Abstract
The innate immune response plays a major role in defense against mastitis causing pathogens. Identification of existing variation in innate immune signaling among cows and the underlying molecular causes for the variation may help in design of new mastitis control strategies. The dermal fibroblast has been used as a model cell type to explore between-cow variation in the ability of cells to produce IL-8 in response to lipopolysaccharide (LPS) treatment and this response appears related to an animal’s ability to respond to in vivo challenge with LPS or Escherichia coli mastitis. In this study, primary dermal fibroblast cultures of cows and microarray-based genomic analysis were used to investigate the cause(s) for the variable response to LPS. Fibroblast cultures from two cows, one with a low response phenotype (LRarray) and another with a high response phenotype (HRarray) were selected from our collection of fibroblast cultures established from 88 cows. The LR array fibroblast culture produces approximately five-fold less IL-8 as well as IL-6 proteins in response to 24 h LPS treatment than the HRarray fibroblast culture. Genomic analysis of RNA obtained from three replicates of the two cultures before and after 8 h LPS treatment revealed a combined LPS-induced differential expression of 321 transcripts indicating the robust response capability of the fibroblast cell. Under basal conditions, the microarray analysis revealed two-fold less TLR4 expression in the LR array fibroblasts as compared to the HRarray fibroblasts and this was associated with a marked reduction in expression of genes regulated by the TLR4-MyD88-dependent and TLR4-TRIF-dependent pathways (IL-8, IL-6, SAA3, CCL20, MX1, IRF1 and ISG20). The between-culture differential expression of TLR4 was confirmed and extended by quantitative PCR analysis (QPCR) that revealed a 33-fold lower expression of TLR4 in the LRarray fibroblast culture. After LPS treatment the difference in TLR4 expression increased to almost 50-fold, and was associated with more than 8-fold lower expression of IL-8 and IL-6. No DNA sequence variations were identified in the proximal 1300 bp promoter region of the TLR4 gene, and microarray analysis did not reveal a molecular explanation for the reduced TLR4 expression under either basal conditions or following exposure to LPS. It appears that the attenuated innate immune response of the LR array fibroblast culture to LPS may be caused by reduced TLR4 receptor expression. These results suggest that the primary dermal fibroblast cells can be used to examine underlying causes for between-cow variations in key immune response pathways.
Keywords: dairy cow, toll-like receptor pathway, interleukin-8
INTRODUCTION
Mastitis often leads to heavy loss of milk production, poor milk quality, damage to mammary tissue, and sometimes premature culling of affected cows. Enhancing host resistance to mastitis through selective breeding is being considered as a method to reduce mastitis incidence (Detilleux, 2009), and some progress is being made (Heringstad et al., 2007). However, several factors impose limitations on the speed of genetic gains that can be made from traditional breeding strategies based on analysis of large datasets containing pedigree information and phenotypic measurements (Berry et al., 2011). Among the limiting factors are difficulties in accurately defining the phenotype without a bacterial challenge, the poor recording of mastitis incidence, the inability to determine the phenotype in males or in pre-parturient females, and the low heritability of clinical mastitis (Berry et al., 2011, Detilleux, 2009). Marker-assisted selection (MAS) also has great potential in selecting cows with enhanced mastitis resistance but is still dependent on defining the appropriate disease resistant phenotype (Griesbeck-Zilch et al., 2009). The accuracy of MAS could potentially be improved if it were possible to locate functional polymorphisms in the actual genes impacting mastitis resistance.
Protective mammary gland defense and resolution of infection are dependent on the appropriate innate immune response that occurs immediately after bacterial penetration of the gland (Bannerman, 2009). Failure to induce an adequate innate immune response has been proposed to be the main factor for the development of persistent mammary gland infections (Gunther et al., 2011, Yang et al., 2008). Deficiency in innate immune response is implicated in delayed influx of neutrophils from blood into the mammary gland and impaired pathogen clearance. Burvenich et al. (2007) proposed that a one-hour delay in neutrophil transmigration during E. coli infection of the mammary gland would cause an eight-fold increase in bacterial number and a subsequent high level of endotoxin release. It seems apparent that rapid detection of pathogens and subsequent induction of a contained innate immune response is essential for effective defense and quick resolution of infection.
Pathogen recognition and induction of the innate immune response after an infection is a complex process. The innate immune response is mainly initiated through a class of pattern recognition receptors called toll-like receptors (TLR) (Medzhitov, 2007). Once receptors have been activated, downstream signaling occurs through myeloid differentiation primary response gene (88) (MyD88)-dependent and Toll/Interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathways with activation of associated kinases leading to translocation of nuclear factor-κB (NF-κB) transcription factors and activated interferon regulatory factor 3 (IRF3) into the nucleus (Boulanger et al., 2003, Kawai and Akira, 2010, Zhang and Ghosh, 2001). Subsequent expression and secretion of several innate immune response mediators play important roles in the host response by potentiating the recruitment and activation of neutrophils, the secretion of anti-bacterial factors, and the production of a febrile response in the animal (Bannerman, 2009).
Many previous studies have reported the existence of substantial between-animal variation in the severity of the response to intramammary challenge with E. coli (Burvenich et al., 2003, Kornalijnslijper et al., 2004, Van Werven et al., 1997). It is likely that a portion of this variation is due to environmental factors affecting the immunological status of the animals at the time of infection (Wenz et al., 2006) but also likely are genetic differences between cows affecting mastitis susceptibility. Genetic variation in innate immune signaling pathways can potentially be identified by using a relevant cell model under laboratory conditions. Dermal fibroblast cells have been used to locate and study key defects in pathogen detection and signaling pathways in humans (Davidson et al., 2006, Picard et al., 2003, von Bernuth et al., 2008). In one study, Von Bernuth et al. (2008) discovered MyD88 mutations in nine patients with invasive pyogenic bacterial disease that resulted in reduced production of IL-6 and IL-8 by the patient’s dermal fibroblasts. Using bovine dermal fibroblasts we have recently examined the relationship between an animal’s dermal fibroblast response to LPS in vitro and the animal’s in vivo response to either an intravenous injection of LPS (Green et al., 2011) or intramammary challenge with E. coli (Kandasamy et al., 2011). In fibroblasts obtained from 15 heifers at 5, 11, and 16 months of age, the relative ranking between animals in the magnitude of IL-8 produced by their cultured fibroblasts in response to LPS was stable (Green et al., 2011). After being challenged with intravenous LPS at 14 months of age, the four highest ranking animals developed higher plasma concentration of IL-8 and tumor necrosis factor-α (TNF-α) than the four lowest ranking heifers. Kandasamy et al. (2011) obtained fibroblast samples from 43 mid-lactation cows and found that there was considerable between-animal variation in the magnitude of the fibroblast production of IL-8 in response to LPS. Four of the high- and four of the low-ranked animals were then challenged with intramammary E. coli and while both groups cleared the resulting infection is a similar fashion, the high ranked animals had more BSA in milk, and a delayed resolution of inflammation as indicated by longer periods of elevated SCC and lower milk production. These studies suggest that dermal fibroblasts may be used as a model in comparative basal and LPS-activation state analysis to detect genetic differences between animals in their innate immune response potential. In the current study, we used microarray-based genomic analysis and dermal fibroblast cells of selected cows in an attempt to locate functional differences in components of the LPS detection and response pathways.
MATERIALS AND METHODS
Dermal fibroblast cell cultures
Primary dermal fibroblast cell cultures were selected from our collection of cultures that had been established and cryopreserved from 88 Holstein cows. The cells were derived by collagenase digestion of skin biopsies obtained from the shoulder region of lactating cows as previously described (Kandasamy et al., 2011). Cows from the University of Vermont herd and a local collaborator’s herd were selected for sampling based on their calving date without regard for milk production or previous disease history. All fibroblast cultures were screened for their ability to produce IL-8 in response to LPS (described below). Based on this initial screening assay we selected three cultures from the lowest responding 10% and three from the highest responding 10% for further analysis. Confirmation of the initially determined low and high response phenotype was done as follows. Fourth passage dermal fibroblast cells were seeded at a density of 2×105 cells/well in six-well culture plates in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Grand Island, NY) supplemented with 5% FBS, 1% insulin-transferrin-selenium supplement (Invitrogen), and penicillin/streptomycin/Fungizone (Thermo Scientific) and cultured for 48 h. Subsequently, cells in one well were treated with either LPS (100 ng/mL) (L4391, Sigma, Saint Louis, MO), recombinant bovine IL-1β (10ng/mL) (AbD Serotec, Raleigh, NC), or vehicle (endotoxin free PBS) alone as control. After 24 h, the conditioned media was centrifuged and supernatant stored at −20°C for IL-8 and IL-6 protein analysis using ELISA. This experiment was replicated three times.
IL-8 and IL-6 ELISA
The concentration of IL-8 in conditioned media was determined by sandwich ELISA as previously described (Kandasamy et al., 2011) using an anti-bovine IL-8 monoclonal antibody (gifted by Dr. Samuel K. Maheswaren, University of Minnesota) as the capture antibody, recombinant bovine IL-8 (Thermo Scientific) as standard, biotinylated anti-bovine IL-8 polyclonal antibody (BAF208, R&D Systems, Minneapolis, MN) as the detection antibody, and streptavidin-horseradish peroxidase (Sigma-Aldrich) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich) for detection. The bovine IL-6 assay was similarly performed using bovine IL-6 specific capture and detection antibodies (Thermo Scientific), and recombinant bovine IL-6 (Thermo Scientific) as standard. All samples were assayed in one assay/protein, and the minimum detectable concentration of both assays was approximately 100 pg/ml.
Gene Expression Analysis
One low responding (LRarray) and one high responding (HRarray) fibroblast culture were selected for genome-wide expression analysis using bovine genome arrays (Affymetrix, Santa Clara, CA). These two cultures were by recovered from cryopreservation, expanded, and seeded at 1 × 106 cells per 25 cm2 flask and incubated at 37° C for 24 h. The cells were then treated with LPS (100 ng/mL) or vehicle alone for 8 h after which the media was collected for protein analysis and the adherent cells were lysed using cell lysis buffer for isolation of total RNA (PerfectPure RNA Cultured Cell Kit, 5 PRIME, Gaithersburg, MD). On-column DNase digestion was performed to remove DNA during the RNA isolation procedure. Quality and quantity of the isolated RNA samples was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and a NanoDrop Spectrophotometer (Thermo Scientific), respectively. Subsequently, gene expression analysis was conducted at the University of Vermont’s core microarray facility. A total of 12 microarray slides were probed (2 fibroblast cultures × 2 treatments × 3 replicated experiments). Hybridization mixes were prepared and hybridized to the arrays as per manufacturer’s recommendations using the Affymetrix 450 fluidics station and scanned with the Affymetrix 3000-G7 scanner.
Microarray analysis
Probe statistics (CEL files) were calculated from scanned images using Affymetrix GCOS software. All other calculations were performed using R (Team, 2009) / Bioconductor (Gentleman, 2005, Gentleman et al., 2004) tools. Probe set and sample matrix expression statistics were calculated using the Robust Multichip Average (RMA) method of Speed and coworkers (Bolstad et al., 2003), implemented in the aroma.affymetrix package (Bengtsson et al., 2008). Quality statistics were calculated using the simpleaffy (Wilson and Miller, 2005) and affyQCReport (Parman et al., 2005) packages. Linear modeling was performed using the method developed by Smythe available in the Bioconductor limma package (Smyth, 2005). Contrasts examined included the main effects fibroblast culture (LR array minus HRarray) and treatment with LPS (LPS minus control), the effects of either fibroblast culture or treatment in the context of one of the other variables (LPS minus control for LRarray, LPS minus control for HRarray, LRarray control minus HRarray control, LRarray LPS minus HRarray LPS), and the interaction between the fibroblast cultures and the treatment. For all contrasts, differentially expressed genes that passed the FDR (P≤0.01) and P-value/fold-change (P≤0.01 and ≥1.5 fold change) thresholds were selected for further analysis. P-value (for each probe set) was calculated for each pair-wise comparison (for example, LPS minus control). Several genes are represented by redundant probe sets on the microarray chip. In such cases, the reference sequence of each probe set was subjected to BLAST analysis and then the probe set corresponding to the reference sequence with highest bit score was selected to represent the particular gene.
Validation of microarray results by quantitative RT-PCR (QPCR)
RNA samples were converted into complementary DNA using RevertAid kit (Promega, Madison, WI). Subsequently, QPCR was performed for IL-8, IL-6, TLR4, and β-actin genes using gene specific primers (Table 1). QPCR was performed using 1X PerfeCTa SYBR Green SuperMix, Low ROX (Quanta BioSciences, Gaithersburg, MD) and one μl of cDNA. The reactions were performed using 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA) and cycling conditions were, initial denaturation at 95° C for 2 minutes; then 40 cycles consisting of denaturation at 95° C for 15 seconds, annealing at 60° C for 30 seconds and extension at 72° C for 1 minute. Melt curve analysis was also performed to check amplification of the desired gene product. The β-actin gene was used as reference gene for normalization procedure. Threshold cycles (Ct) generated using real time PCR experiment for each sample were analyzed using the delta Ct method.
Table 1.
Details of primer sequences used for QPCR experiments.
| Gene symbol | Forward and reverse primer sequences (5′→3′) | Amplicon length (bp) |
Accession number |
Reference |
|---|---|---|---|---|
| Primers used for QPCR experiment | ||||
| ß-actin | 5′- GCAAATGCTTCTAGGCGGACT-3′ 5′- CAATCTCATCTCGTTTTCTGC G-3′ |
85 | BT030480 | Pareek et al. 2005 |
| IL-8 | 5′- GCTGGCTGTTGCTCTCTTG-3′ 5′- AGGTGTGGAATGTGTTTTTATGC-3′ |
118 | EU276073 | Pareek et al. 2005 |
| IL-6 | 5′-TGAGGGAAATCAGGAAAATGT -3′ 5′-CAGTGTTTGTGGCTGGAGTG -3′ |
110 | EU276071 | Pareek et al. 2005 |
| TLR4 | 5′- ACTGCAGCTTCAACCGTATC-3′ 5′- TAAAGGCTCTGCACACATCA -3′ |
190 | AB056444 | Ibeagha-Awemu et al. 2007 |
| CXCL12 | 5′- ATTTAGGCCATGGAGGCTCT -3′ 5′- GAAACTGTGCTGTGGCTTCA -3′ |
117 | NM_001113174 | - |
| SST | 5′- TGGAGCCTGAAGATTTGTCC -3′ 5′- GAAATTCTTGCAGCCAGCTT -3′ |
122 | NM_173960 | - |
| MX1 | 5′- ACAGATGCGTCAGTGGTCAG -3′ 5′- TGGAGAGAGTTCAGGGATGG -3′ |
133 | NM_173940 | - |
| IL1β | 5′- CAAGGAGAGGAAAGAGACA -3′ 5′- TGAGAAGTGCTGATGTACCA G-3′ |
209 | M37211 | Konnai et al. 2003 |
| Keratin 5 | 5′- CAAGGTCCTGGACACCAAGT-3′ 5′- TCCAGCTGTCTCCTGAGGTT-3′ |
114 | NM_001008663 | |
| TLR4 amplification and sequencing primers | ||||
| TLR4 promoter (Amplification) |
5′- CAAGATTTCACAAAATTGGACAGTGCC -3′ 5′- TACCTGTACGCAAGGGTCCCAA -3′ |
1870 | NC_000070 | - |
| TLR4 promoter (Sequencing) |
5′- TCCCTTGGACTGCAAGGAGATCCA -3′ 5′- AGGCACAGGGAGAAGACAGCCA -3′ |
- | NC_000070 | - |
| TLR4 exon 1 |
5′- CCTCTCACTTCCTCTCCTCCCGTAAC -3′ 5′- CCTCGGGCTGCCTGTTAATGC -3′ |
434 | NM_174198 | - |
| TLR4 exon 2 |
5′- GTGACTTATGATTGGATGGA -3′ 5′- GTAAGATGTCTGTTGAGTGA -3′ |
458 | NM_174198 | - |
| TLR4 exon 3 |
5′- ATATCTGTGTGGAGACCTAGATGACTGGG -3′ 5′- GAACACGCCCTGCATCCATCTG -3′ |
2414 | NM_174198 | - |
Microarray-based gene expression pattern was analyzed only in two fibroblast cultures. Presence of a similar gene expression pattern in other fibroblast cultures with similar phenotypes was analyzed as follows using QPCR. A new set of four low-responding and four-high responding cultures were selected from our bank of primary dermal fibroblast cultures. Fourth passage fibroblast cells were cultured as previously described and treated with either LPS (100 ng/mL) or vehicle alone as control. After 8 h, the adherent cells were lysed using cell lysis buffer for isolation of total RNA. Synthesis of cDNA and QPCR were performed as previously described. Five genes were selected for gene expression analysis using QPCR with the primer sequences presented in Table 1: IL-8, myxovirus (influenza virus) resistance 1 (MX1), chemokine (C-X-C motif) ligand 12 (CXCL12), TLR4, and somatostatin (SST).
TLR4 Sequence analysis
Genomic DNA was isolated from skin tissue or dermal fibroblast cells of the LRarray and HRarray cows using DNeasy Blood & Tissue Kit (Qiagen). The proximal promoter and 5′UTR regions of the TLR4 gene (−1301 to +565) were amplified and then sequenced using gene specific primers (Table 1). TLR4 exons were also amplified and then sequenced using respective gene specific primers (Table 1).
RESULTS
IL-8 response of selected low and high responding fibroblast cultures to LPS or IL-1β
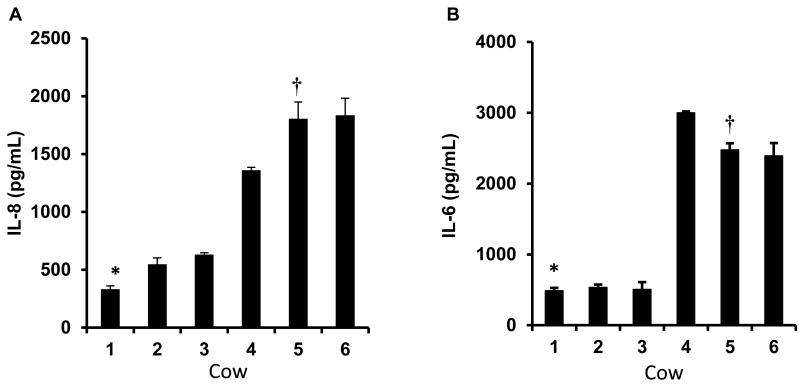
Among the pre-selected high and low response fibroblast cultures there was an expected difference in both IL-8 (Figure 1A) and IL-6 (Figure 1B) secretion in response to the LPS treatment. The low response fibroblast cultures produced approximately five-fold less (P<0.05) IL-8 protein compared to that of the high response cultures. A similar difference (P<0.05) between low and high response cultures was also observed in the IL-6 response to LPS.
Figure 1.
Dermal fibroblasts cells isolated from mid-lactating cows were exposed to LPS (100 ng/mL) for 24 h and the media concentrations of IL-8 (A) and IL-6 (B) were determined by ELISA. The data are from fibroblast cells of six separate cows, with each bar in the chart representing the mean ± S.E of triplicate aliquots of fibroblasts recovered separately from cryopreservation. Based on the IL-8 and IL-6 response to LPS, the lowest responding fibroblast culture (*LRarray) and one of the highest responding fibroblast cultures (†HRarray) were selected for microarray-based gene expression analysis.
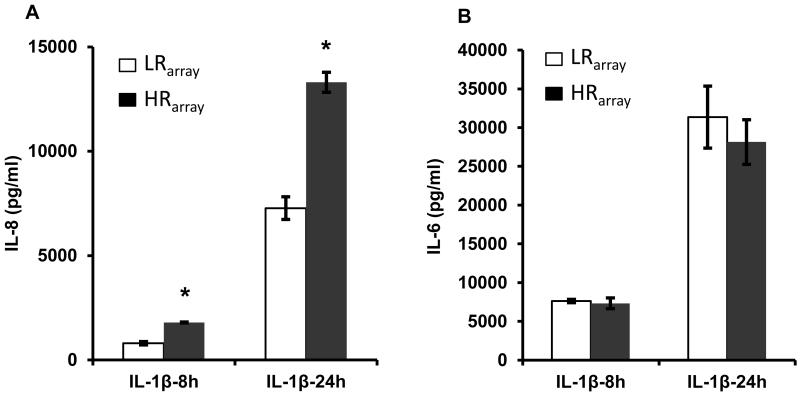
The fibroblast culture from cow #1 (LRarray) and cow #5 (HRarray) were selected for further analysis in an attempt to determine the cause of the differential IL-8 response to LPS. Stimulation with an alternate cytokine-inducing ligand, IL-1β, also revealed significant differences (P<0.05) in IL-8 secretion between the two cultures (Figure 2A). However, in contrast to the response to LPS, there was no significant between-culture difference in IL-1β-induced production of IL-6 (Figure 2B).
Figure 2.
The selected fibroblast cultures (see Figure 1) with the low responder (LRarray) and high responder (HRarray) phenotypes were exposed to IL-1β for 8h or 24h and the media concentrations of IL-8 (A) and IL-6 (B) were determined by ELISA. Each bar in the chart represents the mean ± S.E. of triplicate aliquots of fibroblasts recovered separately from cryopreservation. *P < 0.05 LR vs. HR.
Genomic analysis of dermal fibroblast response to LPS treatment
Genomic analysis of the two selected cultures in response to LPS was characterized by differential expression (fold change > 1.5, p< 0.01) of 395 transcripts in the LR array fibroblasts, 585 transcripts in HRarray fibroblasts, with changes in 321 transcripts being common to both cultures (Supplementary Tables 1 and 2). Surprisingly, LPS treatment did not induce any up-regulation of TNF-α or IL-1β transcripts in either LRarray or HRarray fibroblast cells, although IL-1α was induced in both. In addition to stimulation of pro-inflammatory genes, LPS treatment did cause an up-regulation of, the gene encoding the potent anti-inflammatory, IL-1 receptor antagonist (IL1RN).
Genes differentially expressed between LRarray and HRarray fibroblast cells under basal conditions
Differential gene expression (>1.5 fold, P<0.05) between LRarray and HRarray fibroblasts was determined during the basal state and after LPS exposure. During the basal state, 209 transcripts were up-regulated and 179 transcripts were down-regulated in LRarray fibroblasts relative to HRarray fibroblasts (Supplementary Table 3). In general, relatively few immune-related genes were differentially expressed between the LRarray and HRarray cultures under basal conditions (Table 3). Of genes represented on the array related to the TLR/IL-1β response pathway, one prominent difference between the LRarray and HRarray fibroblasts under control conditions was in the expression of TLR4. The expression of this gene was 1.9 fold less in the LRarray fibroblasts as compared to the HRarray fibroblasts (Table 3). The LRarray fibroblast cells also had significantly lower levels of CXCL10, CXCL11 and CCL19 expressions relative to HRarray fibroblast cells. Other notable genes that were expressed at a significantly lower level in LRarray relative to HRarray fibroblast under basal conditions were TLR3, MX1 and 2′-5′-oligoadenylate synthetase 2 (OAS2) (Table 3). The MX1 and OAS2 proteins are involved in anti-viral response and expression of these genes is regulated through the TRIF-dependent pathway.
Table 3.
Differential expression (fold change ≥ 1.5; P ≤ 0.01) innate immune-related genes between LRarray and HRarray fibroblast cultures maintained under control conditions. Data were obtained by microarray analysis and represent mean fold change of three replicated experiments.
| Probe ID | Gene symbol | Fold change |
|---|---|---|
| Genes down regulated in low responder relative to high responder | ||
| Bt.26655.1.S1_at | CCL19 | −1.6 |
| Bt.28393.1.S1_at | CD55 | −1.5 |
| Bt.16966.1.S1_at | CXCL10 | −3.1 |
| Bt.18368.1.S1_at | CXCL11 | −1.7 |
| Bt.4675.1.S1_a_at | MX1 | −2.1 |
| Bt.20891.1.S1_at | OAS2 | −2.2 |
| Bt.5054.3.S1_at | TGFBR3 | −1.5 |
| Bt.12298.1.S1_at | TLR3 | −1.6 |
| Bt.9030.1.S1_at | TLR4 | −1.9 |
| Bt.12694.1.S1_at | TNFRSF21 | −1.6 |
| Bt.8400.1.A1_at | TOX | −1.7 |
| Genes up- regulated in low responder relative to high responder | ||
| Bt.26983.1.S1_at | CASP1 | 1.7 |
| Bt.552.1.S1_at | CCL5 | 1.6 |
| Bt.5392.1.S1_at | CD36 | 2.0 |
| Bt.7425.1.S1_at | CXCL12 | 3.9 |
| Bt.610.1.A1_at | CXCL2 | 1.7 |
| Bt.26695.1.S1_at | ICOSLG | 1.5 |
| Bt.19889.1.A1_at | IGSF1 | 1.5 |
| Bt.15910.1.S1_at | IL1F6 | 5.1 |
| Bt.4199.1.S1_at | IL1RN | 1.7 |
| Bt.3862.1.S1_a_at | PPARG | 2.2 |
| Bt.456.1.S1_at | SST | 3.6 |
Several genes were up-regulated in LRarray fibroblasts relative to HRarray fibroblasts under basal conditions. The up-regulated immune-related genes included CXCL12, CCL5, SST, peroxisome proliferator-activated receptor gamma (PPARG), CD36, and IL1RN. Although the GeneChip used does not represent the entire bovine genome, it does contain appropriate probe sets to enable detection of many genes involved in the TLR4 signaling pathway, including TLR4, CD14, myeloid differentiation protein-2 (MD-2), MYD88, interleukin-1 receptor-associated kinase 4 (IRAK4), IRAK1, toll-like receptor adaptor molecule 2 (TICAM2), TNF receptor-associated factor 6 (TRAF6), nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (NFKBIA), v-rel reticuloendotheliosis viral oncogene homolog A (RELA), RELB, nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NFKB1), and NFKB2. Of these, only TLR4 was differentially expressed (down-regulated in LR) between LRarray and HRarray fibroblast cells during basal state.
Apart from the above gene list, a greater expression of several keratin genes transcripts was observed in HRarray fibroblast cells relative to LRarray fibroblast cells under both basal and LPS-stimulated states. This indicates that the HRarray fibroblast cells have some keratinocyte contamination. However, there was no visible morphological difference between the cultures and the characteristic spindle shape morphology of the cells combined with their intense staining for vimentin suggests that the vast majority of the cells were fibroblasts. Subsequently, we examined whether the keratinocyte contamination had any effect on either basal TLR4 mRNA expression or IL-8 response of our fibroblast cultures. To do this, a new set of eight-low and eight-high responding fibroblast cultures were selected and then keratinocyte levels of those fibroblast cultures was indirectly determined by quantifying keratin 5 mRNA by QPCR. Subsequent analysis revealed no significant association or correlation between keratinocyte level with either basal TLR4 mRNA expression (P>0.05, r=0.06) or IL-8 response phenotype (P>0.05, r=0.06) (data not shown).
Genes differentially expressed between LRarray and HRarray fibroblast cells after LPS treatment
Following LPS treatment, 448 genes were differentially expressed between LRarray and HRarray fibroblast cultures (Supplementary table 4). For many TLR regulated genes, such as IL-8, IL-6, SAA3, and CCL20, their expression following LPS treatment was several-fold less in the LRarray fibroblasts than in the HRarray fibroblasts (Table 4). As in the basal state, a lower expression of TLR4 following LPS treatment was observed in LRarray fibroblasts compared HRarray fibroblasts.
Immune related genes that were more expressed in the LRarray fibroblasts than the HRarray fibroblasts following LPS treatment included CXCL12, IL1F6, IL1RN, PRARG, and SST. All of these genes were also up-regulated in LRarray fibroblasts under basal conditions.
Validation of microarray results by quantitative RT-PCR
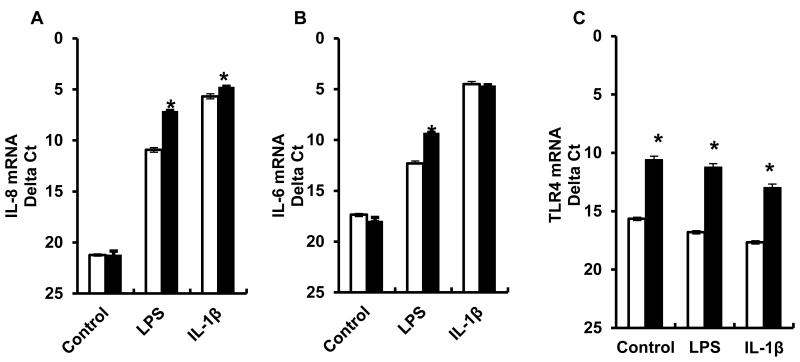
QPCR analysis of the same RNA samples used for the microarray confirmed that basal expression of TLR4 was reduced in the LRarray fibroblasts compared to the HRarray fibroblasts (Figure 3C). However, the more sensitive QPCR analysis revealed that the magnitude of the differential TLR4 expression (33 fold) was much greater than that found by microarray analysis (1.8 fold). This difference in TLR4 expression between the cultures further increased to 47 fold following LPS stimulation. Expression of IL-8 was very low (Δct > 36) in control conditions in both cultures. After LPS treatment there was a marked induction of IL-8 in both cultures with expression in the HRarray being 14 fold greater than that of the LRarray. Similar results were obtained for IL-6 expression with an 8 fold between-culture differential expression following LPS treatment.
Figure 3.
Expression of IL-8 (A), IL-6 (B) and TLR4 (C) mRNA in the low responding (LRarray) and the high responding (HRarray) fibroblast cultures that were exposed to either media alone, LPS (100 ng/mL), or IL-1β (10 ng/mL) for 8 h. Results are from three replicates of cryopreserved cells/cow thawed on separate days (Mean ± S.E.). *P < 0.05 LR vs. HR.
The LRarray and HRarray fibroblast cultures were also treated with IL-1β (10 ng/mL) for eight hours. QPCR analysis revealed a significant difference (2 fold) in IL-8 mRNA between IL-1β treated LRarray and HRarray fibroblast cells (Figure 3A). There was no difference in IL-6 expression between the IL-1β-stimulated cultures (Fig 3B), while treatment with IL-1β had little effect on the differential expression of TLR4 (Figure 3C) observed in control conditions between the LRarray and HRarray fibroblasts.
The expression pattern of selected genes (IL-8, MX1, CXCL12, TLR4, SST) in a new set of four-low and four-high responding fibroblast cultures from our collection was also determined (Table 5). QPCR analysis revealed that expression of IL-8 and MX1 were significantly affected by the LPS treatment and by the low vs. high grouping. In both instances a significant interaction was found that was likely due to the group effects being much more apparent in the LPS-stimulated state. The LPS treatment also caused a significant increase in CXCL12 expression in both LR and HR groups. Expression of TLR4 and SST genes was not different between the LR and HR groups, and was not affected by LPS treatment. Of note is that the expression of TLR4 in this group of eight fibroblast cultures was similar to that of the HRarray fibroblast culture and much greater than that of the LRarray fibroblasts (Figure 3C).
Table 5.
Messenger RNA expression profile of IL-8, MX1, CXCL12, TLR4, and SST genes in an additional set of four low responding (LR) and four high responding (HR) fibroblast cultures. RNA was collected from cells that were exposed to either media alone or LPS (100 ng/mL) for 8 h. Gene expression changes are expressed as delta Ct (Mean ± S.E.).
| Gene | Control |
LPS |
P Value |
||||
|---|---|---|---|---|---|---|---|
| LR | HR | LR | HR | Treatment (LPS) | Group | Interaction | |
| IL-8 | 20.7 ± 0.5 | 20.9 ± 0.3 | 9.3 ± 0.2 | 6.2 ± 0.1 | 0.0001 | 0.001 | 0.0003 |
| MX1 | 19.6 ± 0.9 | 19.0 ± 1.2 | 13.1 ± 0.3 | 9.2 ± 0.1 | 0.0001 | 0.014 | 0.048 |
| CXCL12 | 13.1 ± 0.9 | 14 ± 0.8 | 11.9 ± 0.4 | 12.1 ± 0.5 | 0.046 | 0.484 | 0.647 |
| TLR4 | 12.0 ± 0.5 | 11.6 ± 0.1 | 11.3 ± 0.2 | 11.1 ± 0.5 | 0.118 | 0.412 | 0.916 |
| SST | 3.8 ± 0.6 | 5.2 ± 0.5 | 4.7 ± 0.8 | 5.5 ± 0.5 | 0.345 | 0.078 | 0.624 |
Partial DNA sequencing of the TLR4 gene
No sequence differences were found in the proximal promoter or coding regions of the TLR4 gene between the LRarray and HRarray fibroblasts.
DISCUSSION
Selection of cows with enhanced disease resistance is an evolving strategy to control economically important diseases including mastitis (Heringstad et al., 2007). However, direct selection for disease resistance is difficult because of confounding environmental effects that limit the ability to assign an accurate phenotype. Some progress is being made with marker-assisted selection (MAS) (Kuhn et al., 2008, Rupp and Boichard, 2003), and improvements in this strategy may result from locating polymorphisms in key innate immune response genes to further improve the accuracy of selection. Many studies have reported associations of specific polymorphisms in such genes with the incidence of clinical mastitis or average SCC (Galvão et al., 2011, Sharma et al., 2006, Sugimoto et al., 2006).
The low heritability of mastitis related traits indicate the major role that environmental influences have on mastitis incidence and this negatively affects the ability to detect a mastitis resistant phenotype. A more accurate assignment of a mastitis resistant phenotype resulting from well-controlled challenge studies would aid in the search for causative genetic polymorphisms. However, the ability to contain environmental influences during bacterial challenge studies with lactating cows is limited by factors such as the animal’s disease history and immunological status at the time of challenge, and the availability of a housing facility that would enable large numbers of repeatable challenges. Another approach to facilitate the detection of functional genetic differences between animals is to perform in vitro challenge studies under well-defined conditions on a model cell type. In our previous studies, we have observed substantial between-cow variation in their fibroblast response to LPS, using production of IL-8 as a marker of the innate immune response, and found that this variation was related to between-cow variation in the response to an experimental mastitis challenge (Kandasamy et al., 2011) or intravenous infusion of LPS (Green et al., 2011). In a related strategy, mammary epithelial cells have been used to investigate molecular causes for differences between cows selected on the basis of a QTL affecting SCS on BTA 18 (Griesbeck-Zilch et al., 2009). In this strategy, epithelial cells were obtained from mammary tissue from six animals in early lactation that had inherited a paternal chromosome region associated with decreasing SCS (MAS-Q) and compared to cells obtained from five animals that had inherited the chromosomal region associated with increasing SCS (MAS-q). Following in vitro stimulation for 6 h or 24 h with heat-inactivated E. coli or S. aureus, a markedly higher expression of innate immune response genes, such as IL-8, IL1β, and TNF-α was observed in the cells from the MAS-Q animals. Thus, enhanced production of innate response genes is the presumptive cause for the effects of the QTL on SCS, but the mechanisms leading to the differential expression remain to be defined. In the current study we employed genome-wide microarray analysis in an attempt to locate a cause for the differential ability of selected low and high responding fibroblast cultures to produce IL-8 in response to LPS.
A major goal of the current study was to analyze the potential cause of the attenuated response of fibroblasts from a particular cow (LRarray) to LPS. The fibroblasts from this cow consistently showed the lowest production of IL-8 and IL-6 in response to LPS stimulation out of our 88-cow collection. The coordinate attenuation of IL-8 and IL-6 production suggests reduced signal transduction via the TLR4 pathway. This lowered responsiveness was further examined using IL-1β as the stimulatory ligand in an attempt to narrow the molecular possibilities for the attenuated response to LPS. While IL-1β induces its response through the IL-1 receptor, and LPS acts through the TLR4 receptor, both receptors contain a TLR/Interleukin-1 receptor domain and signal through the MyD88-dependent signaling pathway (Arend et al., 2008). However, IL-1β does not require the TLR4 accessory proteins (CD14, lipopolysaccharide binding protein (LBP), MD-2), and does not appear to signal through the TLR4-TRIF dependent pathway (von Bernuth et al., 2008). We observed that, as with LPS stimulation, the LRarray fibroblasts produced less IL-8 than the HRarray fibroblasts when challenged with IL-1β. However, in contrast to LPS, the LRarray and HRarray fibroblasts produced similar amounts of IL-6 in response to IL-1β. The similar IL-1β-induced production of IL-6 between the cultures is not easily explained given the disparate IL-1β-induced production of IL-8.
Results of the microarray analysis revealed a significant reduction in TLR4 gene expression in LRarray fibroblasts compared to that of HRarray fibroblasts in both the basal state and after LPS treatment. The more sensitive QPCR assay indicated that the TLR4 expression in the LRarray fibroblasts was in the order of 40-fold less than either the HRarray fibroblast culture or the other eight fibroblast cultures examined in this study. It is quite possible that this reduction TLR4 expression contributed to the attenuated response of the LRarray fibroblasts LPS. However, the reason for the decreased TLR4 receptor expression is yet to be determined. The proximal promoter region of the TLR4 gene has several transcription factor binding sites including AP-1, c-Ets-1, AP-4, ADR1, MZF1, Oct-1, and CRE-binding protein (Sharma et al., 2006). Any changes in these sites may cause altered DNA binding capacity of the transcription factors and then lead to altered TLR4 expression. However, DNA sequence analysis of the proximal 1301 bp of promoter region of TLR4 gene revealed no differences between the LRarray and HRarray genes. More extensive sequencing may have been informative as it has been reported that human TLR4 expression is affected by combinations of eight SNPs contained between bp -4038 and -1607 relative to the translation start site (Ragnarsdóttir et al., 2010). Unfortunately, the genomic analysis of differential gene expression did not reveal obvious candidates that could explain the differing expression of the TLR4 gene between the LRarray and HRarray fibroblasts.
In contrast to a general reduction in chemokine expression profiles, a higher level of CXCL12 expression was observed in LRarray fibroblasts in comparison to HRarray fibroblasts in the presence or absence of LPS. CXCL12 is constitutively expressed in human dermal fibroblasts (Avniel et al., 2006) and gingival fibroblasts (Hosokawa et al., 2005) and it is involved in B-cell lymphopoiesis (Nagasawa et al., 1996) and homing of mononuclear cells (Bleul et al., 1996). The underlying cause as well as the functional consequences of the differential CXCL12 expression between LRarray and HRarray fibroblast cultures remains to be determined. Similar to CXCL12, basal expression of SST was also significantly higher in LRarray fibroblasts in comparison to HRarray fibroblasts. Expression of SST in B- and T-lymphocytes was also reported (Lichtenauer-Kaligis et al., 2004), but its functional significance in immune function is not known.
A second goal of the current study was to evaluate the robustness of the response of LPS-stimulated dermal fibroblasts using a microarray-based approach. Numerous immune related genes were induced following the 8 h exposure to LPS suggesting the usefulness of these cells to evaluate innate immune response pathways. Furthermore, the expression profile of the fibroblast response to 8 h LPS treatment was highly reflective of that of primary cultures of bovine mammary epithelial cells exposed to heat-killed E. coli for 6 h and examined with the same microarray chip (Gunther et al., 2011). Some data from Gunther et al. (2011) is included in Table 2 for illustrative purposes. Of note is the finding that all genes up-regulated by more than five-fold in LPS-treated HRarray fibroblasts were also up-regulated by more than five-fold in the E. coli-stimulated epithelial cells. Fibroblast microarray analysis did not detect induction of either TNF-α or IL-1β after 8 h of LPS treatment. However, it is quite possible that transient induction of these key mediators of inflammation had occurred prior to our observation time point. More-acute, transient up-regulation of TNF-α and IL-1β was observed after 1 h and 3 h of E. coli treatment of mammary epithelial cells (Gunther et al., 2011).
Table 2.
Differential expression (fold change ≥ 1.5; P ≤ 0.01) of selected immune-related genes between control and LPS-stimulated (100 ng/ml; 8 h) fibroblast cultures. Data were obtained by microarray analysis and represent mean fold change (control vs. LPS) from triplicate replicates of low responding (LRarray) or a high responding (HRarray) cultures (Indicated in Fig 1). Corresponding differential gene expression by bovine mammary epithelial cells following 6 h exposure to heat killed E. coli from Gunther et al. 2011.
| Fold change | ||||
|---|---|---|---|---|
|
|
||||
| Probe ID | Gene symbol | LR fibroblast culture | HR fibroblast culture | Mammary epithelial cell response to 6 h heat-killed E. coli Data from Gunther et al. 2011a |
| Genes that are up-regulated in response to LPS | ||||
| Bt.2408.1.S1_s_at | CCL2 | 10.1 | 13.9 | 7 |
| Bt.9560.1.S1_at | CCL20 | 10.3 | 47.7 | 137 |
| Bt.552.1.S1_at | CCL5 | 28.9 | 43.8 | 175 |
| Bt.13130.1.S1_at | CD40 | 3.9 | 7.8 | 10 |
| Bt.5494.1.S1_at | CD44 | 1.6 | 1.7 | - |
| Bt.15742.1.S1_at | CD47 | 1.5 | 1.6 | - |
| Bt.3841.1.S1_at | CD83 | 3.1 | 4.3 | - |
| Bt.20259.1.S1_at | CDC42EP2 | 1.7 | 2.3 | - |
| Bt.13542.1.S1_at | CFB | 21.0 | 79.4 | 10 |
| Bt.1275.1.S1_at | CX3CL1 | 5.7 | 14.4 | 7 |
| Bt.16966.1.S1_at | CXCL10 | 5.0 | 1.8 | - |
| Bt.610.1.A1_at | CXCL2 | 87.4 | 175.3 | 144 |
| Bt.7165.1.S1_at | CXCL6 | 5.1 | 7.3 | 18 |
| Bt.22021.1.S1_at | IFI16 | 2.5 | 2.4 | 8 |
| Bt.20785.2.S1_at | IFI44 | 3.5 | 6.0 | 20 |
| Bt.24098.1.A1_at | IFIH1 | 15.0 | 14.5 | 11 |
| Bt.234.1.S1_at | IL18 | 1.5 | 1.5 | - |
| Bt.29883.1.A1_at | IL18R1 | 1.9 | 2.0 | - |
| Bt.191.1.S2_at | IL1A | 1.5 | 3.9 | 5 |
| Bt.15910.1.S1_at | IL1F6 | 4.7 | 8.2 | - |
| Bt.4199.1.S1_at | IL1RN | 2.6 | 2.4 | - |
| Bt.3686.1.S1_at | IL6 | 12.1 | 35.0 | 13 |
| Bt.155.1.S1_at | IL8 | 64.4 | 223.4 | 103 |
| Bt.10077.1.S3_at | IRF1 | 2.0 | 3.1 | 3 |
| Bt.5768.1.S1_at | IRF7 | 1.6 | 2.4 | 4 |
| Bt.12304.1.S1_at | ISG15 | 36.7 | 33.0 | 28 |
| Bt.22275.1.A1_at | ISG20 | 2.0 | 3.0 | 7 |
| Bt.4675.1.S1_a_at | MX1 | 10.6 | 11.2 | 20 |
| Bt.20288.1.S1_at | NFKB2 | 1.5 | 2.1 | 3 |
| Bt.9027.1.S1_at | NFKBIA | 7.8 | 11.3 | 11 |
| Bt.8227.1.S1_at | NFKBIZ | 1.8 | 2.9 | 8 |
| Bt.20891.1.S1_at | OAS2 | 19.8 | 17.7 | 18 |
| Bt.10398.1.S1_at | PTX3 | 6.3 | 21.7 | - |
| Bt.24467.1.S1_at | RSAD2 | 15.9 | 13.1 | 57 |
| Bt.278.1.S1_at | SAA3 | 6.3 | 29.2 | 299 |
| Bt.27681.1.A1_at | TICAM2 | 1.6 | 2.0 | - |
| Bt.7043.2.S1_a_at | VCAM1 | 8.4 | 12.4 | - |
| Genes that are down-regulated in response to LPS | ||||
| Bt.13934.1.S1_at | CD3G | −2.1 | −2.9 | - |
| Bt.22699.1.S1_at | NFIA | −1.7 | −1.9 | - |
| Bt.21763.3.S1_at | NFIB | −1.5 | −1.9 | - |
not significantly regulated.
Thus, even though fibroblasts are not considered as sentinel cells in the response to mammary infection they do seem to model the acute infection process to Gram-negative bacteria. The fibroblasts may also model response to Gram-positive bacteria as they produce similar quantities of IL-8 in response to LPS or the synthetic diacylated lipopeptide, Pam2CSK4, that activates TLR2-dependent signaling in an analogous fashion to gram-positive bacteria (Buwitt-Beckmann et al., 2005, Farhat et al., 2010, Kandasamy et al., 2011).
CONCLUSIONS
Dermal fibroblasts have a robust innate immune response to LPS. An approximately five-fold difference between two fibroblast cultures in the production of IL-8 in response to LPS may have been caused by a greater than 30-fold difference in the expression of TLR4. Future studies will explore this possibility through a complementation approach with transfection of a functional TLR4 expression plasmid.
Supplementary Material
Table 4.
Differential expression (fold change ≥ 1.5; P ≤ 0.01) of innate immune-related genes between LRarray and HRarray fibroblast cultures following exposure to LPS (100 ng/ml; 8 h). Data were obtained by microarray analysis and represent mean fold change of three replicated experiments.
| Probe ID | Gene symbol | Fold change |
|---|---|---|
| Genes down-regulated in low responder relative to high responder | ||
| Bt.2408.1.S1_s_at | CCL2 | −1.5 |
| Bt.24326.1.S1_at | CCL11 | −2.0 |
| Bt.26655.1.S1_at | CCL19 | −1.6 |
| Bt.9560.1.S1_at | CCL20 | −4.2 |
| Bt.13130.1.S1_at | CD40 | −1.8 |
| Bt.50.1.S1_at | CD8A | −1.7 |
| Bt.18368.1.S1_at | CXCL11 | −1.5 |
| Bt.7671.1.S1_at | IFITM3 | −1.5 |
| Bt.5508.1.S1_at | IFNAR2 | −1.7 |
| Bt.3686.1.S1_at | IL6 | −2.5 |
| Bt.155.1.S1_at | IL8 | −3.6 |
| Bt.4675.1.S1_a_at | MX1 | −2.2 |
| Bt.20891.1.S1_at | OAS2 | −2.0 |
| Bt.12148.1.S1_at | PPARGC1A | −1.6 |
| Bt.357.1.S1_at | S100A12 | −1.5 |
| Bt.5970.1.S1_a_at | S100A2 | −2.0 |
| Bt.278.1.S1_at | SAA3 | −5.3 |
| Bt.8945.1.S1_at | TLR2 | −1.9 |
| Bt.12298.1.S1_at | TLR3 | −1.5 |
| Bt.9030.1.S1_at | TLR4 | −2.2 |
| Bt.26969.1.A1_at | TNFAIP8L3 | −1.5 |
| Bt.12694.1.S1_at | TNFRSF21 | −2.3 |
| Genes up-regulated in low responder relative to high responder | ||
| Bt.5392.1.S1_at | CXCL12 | 1.9 |
| Bt.7425.1.S1_at | IL1F6 | 3.0 |
| Bt.15910.1.S1_at | IL1RN | 2.9 |
| Bt.4199.1.S1_at | PPARG | 1.9 |
| Bt.456.1.S1_at | SST | 4.3 |
ACKNOWLEDGEMENTS
This research was supported by a grant from the USDA National Institute of Food and Agriculture (Award #2010-65119-20495). The University of Vermont’s core microarray facility was supported by the Vermont Genetics Network through Grant Number P20 RR16462 from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This work was supported by the Molecular Bioinformatics Shared Resource of the College of Medicine of the University of Vermont.
REFERENCES
- Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- Avniel S, Arik Z, Maly A, Sagie A, Basst HB, Yahana MD, Weiss ID, Pal B, Wald O, Ad-El D, Fujii N, Arenzana-Seisdedos F, Jung S, Galun E, Gur E, Peled A. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J. Invest. Dermatol. 2006;126:468–476. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- Bannerman DD. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 2009;87:10–25. doi: 10.2527/jas.2008-1187. [DOI] [PubMed] [Google Scholar]
- Bengtsson H, Simpson K, Bullard J, Hansen K. aroma.affymetrix: A generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory. Department of Statistics, University of California; Berkeley: 2008. Tech Report #745. [Google Scholar]
- Berry DP, Bermingham ML, Good M, More SJ. Genetics of animal health and disease in cattle. Ir Vet J. 2011;64:5. doi: 10.1186/2046-0481-64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J. Exp. Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Boulanger D, Bureau F, Melotte D, Mainil J, Lekeux P. Increased Nuclear Factor [kappa] b Activity in Milk Cells of Mastitis-Affected Cows. J. Dairy Sci. 2003;86:1259–1267. doi: 10.3168/jds.S0022-0302(03)73710-2. [DOI] [PubMed] [Google Scholar]
- Burvenich C, Bannerman DD, Lippolis JD, Peelman L, Nonnecke BJ, Kehrli ME, Jr., Paape MJ. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 2007;90(Suppl 1):E39–54. doi: 10.3168/jds.2006-696. [DOI] [PubMed] [Google Scholar]
- Burvenich C, Merris V. r. V., Mehrzad J, Diez-Fraile A, Duchateau L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003;34:521–564. doi: 10.1051/vetres:2003023. [DOI] [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 2005;35:282–289. doi: 10.1002/eji.200424955. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Currie AJ, Bowdish DM, Brown KL, Rosenberger CM, Ma RC, Bylund J, Campsall PA, Puel A, Picard C, Casanova JL, Turvey SE, Hancock RE, Devon RS, Speert DP. IRAK-4 mutation (Q293X): rapid detection and characterization of defective post-transcriptional TLR/IL-1R responses in human myeloid and non-myeloid cells. J. Immunol. 2006;177:8202–8211. doi: 10.4049/jimmunol.177.11.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detilleux JC. Genetic factors affecting susceptibility to udder pathogens. Vet. Microbiol. 2009;134:157–164. doi: 10.1016/j.vetmic.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Farhat K, Riekenberg S, Jung G, Wiesmüller K-H, Jungi TW, Ulmer AJ. Identification of full length bovine TLR1 and functional characterization of lipopeptide recognition by bovine TLR2/1 heterodimer. Vet. Res. 2010;41:34. doi: 10.1051/vetres/2010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão KN, Pighetti GM, Cheong SH, Nydam DV, Gilbert RO. Association between interleukin-8 receptor-α (CXCR1) polymorphism and disease incidence, production, reproduction, and survival in Holstein cows. J. Dairy Sci. 2011;94:2083–2091. doi: 10.3168/jds.2010-3636. [DOI] [PubMed] [Google Scholar]
- Gentleman R. Bioinformatics and computational biology solutions using R and Bioconductor. Springer Verlag. 2005 [Google Scholar]
- Gentleman R, Carey V, Bates D, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J. Bioconductor: open software development for computational biology and bioinformatics Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Kandasamy S, Elsasser TH, Kerr DE. The use of dermal fibroblasts as a predictive tool of the toll-like receptor 4 response pathway and its development in Holstein heifers. J. Dairy Sci. 2011;94:5502–5514. doi: 10.3168/jds.2011-4441. [DOI] [PubMed] [Google Scholar]
- Griesbeck-Zilch B, Osman M, Kuhn C, Schwerin M, Bruckmaier RH, Pfaffl MW, Hammerle-Fickinger A, Meyer HHD, Wellnitz O. Analysis of key molecules of the innate immune system in mammary epithelial cells isolated from marker-assisted and conventionally selected cattle. J. Dairy Sci. 2009;92:4621–4633. doi: 10.3168/jds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Gunther J, Esch K, Poschadel N, Petzl W, Zerbe H, Mitterhuemer S, Blum H, Seyfert HM. Comparative kinetics of Escherichia coli- and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infect. Immun. 2011;79:695–707. doi: 10.1128/IAI.01071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringstad B, Klemetsdal G, Steine T. Selection responses for disease resistance in two selection experiments with Norwegian red cows. J. Dairy Sci. 2007;90:2419–2426. doi: 10.3168/jds.2006-805. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Murakami K, Miyake Y, Matsuo T. CXCL12 and CXCR4 expression by human gingival fibroblasts in periodontal disease. Clin. Exp. Immunol. 2005;141:467–474. doi: 10.1111/j.1365-2249.2005.02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy S, Green BB, Benjamin AL, Kerr DE. Between-cow variation in dermal fibroblast response to lipopolysaccharide reflected in resolution of inflammation during Escherichia coli mastitis. J. Dairy Sci. 2011;94:5963–5975. doi: 10.3168/jds.2011-4288. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Konnai S, Usui T, Ohashi K, Onuma M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Vet. Microbiol. 2003;94:283–294. doi: 10.1016/s0378-1135(03)00119-6. [DOI] [PubMed] [Google Scholar]
- Kornalijnslijper JE, Daemen AJ, van Werven T, Niewold TA, Rutten VP, Noordhuizen-Stassen EN. Bacterial growth during the early phase of infection determines the severity of experimental Escherichia coli mastitis in dairy cows. Vet. Microbiol. 2004;101:177–186. doi: 10.1016/j.vetmic.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Reinhardt F, Schwerin M. Marker assisted selection of heifers improved milk somatic cell count compared to selection on conventional pedigree breeding values. Arch Tierz. 2008;51:23–32. [Google Scholar]
- Lichtenauer-Kaligis EG, Dalm VA, Oomen SP, Mooij DM, van Hagen PM, Lamberts SW, Hofland LJ. Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur. J. Endocrinol. 2004;150:565–577. doi: 10.1530/eje.0.1500565. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Parman C, Halling C, Gentleman R. R package version 1. 2005. affyQCReport: QC Report Generation for affyBatch objects. [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al-Ghonaium A, Al-Rayes H, Al-Jumaah S, Al-Hajjar S, Al-Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- Ragnarsdóttir B, Jönsson K, Urbano A, Grönberg-Hernandez J, Lutay N, Tammi M, Gustafsson M, Lundstedt A-C, Leijonhufvud I, Karpman D, Wullt B, Truedsson L, Jodal U, Andersson B, Svanborg C. Toll-Like Receptor 4 Promoter Polymorphisms: Common <italic>TLR4</italic> Variants May Protect against Severe Urinary Tract Infection. PLoS ONE. 2010;5:e10734. doi: 10.1371/journal.pone.0010734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R, Boichard D. Genetics of resistance to mastitis in dairy cattle. Vet. Res. 2003;34:671–688. doi: 10.1051/vetres:2003020. [DOI] [PubMed] [Google Scholar]
- Sharma BS, Leyva I, Schenkel F, Karrow NA. Association of Toll-Like Receptor 4 Polymorphisms with Somatic Cell Score and Lactation Persistency in Holstein Bulls. J. Dairy Sci. 2006;89:3626–3635. doi: 10.3168/jds.S0022-0302(06)72402-X. [DOI] [PubMed] [Google Scholar]
- Smyth G. Limma: linear models for microarray data. Bioinformatics and computational biology solutions using R and Bioconductor. 2005:397–420. [Google Scholar]
- Sugimoto M, Fujikawa A, Womack JE, Sugimoto Y. Evidence that bovine forebrain embryonic zinc finger-like gene influences immune response associated with mastitis resistance. Proceedings of the National Academy of Sciences. 2006;103:6454–6459. doi: 10.1073/pnas.0601015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A Language and Environment for Statistical Computing. 2009 Available from: http://www.R-project.org.
- Van Werven T, Noordhuizen-Stassen EN, Daemen AJ, Schukken YH, Brand A, Burvenich C. Preinfection in vitro chemotaxis, phagocytosis, oxidative burst, and expression of CD11/CD18 receptors and their predictive capacity on the outcome of mastitis induced in dairy cows with Escherichia coli. J. Dairy Sci. 1997;80:67–74. doi: 10.3168/jds.s0022-0302(97)75913-7. [DOI] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yague J, Anton J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Marodi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz JR, Barrington GM, Garry FB, Ellis RP, Magnuson RJ. Escherichia coli isolates’ serotypes, genotypes, and virulence genes and clinical coliform mastitis severity. J. Dairy Sci. 2006;89:3408–3412. doi: 10.3168/jds.S0022-0302(06)72377-3. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Miller CJ. Simpleaffy: a BioConductor package for Affymetrix Quality Control and data analysis. Bioinformatics. 2005;21:3683. doi: 10.1093/bioinformatics/bti605. [DOI] [PubMed] [Google Scholar]
- Yang W, Zerbe H, Petzl W, Brunner RM, Gunther J, Draing C, von Aulock S, Schuberth HJ, Seyfert HM. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-kappaB in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008;45:1385–1397. doi: 10.1016/j.molimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Toll-like receptor-mediated NF-B activation: a phylogenetically conserved paradigm in innate immunity. J. Clin. Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.