% of them were devoid of inflammation. Adverse effects include reversible bone marrow suppression, low WBC count, gonadal dysfunction and opportunistic infections [189].
Emerging Anti-Uveitic Drugs
AIN457
Biological agents such as AIN457 are being developed as rescue therapy for the patients who do not respond to standard corticosteroids and immunosuppressive drugs. It is a high affinity human monoclonal antibody that finds application in the treatment of non-infectious uveitis. This compound neutralizes the bioactivity of proinflammatory cytokine IgG1 isotype IL-17 [190]. From the Novartis unpublished data it was discovered that the molecule possesses limited potential for binding with normal and uninflammed tissues. Phase II open label pilot study included a total of 16 patients with anterior, intermediate and posterior uveitis of diverse etiologies. Intravenous administration of 10 mg/kg dose of AIN457 significantly reduced inflammation compared to the placebo group. Uveitis response was successfully treated over 8 weeks in 73% of patients [191]. Efficacy of AIN457 was assessed with improved visual acuity, resolved vitreous haze/posterior inflammation and ability to discontinue topical and systemic corticosteroids administrations. Adverse effects such as severe pruritus, mild upper respiratory tract infections, headache, upper abdominal pain and conjunctival hyperemia were observed. Their rates of occurrence were similar in both the AIN457 and placebo groups. No death occurred with intravenous administration in the treated patients and no antibodies were detected for AIN457 [192]. This agent has successfully completed its Phase II studies and Phase III studies are on-going. AIN457 is currently being evaluated in three different studies namely, SHIELD, ENDURE and INSURE. In SHIELD study, patients are enrolled to determine the safety and efficacy of AIN457 compared to placebo and adjunctive immunosup-pressive therapy in Behçet’s syndrome individuals with panuveitis or posterior uveitis. ENDURE study is on-going to determine the safety and efficacy of AIN457 for quiescent non-infectious intermediate and posterior uveitis. INSURE is a dose ranging placebo controlled study wherein AIN457 compared to placebo will be evaluated to maintain suppression of uveitis in adults with active non-infectious intermediate, posterior or panuveitis [193].
Apremilast
Apremilast ((S)-N-(2-[1-(3-Ethoxy-4-methoxy-phenyl)-2-methanesulfonylethyl]-1,2-dioxo-2,3-dihydro-1H-isoindol-4-yl) acetamide) is a phosphodiester 4 (PDE4) inhibitor [194]. Elevated tumor necrosis factor-α (TNF-α) levels are associated with several inflammatory diseases. TNF-α is the product of secretions from monocytes and macrophages. Among various PDE enzymes, PDE4 is the major enzyme which is involved in TNF-α production. Inhibition of PDE4 enzyme can suppress the TNF-a production from the stimu-lated human peripheral blood monocytes and macrophages and thus suppress immune response. This drug is expected to provide an oral medication that would avoid the complications associated with anti-TNF-a injection. Phase II clinical trials are on-going with this medicament. No clinical trials for uveitis are conducted but this drug may be clinically useful for uveitis treatment.
Voclosporin [LX-211]
Voclosporin, a chemical derivative of cyclosporine, is a calcineurin inhibitor currently in Phase III studies for non-infectious uveitis. It is 4 times more potent than CsA [195]. It acts by blocking the activity of calcium regulated serine threonine phosphatase calcineurin that results in prevention of proinflammatory cytokine release and inhibition of T-cell proliferation [196]. Also, it inhibits the lymphokine production, release, fibroblast proliferation and VEGF expression [197]. Clinical trials with voclosporin were evaluated in six Phase I studies with a single dose of ranging from 0.25 - 4.5 mg/kg and in multiple doses ranging 0.25 - 1.5 mg/kg bid. Voclosporin was well absorbed with higher drug accumulations in blood. Safety profile for voclosporin was determined and found to be safe in all single and multiple dose Phase I clinical trials. Dose dependent adverse effects such as diarrhea, headache and hypertension were observed. Although reports exist for decrease in renal function, only 4% of the patients were found with clinically significant changes in kidney function.
A randomized, double blind, placebo-controlled LUMI-NATE program was conducted. This program contained three studies; Study 1, LUMINATE Active; quiescent inter- mediate-, anterior- and intermediate-, posterior- or panuveitis patients requiring systemic immunosuppressant therapy; Study 2, LUMINATE Maintenance and Study 3 LUMI- NATE Anterior. The study was successfully completed and Phase III studies were initiated in 2009. Recently, Phase III studies have been completed and the reports yet to be released.
ESBA-105
ESBA-105 is a novel potent humanized small chain anti-body variable (scFv) fragment. This molecule consists of 246 amino acids with a molecular weight of 26,255 kDa [198]. The function of ESBA-105 is similar to the full IgG antibody infliximab against TNF-α [199]. Topical administration of the ESBA-105 achieved therapeutic concentrations in all ocular tissues with very low systemic exposure. Topical administration improved the half life of the drug in vitreous (16-24 hours) relative to intravenous administration (7 hours). After topical administration, the drug was found to diffuse into the vitreous in high concentrations after one hour of first drop. It is suggested that the drug may bypass the cornea and anterior chamber to reach vitreous. Transport pathway involves diffusion through limbus, migration into sclera and directly to vitreous humor and retina [198]. ESBA-105 reached ocular tissues efficiently without the aid of any penetration enhancer [199].
Canakinumab
Canakinumab (Ilaris®) is a high affinity and specific human IgGK monoclonal antibody that selectively blocks IL-1β. It is the third IL-1 blocker that received FDA approval in 2009. Currently, this novel antibody is being evaluated in chronic obstructive pulmonary disease, diabetes and AMD. Phase I studies to evaluate the safety, tolerance, pharmacokinetics and pharmacodynamics are currently on-going. The studies are being conducted in 800 enrolled Japanese patients and till now 2.1% of patients are reported with serious adverse reactions. Clinical investigations are still on-going [200]. This drug may have potential utility in treating ocular inflammatory disease.
Recent Patents for the Treatment of Uveitis
An US patent application by Wannamaker et al., disclosed design, synthesis and oral bioavailability of novel prodrugs of IL-1β converting enzyme (ICE) inhibitor [201]. These compounds act by inhibiting the activity of ICE enzyme, decreasing the production of interferon-y inducing factor (IL-18) or interferon-γ (INF-γ) or interleukin-1. Peptide, peptidyl inhibitors and non-peptidyl compounds were reported earlier which can inhibit ICE. Low cellular uptake, lower oral absorption and rapid metabolism restricted the use of these inhibitors. Oral studies were conducted in male Sprague-Dawley® rats. Novel prodrug and their closely related prodrugs exhibited better in vivo activity than the active parent drug, aspartic acid II. The new prodrug which exhibited better pharmacological properties and higher in vivo bioavailability was 1-[2-(4-amino-3-chloro-benzoylamino)-3, 3-dimethyl-butyrl]-pyrrolidine-2-carboxylic acid (2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide. This new prodrug displayed better pharmacological properties, higher oral bioavailability and finds application in various inflammatory disease treatments including uveitis.
A recent US patent application [202] discusses the synthesis, characterization and pharmacological properties of new kinase inhibitors. These compounds have been reported to act by inhibiting various kinase and kinase-mediated events in the signaling process within the cell. Inhibition of these signaling pathways reduces cytokine production, angiogenesis causing arrest of cell division and consequently diminishes inflammation. Various in vitro assays were conducted to determine biological activities of the compounds. From the list of compounds prepared, compound 72 (IC50 of 2 nM) was found to be a potent inhibitor of the p38u Abelson cytoplasmic tyrosine kinase (Ab1) and Tie-receptor tyrosine kinase pathway. Tolerability studies were conducted in mice with compound 72 at three doses of 10, 30 and 100 mg/kg BID for two weeks. In vivo data revealed a significant inhibition (72 %) of dose dependent angiogenesis with this compound. This new compound is found to be potent in regulating the vascular permeability properties of the endothelial cells and would aid in regulating the inflammatory diseases such as uveitis.
A recent patent [203] disclosed novel phosphotetrahy-dropyran compounds and their derivatives which are similar to mannose-6-phosphate exhibiting higher resistance towards mannosidase enzymes. These compounds find application in preventing T-lymphocyte migration from blood to extravascular tissues. Inhibitory function of these compounds plays a key role in treating various inflammatory disorders such as uveitis, thyroiditis, sialitis, type 1 diabetes and others. T-lymphocyte cell surface expresses receptor for phosphosugars such as mannose-6-phosphate which are thought to play a key role in extravasation in vivo. T-lymphocytes are recirculated in the body by migrating from lymph nodes into the blood stream through efferent lymphatic ducts and return back into lymph nodes from blood stream through post capillary venules. During this process, T-lymphocytes bind with high endothelial venules of peripheral lymph nodes and secondary lymph organs and initiate extravasation. Previous experimental studies showed selective inhibition of lymphocyte binding to high endothelial venules with mannose-6-phosphate and other related carbohydrates which concluded the expression of carbohydrate binding receptor on lymphocyte. In vitro inhibition studies for the new tetrahydropyran derivatives were conducted in brain derived endothelial cells. Mannose-6-phosphate derivatives were found to significantly inhibit T-lymphocyte migration ranging from 53±2 to 90±4%. Intravenous administration of mannose-6-phosphate derivative, 1-(2,4-dimethyl phenyl)-6-phosphono-mannoside inhibited the T-cell migration by 56.1%. Side effects associated with this compound include chills, fever, pain, nausea, tachycardia, hypertension, diarrhea, blurred vision, and gastritis. The efficacy of these new mannose-6-phoshpate derivatives to inhibit the T-cell migration is very high and could be a valuable tool in treating inflammatory diseases or disorders that involves T-lymphocyte migration.
A recent patent [204] disclosed the synthesis, in vitro release kinetics and anti-inflammatory activity of novel aldehyde derivatives. These novel derivatives upon administration release in situ carbon monoxide “CO” at the inflammatory site and aid in reducing inflammations. These new derivatives are referred to as carbon monoxide release molecules (CORMS). CO is an endogenous metabolite which finds potential application in treating inflammations and in adaptive body response to various types of stress. CO has an inhibitory effect on the production of TNF, other proinflammatory cytokines such as IL-1, IL-6, MIP-1 and thereby modulates immune response. Also, CO with inducible nitric oxide synthetase is shown to inhibit excess production of NO and inhibit mast cell activation. Under pathological conditions high levels of ROS are generated. In vitro release studies showed that CORMS or aldehyde derivatives undergo hydrolysis and release CO at particular sites where high levels of ROS are located. These derivatives are safe and effective for the treatment of inflammatory diseases and are not associated with adverse side effects of drugs such as steroids. These compounds could be of utility in the treatment of inflammatory conditions such as uveitis.
A recent patent [205] disclosed compositions that finds application in treating ocular disorders. Induced oxidative stress is implicated to develop ocular diseases such as AMD, uveitis, and various retinopathies. Use of anti-oxidants was investigated to reduce the oxidative stress and thereby reduce/prevent inflammations. Novel hydroxylamines derivatives possess radical scavenging property, mimic the activity of superoxide dismutase and exert an anti-inflammatory effect. In vivo studies with topical application of these compositions were conducted in rabbit animal model. Studies conducted to identify the metabolites of the compound revealed that the compound was not detected and rapidly converted into its corresponding metabolites. Preliminary ocular tolerance studies revealed that the compositions were well tolerated with no observed adverse effects. Hydroxylamine compositions are being evaluated to serve the purpose of therapeutic agents. Results obtained indicate that these molecules could penetrate the cornea and get converted to desired hydroxylamine (H-hydroxypiperidine) by enzymatic activity. In vivo studies demonstrated considerable amounts of drug concentrations in the back of the eye, vitreous humor and blood, post topical drop application. The inventors claimed that these compositions may serve as alternatives for the treatment of cataract and other inflammatory diseases.
CURRENT & FUTURE DEVELOPMENTS
Significant advances have been made towards the design and development of novel ocular therapeutics. Researchers have focused on identifying novel targets for which newer drugs could be designed. Innovative outcomes in pharmaceutical biotechnology and molecular research has allowed for significant improvements in the availability of newer therapeutics including siRNA, RNAi, and antibodies. Continuous advancements in gene delivery appear to be very exciting for the treatment of a gamut of ophthalmic diseases. A comprehensible understanding of the complexities associated with tissues in normal or diseased conditions, physiological barriers, tissue distribution and pharmacokinetics would significantly accelerate the development of new therapeutics. Current literature pertaining to ophthalmic field exhibits a drastic increase in the number of patents granted as well as applications published which disclose various therapeutic compounds acting on novel targets. Hopefully, the current pace in which these new molecules are being developed would lead to block buster drugs in the pharmaceutical market.
ACKNOWLEDGMENTS
The authors would like to acknowledge NIH grants R01EY010659-14 and R01EY09171-16 for financial support.
Footnotes
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–60. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4(4):371–88. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- [3].Janoria KG, Hariharan S, Dasari CR, Mitra AK. Recent patents and advances in ophthalmic drug delivery. Recent Pat Drug Deliv Formul. 2007;1(2):161–70. doi: 10.2174/187221107780831923. [DOI] [PubMed] [Google Scholar]
- [4].Dingeldein SA, Klyce SD. Imaging of the cornea. Cornea. 1988;7(3):170–82. [PubMed] [Google Scholar]
- [5].Majumdar S, Duvvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: strategies for human and veterinary drug development. Adv Drug Deliv Rev. 2004;56(10):1437–52. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [6].Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23(5):279–96. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- [7].Caprioli J, Coleman AL. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol. 2010;149(5):704–12. doi: 10.1016/j.ajo.2010.01.018. [DOI] [PubMed] [Google Scholar]
- [8].Osborne NN. Pathogenesis of ganglion "cell death" in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008;173:339–52. doi: 10.1016/S0079-6123(08)01124-2. [DOI] [PubMed] [Google Scholar]
- [9].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38(1):83–91. [PubMed] [Google Scholar]
- [11].Desai PV, Caprioli J. The treatment of normal-tension glaucoma. Prog Brain Res. 2008;173:195–210. doi: 10.1016/S0079-6123(08)01114-X. [DOI] [PubMed] [Google Scholar]
- [12].Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2011;19(2):85–8. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- [13].Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–77. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alm A, Nilsson SF. Uveoscleral outflow--a review. Exp Eye Res. 2009;88(4):760–8. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [15].Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci. 2006;47(10):4181–7. doi: 10.1167/iovs.06-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bahrami H. Causal inference in primary open angle glaucoma: specific discussion on intraocular pressure. Ophthalmic Epidemiol. 2006;13(4):283–9. doi: 10.1080/09286580600681339. [DOI] [PubMed] [Google Scholar]
- [17].Coakes RL, Brubaker RF. The mechanism of timolol in lowering intraocular pressure. In the normal eye. Arch Ophthalmol. 1978;96(11):2045–8. doi: 10.1001/archopht.1978.03910060433007. [DOI] [PubMed] [Google Scholar]
- [18].Battershill PE, Sorkin EM. Ocular metipranolol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in glaucoma and ocular hypertension. Drugs. 1988;36(5):601–15. doi: 10.2165/00003495-198836050-00004. [DOI] [PubMed] [Google Scholar]
- [19].Wandel T, Charap AD, Lewis RA, Partamian L, Cobb S, Lue JC, et al. Glaucoma treatment with once-daily levobunolol. Am J Ophthalmol. 1986;101(3):298–304. doi: 10.1016/0002-9394(86)90823-8. [DOI] [PubMed] [Google Scholar]
- [20].Kaiserman I, Fendyur A, Vinker S. Topical beta blockers in asthmatic patients-is it safe? Curr Eye Res. 2009;34(7):517–22. doi: 10.1080/02713680902989337. [DOI] [PubMed] [Google Scholar]
- [21].Nieminen T, Uusitalo H, Turjanmaa V, Bjarnhall G, Hedenstrom H, Maenpaa J, et al. Association between low plasma levels of ophthalmic timolol and haemodynamics in glaucoma patients. Eur J Clin Pharmacol. 2005;61(5-6):369–74. doi: 10.1007/s00228-005-0945-2. [DOI] [PubMed] [Google Scholar]
- [22].Harris LS, Shimmyo M, Mittag TW. Cholinesterases and contractility of cat irides. Effect of echothiophate iodide. Arch Ophthalmol. 1973;89(1):49–51. doi: 10.1001/archopht.1973.01000040051012. [DOI] [PubMed] [Google Scholar]
- [23].Mills RP, Janz NK, Wren PA, Guire KE. Correlation of visual field with quality-of-life measures at diagnosis in the Collaborative Initial Glaucoma Treatment Study (CIGTS) J Glaucoma. 2001;10(3):192–8. doi: 10.1097/00061198-200106000-00008. [DOI] [PubMed] [Google Scholar]
- [24].Naveh-Floman N, Stahl V, Korczyn AD. Effect of pilocarpine on intraocular pressure in ocular hypertensive subjects. Ophthalmic Res. 1986;18(1):34–7. doi: 10.1159/000265411. [DOI] [PubMed] [Google Scholar]
- [25].Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–81. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- [26].Hollo G, Chiselita D, Petkova N, Cvenkel B, Liehneova I, Izgi B, et al. The efficacy and safety of timolol maleate versus brinzolamide each given twice daily added to travoprost in patients with ocular hypertension or primary open-angle glaucoma. Eur J Ophthalmol. 2006;16(6):816–23. doi: 10.1177/112067210601600606. [DOI] [PubMed] [Google Scholar]
- [27].Stewart WC, Day DG, Stewart JA, Holmes KT, Jenkins JN. Short-term ocular tolerability of dorzolamide 2% and brinzolamide 1% vs placebo in primary open-angle glaucoma and ocular hypertension subjects. Eye (Lond) 2004;18(9):905–10. doi: 10.1038/sj.eye.6701353. [DOI] [PubMed] [Google Scholar]
- [28].Silver LH. Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: results from two multicenter comfort studies. Brinzolamide Comfort Study Group. Surv Ophthalmol. 2000;44(Suppl 2):S141–5. doi: 10.1016/s0039-6257(99)00111-3. [DOI] [PubMed] [Google Scholar]
- [29].Silver LH. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Brinzolamide Primary Therapy Study Group. Am J Ophthalmol. 1998;126(3):400–8. doi: 10.1016/s0002-9394(98)00095-6. [DOI] [PubMed] [Google Scholar]
- [30].O'Connor DJ, Martone JF, Mead A. Additive intraocular pressure lowering effect of various medications with latanoprost. Am J Ophthalmol. 2002;133(6):836–7. doi: 10.1016/s0002-9394(02)01418-6. [DOI] [PubMed] [Google Scholar]
- [31].Burke J, Schwartz M. Preclinical evaluation of brimonidine. Surv Ophthalmol. 1996;41(Suppl 1):S9–18. doi: 10.1016/s0039-6257(96)82027-3. [DOI] [PubMed] [Google Scholar]
- [32].Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for glaucoma. Expert Opin Drug Saf. 2010;9(3):483–91. doi: 10.1517/14740331003709736. [DOI] [PubMed] [Google Scholar]
- [33].Schmier JK, Covert DW, Robin AL. First-year treatment costs among new initiators of topical prostaglandin analog identified from November 2007 through April 2008. Curr Med Res Opin. 2010;26(12):2769–77. doi: 10.1185/03007995.2010.531254. [DOI] [PubMed] [Google Scholar]
- [34].Sharif NA, Kelly CR, Crider JY. Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest Ophthalmol Vis Sci. 2003;44(2):715–21. doi: 10.1167/iovs.02-0323. [DOI] [PubMed] [Google Scholar]
- [35].Hellberg MR, Sallee VL, McLaughlin MA, Sharif NA, Desantis L, Dean TR, et al. Preclinical efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist. J Ocul Pharmacol Ther. 2001;17(5):421–32. doi: 10.1089/108076801753266802. [DOI] [PubMed] [Google Scholar]
- [36].Cantor LB. Clinical pharmacology of bimatoprost. Expert Opin Drug Metab Toxicol. 2005;1(1):151–7. doi: 10.1517/17425255.1.1.151. [DOI] [PubMed] [Google Scholar]
- [37].Krauss AH, Woodward DF. Update on the mechanism of action of bimatoprost: a review and discussion of new evidence. Surv Ophthalmol. 2004;49(Suppl 1):S5–11. doi: 10.1016/j.survophthal.2003.12.014. [DOI] [PubMed] [Google Scholar]
- [38].Woodward DF, Krauss AH, Chen J, Lai RK, Spada CS, Burk RM, et al. The pharmacology of bimatoprost (Lumigan) Surv Ophthalmol. 2001;45(Suppl 4):S337–45. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- [39].Sharif NA, Kelly CR, Williams GW. Bimatoprost (Lumigan((R))) is an agonist at the cloned human ocular FP prostaglandin receptor: real-time FLIPR-based intracellular Ca(2+) mobilization studies. Prostaglandins Leukot Essent Fatty Acids. 2003;68(1):27–33. doi: 10.1016/s0952-3278(02)00232-6. [DOI] [PubMed] [Google Scholar]
- [40].Maxey KM, Johnson JL, LaBrecque J. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol. 2002;47(Suppl 1):S34–40. doi: 10.1016/s0039-6257(02)00323-5. [DOI] [PubMed] [Google Scholar]
- [41].Sharif NA, Williams GW, Kelly CR. Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur J Pharmacol. 2001;432(2-3):211–3. doi: 10.1016/s0014-2999(01)01486-8. [DOI] [PubMed] [Google Scholar]
- [42].Bahler CK, Howell KG, Hann CR, Fautsch MP, Johnson DH. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008;145(1):114–9. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hylton C, Robin AL. Update on prostaglandin analogs. Curr Opin Ophthalmol. 2003;14(2):65–9. doi: 10.1097/00055735-200304000-00001. [DOI] [PubMed] [Google Scholar]
- [44].Gaton DD, Sagara T, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch Ophthalmol. 2001;119(8):1165–70. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- [45].Walters TR, DuBiner HB, Carpenter SP, Khan B, VanDenburgh AM. 24-Hour IOP control with once-daily bimatoprost, timolol gelforming solution, or latanoprost: a 1-month, randomized, comparative clinical trial. Surv Ophthalmol. 2004;49(Suppl 1):S26–35. doi: 10.1016/j.survophthal.2003.12.017. [DOI] [PubMed] [Google Scholar]
- [46].Hellberg MR, McLaughlin MA, Sharif NA, DeSantis L, Dean TR, Kyba EP, et al. Identification and characterization of the ocular hypotensive efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist, and AL-6598, a DP prostaglandin receptor agonist. Surv Ophthalmol. 2002;47(Suppl 1):S13–33. doi: 10.1016/s0039-6257(02)00293-x. [DOI] [PubMed] [Google Scholar]
- [47].Netland PA, Landry T, Sullivan EK, Andrew R, Silver L, Weiner A, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132(4):472–84. doi: 10.1016/s0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- [48].Grierson I, Millar L, De Yong J, Day J, McKechnie NM, Hitchins C, et al. Investigations of cytoskeletal elements in cultured bovine meshwork cells. Invest Ophthalmol Vis Sci. 2011;27(9):1318–30. [PubMed] [Google Scholar]
- [49].Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin Emerg Drugs. 2011;16(1):137–61. doi: 10.1517/14728214.2011.521631. [DOI] [PubMed] [Google Scholar]
- [50].Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47(5):1991–8. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- [52].Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82(2):236–46. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- [53].Peterson JA, Tian B, McLaren JW, Hubbard WC, Geiger B, Kaufman PL. Latrunculins' effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41(7):1749–58. [PubMed] [Google Scholar]
- [54].Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–7. discussion 7-9. [PMC free article] [PubMed] [Google Scholar]
- [55].Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res. 2009;88(4):713–7. doi: 10.1016/j.exer.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. Pt 5. [DOI] [PubMed] [Google Scholar]
- [57].Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- [58].Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20(2):242–8. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Strutton DR, Walt JG. Trends in glaucoma surgery before and after the introduction of new topical glaucoma pharmacotherapies. J Glaucoma. 2004;13(3):221–6. doi: 10.1097/00061198-200406000-00008. [DOI] [PubMed] [Google Scholar]
- [60].Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21(3):167–77. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- [61].Nakajima E, Nakajima T, Minagawa Y, Shearer TR, Azuma M. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J Pharm Sci. 2005;94(4):701–8. doi: 10.1002/jps.20285. [DOI] [PubMed] [Google Scholar]
- [62].Fukiage C, Mizutani K, Kawamoto Y, Azuma M, Shearer TR. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem Biophys Res Commun. 2001;288(2):296–300. doi: 10.1006/bbrc.2001.5751. [DOI] [PubMed] [Google Scholar]
- [63].Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, et al. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281(1):260–8. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- [64].Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–98. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- [65].Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- [66].Tokushige H, Inatani M, Nemoto S, Sakaki H, Katayama K, Uehata M, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48(7):3216–3216. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]
- [67].DeLong MASJ, Royalty SM, et al. Discovery and in vitro SAR of AR-12286, a potent kinase inhibitor for the treatment of glaucoma. 240th American Chemical Society National Meeting; Boston, MA, US. Aug, 2010. pp. 22–26. [Google Scholar]
- [68].Wang R-FSJ, Kopczynski C. Effect of 0.6% AR-12286 on aqueous humor dynamics in 6 normotensive monkey eyes. 2009 doi: 10.1097/IJG.0b013e3182952213. ARVO E-Abstract 1465. [DOI] [PubMed] [Google Scholar]
- [69].Mizuno K, Koide T, Fujieda Y, Mori J, Kondo S-I, Matsumoto J, et al. Ocular Hypotensive and Neuroprotective Effects of K-115, a Novel Rho-Kinase Inhibitor. Invest. Ophthalmol. Vis. Sci. 2007;48(5):4805. [Google Scholar]
- [70].Karl MO, Peterson-Yantorno K, Civan MM. Cell-specific differential modulation of human trabecular meshwork cells by selective adenosine receptor agonists. Exp Eye Res. 2007;84(1):126–34. doi: 10.1016/j.exer.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fleischhauer JC, Mitchell CH, Stamer WD, Karl MO, Peterson-Yantorno K, Civan MM. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membr Biol. 2003;193(2):121–36. doi: 10.1007/s00232-002-2013-5. [DOI] [PubMed] [Google Scholar]
- [72].Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316(8):1284–8. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- [74].Kim NCC, Lam T, et al. INO-8875, an adenosine A1 agonist, lowers intraocular pressure through the conventional outflow pathway. Invest Ophthalmol Vis Sci. 2010;51 (ARVO E-Abstract 3238) [Google Scholar]
- [75].Webb RL, Sills MA, Chovan JP, Peppard JV, Francis JE. Development of tolerance to the antihypertensive effects of highly selective adenosine A2a agonists upon chronic administration. J Pharmacol Exp Ther. 1993;267(1):287–95. [PubMed] [Google Scholar]
- [76].Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, et al. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010;117(7):1287–93. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117(1):123–40. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [78].Okamura T, Kurogi Y, Hashimoto K, Sato S, Nishikawa H, Kiryu K, et al. Structure-activity relationships of adenosine A3 receptor ligands: new potential therapy for the treatment of glaucoma. Bioorg Med Chem Lett. 2004;14(14):3775–9. doi: 10.1016/j.bmcl.2004.04.099. [DOI] [PubMed] [Google Scholar]
- [79].Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th) 2009;158(Suppl 1):S1–254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108(5):1614–41. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- [81].May JA, McLaughlin MA, Sharif NA, Hellberg MR, Dean TR. Evaluation of the ocular hypotensive response of serotonin 5-HT1A and 5-HT2 receptor ligands in conscious ocular hypertensive cynomolgus monkeys. J Pharmacol Exp Ther. 2003;306(1):301–9. doi: 10.1124/jpet.103.049528. [DOI] [PubMed] [Google Scholar]
- [82].Gabelt BT, Millar CJ, Kiland JA, Peterson JA, Seeman JL, Kaufman PL. Effects of serotonergic compounds on aqueous humor dynamics in monkeys. Curr Eye Res. 2001;23(2):120–7. doi: 10.1076/ceyr.23.2.120.5477. [DOI] [PubMed] [Google Scholar]
- [83].Gabelt BT, Okka M, Dean TR, Kaufman PL. Aqueous humor dynamics in monkeys after topical R-DOI. Invest Ophthalmol Vis Sci. 2005;46(12):4691–6. doi: 10.1167/iovs.05-0647. [DOI] [PubMed] [Google Scholar]
- [84].Allen JA, Yadav PN, Roth BL. Insights into the regulation of 5-HT2A serotonin receptors by scaffolding proteins and kinases. Neuropharmacology. 2008;55(6):961–8. doi: 10.1016/j.neuropharm.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56(5):441–51. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- [86].Bunch TJSJ, Gabelt BT, et al. BVT.28949 as a potential ocular hypotensive agent in monkeys. Invest Ophthalmol Vis Sci. 2004;45 (ARVO E-Abstract 5040) [Google Scholar]
- [87].Tomida I, Pertwee RG, Azuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol. 2004;88(5):708–13. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chien FY, Wang RF, Mittag TW, Podos SM. Effect of WIN 55212-2, a cannabinoid receptor agonist, on aqueous humor dynamics in monkeys. Arch Ophthalmol. 2003;121(1):87–90. doi: 10.1001/archopht.121.1.87. [DOI] [PubMed] [Google Scholar]
- [89].Shepard AR, Clark AF, Klimko PG, Wax MB. Complement C1Q Inhibitors For The Prevention And Treatment Of Glaucoma. 2009 US20090117098. [Google Scholar]
- [90].Park M-G, Kwak T. Pharmaceutical composition for the treatment and prevention of glaucoma. 2011 US20110020448. [Google Scholar]
- [91].Clark AF, Wang W-h, Mcnatt LG, Chatterton JE. RNAi-mediated inhibition of frizzled related protein-1 for treatment of glaucoma. 2011 US7947660. [Google Scholar]
- [92].Hellberg MR. Statins for the treatment of ocular hypertension and glaucoma. 2011 US20110098314. [Google Scholar]
- [93].Dantanarayana AP, May JA. Pyrazolo[3,4-e]benzoxazoles for the treatment of glaucoma. 2006 US20060069096. [Google Scholar]
- [94].Dantanarayana AP, May JA. Substituted 1-alkylamino-1H-indazoles for the treatment of glaucoma. 2008 US7338972. [Google Scholar]
- [95].Dantanarayana AP, May JA. Substituted furo[2,3-g]indazoles for the treatment of glaucoma. 2009 US07476687. [Google Scholar]
- [96].Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- [97].Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- [98].Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127–49. doi: 10.1093/bmb/ldn012. [DOI] [PubMed] [Google Scholar]
- [99].Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- [100].Ayoub T, Patel N. Age-related macular degeneration. J R Soc Med. 2009;102(2):56–61. doi: 10.1258/jrsm.2009.080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bourla DH, Young TA. Age-related macular degeneration: a practical approach to a challenging disease. J Am Geriatr Soc. 2006;54(7):1130–5. doi: 10.1111/j.1532-5415.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- [102].Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109(8):1109–14. [PubMed] [Google Scholar]
- [103].Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993;111(9):1200–9. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- [104].Macular Photocoagulation Study Group Laser photocoagulation of subfoveal recurrent neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1232–41. doi: 10.1001/archopht.1991.01080090056026. [DOI] [PubMed] [Google Scholar]
- [105].Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1220–31. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- [106].Augustin AJ, Scholl S, Kirchhof J. Treatment of neovascular age-related macular degeneration: Current therapies. Clin Ophthalmol. 2009;3:175–82. doi: 10.2147/opth.s3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- [108].Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials--TAP report. Arch Ophthalmol. 1999;117(10):1329–45. [PubMed] [Google Scholar]
- [109].Waisbourd M, Loewenstein A, Goldstein M, Leibovitch I. Targeting vascular endothelial growth factor: a promising strategy for treating age-related macular degeneration. Drugs Aging. 2007;24(8):643–62. doi: 10.2165/00002512-200724080-00003. [DOI] [PubMed] [Google Scholar]
- [110].Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40(3):352–68. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- [111].Gragoudas ES, Adamis AP, Cunningham ET, Jr., Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- [112].Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- [113].Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- [114].Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142(1):1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- [115].Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye (Lond) 2011;25(2):127–39. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumper JM, Gentile RC, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol. 2006;124(11):1532–42. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- [117].Aggio FB, Farah ME, Meirelles RL, Costa RA, Monteiro ML. New stratusoct findings in extrapapillary excavated anomaly and secondary macular complications. Retina. 2007;27(1):113–5. doi: 10.1097/01.iae.0000242762.86901.26. [DOI] [PubMed] [Google Scholar]
- [118].Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs. 2007;21(4):245–57. doi: 10.2165/00063030-200721040-00005. [DOI] [PubMed] [Google Scholar]
- [119].Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 99(17):11393–8. doi: 10.1073/pnas.172398299. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–6. [PubMed] [Google Scholar]
- [121].Brucker A. Small interfering rna (CAND5) for the treatment of subfoveal choroidal neovascularization due to age-related macular degeneration. Retina society; Combined Retina Society/Gonin Society Meeting; Cape Town. Oct 15-20, 2006. [Google Scholar]
- [122].Emerson MV, Lauer AK. Current and emerging therapies for the treatment of age-related macular degeneration. Clinical ophthalmology. 2008;2(2):377–88. doi: 10.2147/opth.s1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13(3):225–34. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- [124].Maier P, Unsoeld AS, Junker B, Martin G, Drevs J, Hansen LL, et al. Intravitreal injection of specific receptor tyrosine kinase inhibitor PTK787/ZK222 584 improves ischemia-induced retinopathy in mice. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):593–593. doi: 10.1007/s00417-004-1021-9. [DOI] [PubMed] [Google Scholar]
- [125].Mori K, Gehlbach P, Ando A, McVey D, Wei L, Campochiaro PA. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43(7):2428–34. [PubMed] [Google Scholar]
- [126].Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90(4):1526–30. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wong WT, Kam W, Cunningham D, Harrington M, Hammel K, Meyerle CB, et al. Treatment of geographic atrophy by the topical administration of OT-551: results of a phase II clinical trial. Invest Ophthalmol Vis Sci. 2010;51(12):6131–9. doi: 10.1167/iovs.10-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Augustin A. Anecortave acetate in the treatment of age-related macular degeneration. Clin Interv Aging. 2006;1(3):237–46. doi: 10.2147/ciia.2006.1.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Howard AN, Landrum JT, Bone RA. Mesozeaxanthin formulations for treatment of retinal disorders. 2001 US6329432. [Google Scholar]
- [130].Marcus DM, Chu CK. Methods and Compositions for Treatment of Macular and Retinal Disease. 2007 US7259180. [Google Scholar]
- [131].Inana G, McLaren M. Methods and compositions for detecting and treating retinal diseases. 2007 US7309487. [Google Scholar]
- [132].Ambati J. Methods And Compositions For The Treatment Of Ocular Neovascularization. 2008 US20080299130. [Google Scholar]
- [133].Tamaki Y, Tahara H. Vaccine therapy for choroidal neovascularization. 2010 US20100297157. [Google Scholar]
- [134].Caspi RR. Mechanisms underlying autoimmune uveitis. Drug Discov. Today: Dis. Mech. 2006;3:199–206. [Google Scholar]
- [135].Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. 2005;50(4):364–88. doi: 10.1016/j.survophthal.2005.04.003. [DOI] [PubMed] [Google Scholar]
- [136].Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion. [DOI] [PubMed] [Google Scholar]
- [137].McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996;121(1):35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- [138].Weiner A, BenEzra D. Clinical patterns and associated conditions in chronic uveitis. Am J Ophthalmol. 1991;112(2):151–8. doi: 10.1016/s0002-9394(14)76694-2. [DOI] [PubMed] [Google Scholar]
- [139].London NJ, Hovakimyan A, Cubillan LD, Siverio CD, Jr, Cunningham ET., Jr Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur J Ophthalmol. 2011 doi: 10.5301/EJO.2011.6403. [DOI] [PubMed] [Google Scholar]
- [140].Ossewaarde-van Norel A, Rothova A. Clinical review: Update on treatment of inflammatory macular edema. Ocul Immunol Inflamm. 2011;19(1):75–83. doi: 10.3109/09273948.2010.509530. [DOI] [PubMed] [Google Scholar]
- [141].Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, et al. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171(3):1058–65. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;101(10):3809–17. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- [143].Urban RC, Jr., Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986;31(2):102–10. doi: 10.1016/0039-6257(86)90077-9. [DOI] [PubMed] [Google Scholar]
- [144].Becker B, Mills DW. Elevated Intraocular Pressure Following Corticosteroid Eye Drops. JAMA. 1963;185:884–6. doi: 10.1001/jama.1963.03060110088027. [DOI] [PubMed] [Google Scholar]
- [145].Simonini G, Cantarini L, Bresci C, Lorusso M, Galeazzi M, Cimaz R. Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmun Rev. 2010;9(10):674–83. doi: 10.1016/j.autrev.2010.05.017. [DOI] [PubMed] [Google Scholar]
- [146].Raizman MB, Donnenfeld ED, Weinstein AJ. Clinical comparison of two topical prednisolone acetate 1% formulations in reducing inflammation after cataract surgery. Curr Med Res Opin. 2007;23(10):2325–31. doi: 10.1185/030079907X226195. [DOI] [PubMed] [Google Scholar]
- [147].Chavan SB, Cummings EJ. An initial evaluation of (aristocort) triamcinolone and its acetonide derivative in the therapy of ocular inflammation. Am J Ophthalmol. 1960;49:516–30. doi: 10.1016/0002-9394(60)91654-8. Part II. Clinical studies. [DOI] [PubMed] [Google Scholar]
- [148].Chavan SB, Cummings EJ. An initial evaluation of (aristocort) triamcinolone in the therapy of ocular inflammation. Am J Ophthalmol. 1960;49:55–64. doi: 10.1016/0002-9394(60)92664-7. Part I: Experimental studies. [DOI] [PubMed] [Google Scholar]
- [149].Scholes GN, O'Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985;103(10):1567–9. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- [150].Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93(4):415–415. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- [151].Tano Y, Sugita G, Abrams G, Machemer R. Inhibition of intraocular proliferations with intravitreal corticosteroids. Am J Ophthalmol. 1980;89(1):131–6. doi: 10.1016/0002-9394(80)90239-1. [DOI] [PubMed] [Google Scholar]
- [152].Graham RO, Peyman GA. Intravitreal injection of dexamethasone. Treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92(2):149–54. doi: 10.1001/archopht.1974.01010010155016. [DOI] [PubMed] [Google Scholar]
- [153].Habot-Wilner Z, Sallam A, Pacheco PA, Do HH, McCluskey P, Lightman S. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol. 2010;21(S6):56–61. doi: 10.5301/EJO.2010.6062. [DOI] [PubMed] [Google Scholar]
- [154].Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–1115. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dyer D, Callanan D, Bochow T, Abraham P, Lambert HM, Lee SY, et al. Clinical evaluation of the safety and efficacy of preservative-free triamcinolone (triesence [triamcinolone acetonide injectable suspension] 40 mg/ml) for visualization during pars plana vitrectomy. Retina. 2009;29(1):38–45. doi: 10.1097/IAE.0b013e318188c6e2. [DOI] [PubMed] [Google Scholar]
- [156].Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41(11):3569–75. [PubMed] [Google Scholar]
- [157].Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6):1020–7. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- [158].Jaffe GJ. Reimplantation of a fluocinolone acetonide sustained drug delivery implant for chronic uveitis. Am J Ophthalmol. 2008;145(4):667–75. doi: 10.1016/j.ajo.2007.11.008. [DOI] [PubMed] [Google Scholar]
- [159].Noble S, Goa KL. Loteprednol etabonate: clinical potential in the management of ocular inflammation. BioDrugs. 1998;10(4):329–39. doi: 10.2165/00063030-199810040-00007. [DOI] [PubMed] [Google Scholar]
- [160].Howes JF. Loteprednol etabonate: a review of ophthalmic clinical studies. Pharmazie. 2000;55(3):178–83. [PubMed] [Google Scholar]
- [161].Bartlett JD, Horwitz B, Laibovitz R, Howes JF. Intraocular pressure response to loteprednol etabonate in known steroid responders. J Ocul Pharmacol. 1993;9(2):157–65. doi: 10.1089/jop.1993.9.157. [DOI] [PubMed] [Google Scholar]
- [162].Comstock TL, Paterno MR, Singh A, Erb T, Davis E. Safety and efficacy of loteprednol etabonate ophthalmic ointment 0.5% for the treatment of inflammation and pain following cataract surgery. Clin Ophthalmol. 2011;5:177–86. doi: 10.2147/OPTH.S16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kim EC, Foster CS. Immunomodulatory therapy for the treatment of ocular inflammatory disease: evidence-based medicine recommendations for use. Int Ophthalmol Clin. 2006;46(2):141–64. doi: 10.1097/00004397-200604620-00013. [DOI] [PubMed] [Google Scholar]
- [164].Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroylglutamic acid. N Engl J Med. 1948;238(23):787–93. doi: 10.1056/NEJM194806032382301. [DOI] [PubMed] [Google Scholar]
- [165].Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797–801. doi: 10.1016/j.ophtha.2008.10.033. [DOI] [PubMed] [Google Scholar]
- [166].Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behcet's syndrome. N Engl J Med. 1990;322(5):281–5. doi: 10.1056/NEJM199002013220501. [DOI] [PubMed] [Google Scholar]
- [167].Mathews JD, Crawford BA, Bignell JL, Mackay IR. Azathioprine in active chronic iridocyclitis. A double-blind controlled trial. Br J Ophthalmol. 1969;53(5):327–30. doi: 10.1136/bjo.53.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Lipsky JJ. Mycophenolate mofetil. The Lancet. 1996;348(9038):1357–9. doi: 10.1016/S0140-6736(96)10310-X. [DOI] [PubMed] [Google Scholar]
- [169].Morris RE, Hoyt EG, Murphy MP, Eugui EM, Allison AC. Mycophenolic acid morpholinoethylester (RS-61443) is a new immunosuppressant that prevents and halts heart allograft rejection by selective inhibition of T- and B-cell purine synthesis. Transplant Proc. 1990;22(4):1659–62. [PubMed] [Google Scholar]
- [170].Baltatzis S, Tufail F, Yu EN, Vredeveld CM, Foster CS. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology. 2003;110(5):1061–5. doi: 10.1016/S0161-6420(03)00092-7. [DOI] [PubMed] [Google Scholar]
- [171].Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporin A therapy in treating chronic, noninfectious uveitis. Ophthalmology. 1996;103(3):365–73. doi: 10.1016/s0161-6420(96)30683-0. discussion 73-4. [DOI] [PubMed] [Google Scholar]
- [172].Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin A therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96(3):275–82. doi: 10.1016/s0002-9394(14)77814-6. [DOI] [PubMed] [Google Scholar]
- [173].Nussenblatt RB, Rodrigues MM, Salinas-Carmona MC, Gery I, Cevario S, Wacker W. Modulation of experimental autoimmune uveitis with cyclosporin A. Arch Ophthalmol. 1982;100(7):1146–9. doi: 10.1001/archopht.1982.01030040124022. [DOI] [PubMed] [Google Scholar]
- [174].Liu J. FK506 and ciclosporin: molecular probes for studying intracellular signal transduction. Trends Pharmacol Sci. 1993;14(5):182–182. doi: 10.1016/0165-6147(93)90206-y. [DOI] [PubMed] [Google Scholar]
- [175].Mathews D, Mathews J, Jones NP. Low-dose cyclosporine treatment for sight-threatening uveitis: efficacy, toxicity, and tolerance. Indian J Ophthalmol. 2010;58(1):55–8. doi: 10.4103/0301-4738.58472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, Jouanneau C, Jaudon MC, Maksud P, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol. 2002;13(12):2962–8. doi: 10.1097/01.asn.0000034945.61533.26. [DOI] [PubMed] [Google Scholar]
- [177].Peters DH, Fitton A, Plosker GL, Faulds D. Tacrolimus. A review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs. 1993;46(4):746–94. doi: 10.2165/00003495-199346040-00009. [DOI] [PubMed] [Google Scholar]
- [178].Friemann S, Feuring E, Padberg W, Ernst W. Improvement of nephrotoxicity, hypertension, and lipid metabolism after conversion of kidney transplant recipients from cyclosporine to tacrolimus. Transplant Proc. 1998;30(4):1240–2. doi: 10.1016/s0041-1345(98)00226-7. [DOI] [PubMed] [Google Scholar]
- [179].Textor SC, Wiesner R, Wilson DJ, Porayko M, Romero JC, Burnett JC, Jr., et al. Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55(6):1332–9. doi: 10.1097/00007890-199306000-00023. [DOI] [PubMed] [Google Scholar]
- [180].Hogan AC, McAvoy CE, Dick AD, Lee RW. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology. 2007;114(5):1000–6. doi: 10.1016/j.ophtha.2007.01.026. [DOI] [PubMed] [Google Scholar]
- [181].Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, et al. Rapamycin reduces VEGF expression in retinal pigment epithelium (RPE) and inhibits RPE-induced sprouting angiogenesis in vitro. FEBS Lett. 2008;582(20):3097–102. doi: 10.1016/j.febslet.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [182].Salzmann J, Lightman S. The potential of newer immunomodulating drugs in the treatment of uveitis: a review. BioDrugs. 2000;13(6):397–408. doi: 10.2165/00063030-200013060-00003. [DOI] [PubMed] [Google Scholar]
- [183].Terada N, Lucas JJ, Szepesi A, Franklin RA, Domenico J, Gelfand EW. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993;154(1):7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- [184].Shanmuganathan VA, Casely EM, Raj D, Powell RJ, Joseph A, Amoaku WM, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89(6):666–9. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Roda Perez E. Nitrogen mustard therapy of uveitis of unknown etiology. Rev Clin Esp. 1952;44(3):173–80. [PubMed] [Google Scholar]
- [186].Okada AA. Immunomodulatory therapy for ocular inflammatory disease: a basic manual and review of the literature. Ocul Immunol Inflamm. 2005;13(5):335–51. doi: 10.1080/09273940590951034. [DOI] [PubMed] [Google Scholar]
- [187].Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991;35(5):369–85. doi: 10.1016/0039-6257(91)90186-j. [DOI] [PubMed] [Google Scholar]
- [188].Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. Arthritis Rheum. 2010;62(1):9–21. doi: 10.1002/art.25061. [DOI] [PubMed] [Google Scholar]
- [189].Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16(2):309–22. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Leah E. Therapy: Anti-inflammatory hope for AIN457. Nat Rev Rheumatol. 2010;6(12):675. [Google Scholar]
- [191].Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52) doi: 10.1126/scitranslmed.3001107. 52ra72. [DOI] [PubMed] [Google Scholar]
- [192].Heiligenhaus A, Thurau S, Hennig M, Grajewski RS, Wildner G. Anti-inflammatory treatment of uveitis with biologicals: new treatment options that reflect pathogenetic knowledge of the disease. Graefes Arch Clin Exp Ophthalmol. 2010;248(11):1531–51. doi: 10.1007/s00417-010-1485-8. [DOI] [PubMed] [Google Scholar]
- [193].Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs. 2011;3(1):76–99. doi: 10.4161/mabs.3.1.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Man HW, Schafer P, Wong LM, Patterson RT, Corral LG, Raymon H, et al. Discovery of (S)-N-[2-[1-(3-ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo-2 ,3-dihydro-1H-isoindol-4-yl] acetamide (apremilast), a potent and orally active phosphodiesterase 4 and tumor necrosis factor-alpha inhibitor. J Med Chem. 2009;52(6):1522–4. doi: 10.1021/jm900210d. [DOI] [PubMed] [Google Scholar]
- [195].Aspeslet L, Freitag D, Trepanier D, Abel M, Naicker S, Kneteman N, et al. ISA(TX)247: a novel calcineurin inhibitor. Transplant Proc. 2001;33(1-2):1048–51. doi: 10.1016/s0041-1345(00)02325-3. [DOI] [PubMed] [Google Scholar]
- [196].Cunningham MA, Austin BA, Li Z, Liu B, Yeh S, Chan CC, et al. LX211 (voclosporin) suppresses experimental uveitis and inhibits human T cells. Invest Ophthalmol Vis Sci. 2009;50(1):249–55. doi: 10.1167/iovs.08-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Anglade E, Aspeslet LJ, Weiss SL. A new agent for the treatment of noninfectious uveitis: rationale and design of three LUMINATE (Lux Uveitis Multicenter Investigation of a New Approach to Treatment) trials of steroid-sparing voclosporin. Clin Ophthalmol. 2008;2(4):693–702. doi: 10.2147/opth.s2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Furrer E, Berdugo M, Stella C, Behar-Cohen F, Gurny R, Feige U, et al. Pharmacokinetics and posterior segment biodistribution of ESBA105, an anti-TNF-alpha single-chain antibody, upon topical administration to the rabbit eye. Invest Ophthalmol Vis Sci. 2009;50(2):771–8. doi: 10.1167/iovs.08-2370. [DOI] [PubMed] [Google Scholar]
- [199].Ottiger M, Thiel MA, Feige U, Lichtlen P, Urech DM. Efficient intraocular penetration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest Ophthalmol Vis Sci. 2009;50(2):779–86. doi: 10.1167/iovs.08-2372. [DOI] [PubMed] [Google Scholar]
- [200].Dhimolea E. Canakinumab. MAbs. 2010;2(1):3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Wannamaker MW, Davies RJ. Prodrug of an ice inhibitor. 2009 US20090012149. [Google Scholar]
- [202].Groneberg R, Burges LE, Harvey D, Laird E, Munson MC, Rizzi JP, et al. Kinase Inhibitors And Methods Of Use Thereof. 2011 US20110124878. [Google Scholar]
- [203].Cowden WB, Bartell GJ, Eschler BM, March DR, Robertson A. Mannose-6-phosphonate compounds for the treatment of inflammatory diseases. 2010 US7648966. [Google Scholar]
- [204].De Matos MN, Romao CC. Methods for treating inflammatory disease by administering aldehydes and derivatives thereof. 2011 US7968605. [Google Scholar]
- [205].Matier WL, Patil G. Amelioration of cataracts, macular degeneration and other ophthalmic diseases. 2010 US7825134. [Google Scholar]