ABSTRACT
Candida albicans is the most prevalent fungal pathogen of humans, causing a variety of diseases ranging from superficial mucosal infections to deep-seated systemic invasions. Mucus, the gel that coats all wet epithelial surfaces, accommodates C. albicans as part of the normal microbiota, where C. albicans resides asymptomatically in healthy humans. Through a series of in vitro experiments combined with gene expression analysis, we show that mucin biopolymers, the main gel-forming constituents of mucus, induce a new oval-shaped morphology in C. albicans in which a range of genes related to adhesion, filamentation, and biofilm formation are downregulated. We also show that corresponding traits are suppressed, rendering C. albicans impaired in forming biofilms on a range of different synthetic surfaces and human epithelial cells. Our data suggest that mucins can manipulate C. albicans physiology, and we hypothesize that they are key environmental signals for retaining C. albicans in the host-compatible, commensal state.
IMPORTANCE
The yeast Candida albicans causes both superficial infections of the mucosa and life-threatening infections upon entering the bloodstream. However, C. albicans is not always harmful and can exist as part of the normal microbiota without causing disease. Internal body surfaces that are susceptible to infection by C. albicans are coated with mucus, which we hypothesize plays an important role in preventing infections. Here, we show that the main components of mucus, mucin glycoproteins, suppress virulence attributes of C. albicans at the levels of gene expression and the corresponding morphological traits. Specifically, mucins suppress attachment to plastic surfaces and human cells, the transition to cell-penetrating hyphae, and the formation of biofilms (drug-resistant microbial communities). Additionally, exposure to mucins induces an elongated morphology that physically resembles the mating-competent opaque state but is phenotypically distinct. We suggest that mucins are potent antivirulence molecules that have therapeutic potential for suppressing C. albicans infections.
INTRODUCTION
Candida albicans is an important opportunistic fungal pathogen in humans that can cause superficial infections, such as vaginitis in women or thrush in babies and HIV patients, and systemic, often fatal, disease in more advanced cases (1). C. albicans possesses a range of virulence traits, including adherence, filamentation, and secretion of proteases (2). At the heart of many infections is the formation of surface-associated C. albicans communities, also termed biofilms, which can form on mucosal epithelial surfaces and on implanted medical devices, such as catheters and heart valves. Biofilms show increased resistance both to the immune system and to antifungal treatment (3). Despite the ability of C. albicans to cause disease, the healthy human body accommodates it as part of the microbiota (4–6). How the body tolerates the continued presence of potentially virulent C. albicans is largely unknown.
Mucus is the slimy coating found on all wet epithelia in the human body, including the eyes, airways, and the gastrointestinal and female genitourinary tracts, and many host-microbe interactions take place in this context. Its major gel-forming components, the mucin glycopolymers, are emerging as important regulators of microbial virulence. For example, the human cell-surface mucin MUC1 can inhibit surface adhesion of the gastric bacterium Helicobacter pylori (7). Moreover, the secreted human mucin Muc5AC can prevent Pseudomonas aeruginosa surface attachment and biofilm formation by promoting a dispersive state of bacteria (8). Other examples include mucus-mediated clearance of the bacterium Streptococcus pneumoniae (9) and modulation of HIV-1 (10) and influenza virus (11) infectivity by mucins. These observations indicate that mucin biopolymers help prevent bacterial and viral infections by regulating cellular processes related to virulence. Fungal pathogens are only distantly related to bacteria and viruses, and little is known about their interactions with mucins.
Here, we investigate the role of mucins as potential regulators of C. albicans virulence. Using a combination of gene expression analysis, mucus-secreting cell lines, and defined in vitro assays with natively purified mucins, we show that exposure to mucins induces a new oval-shaped morphological state in C. albicans in which various virulence traits are downregulated, including surface adhesion, the morphological transition to the filamentous state, and biofilm formation. Our results indicate that mucins are key contributors to host defense against C. albicans and may offer new strategies to target fungal virulence, such as the design of antifungal treatments or coatings for implants.
RESULTS
Mucins regulate C. albicans physiology.
To determine the effects of mucins on C. albicans, the strain SC5314 was cultured in RPMI medium with and without pig gastric mucins (Muc5AC); RPMI favors growth in the filamentous state. Mucins were supplemented in the medium to create a 3-dimensional environment, as is found in the native mucus barrier (8). In the absence of mucins, the cells formed extensive hyphae which clumped together into flocs (Fig. 1A). In contrast, mucin-exposed cells predominantly formed short chains resembling pseudohyphae (Fig. 1A) or unicellular, ellipsoidal cells that are distinct from the round, yeast form cells. An analysis of the growth rate of C. albicans in mucins shows that cells continue to grow over time and even show an enhanced increase in optical density (OD) due to the homogenous suspension of individual cells as opposed to the filamentous flocs formed in RPMI medium alone (see Fig. S1 in the supplemental material).
FIG 1 .
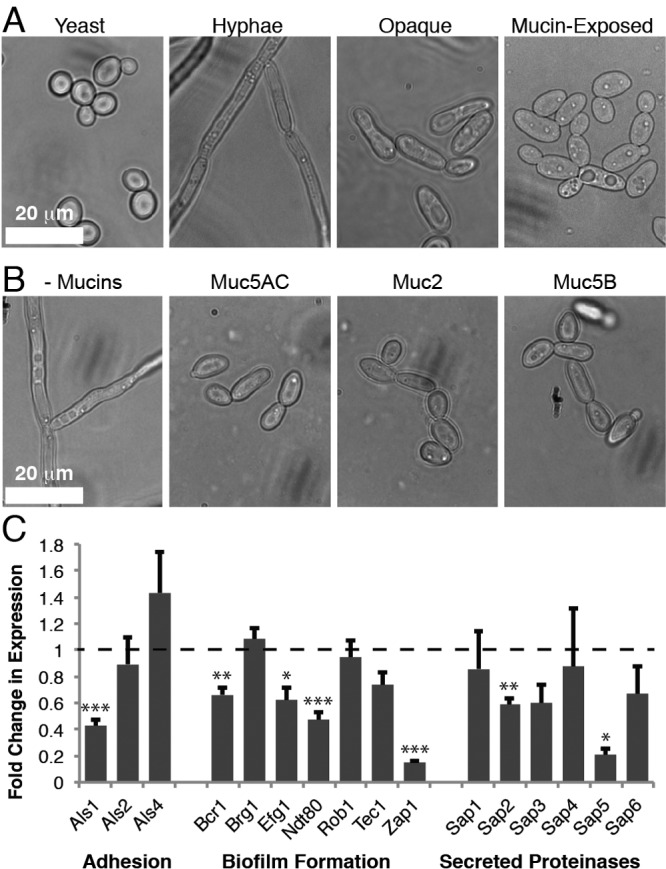
Mucins induce a unique morphological state characterized by suppressed virulence traits. (A) Phase-contrast images of C. albicans in different morphological states. (B) Phase-contrast images of C. albicans after growth in the presence or absence (- Mucins) of one of the following mucins: pig gastric (Muc5AC), pig intestinal (Muc2), or human salivary (Muc5B). (C) Results of qPCR of known C. albicans virulence genes, comparing gene expression levels in RPMI with mucins to their expression levels without mucins. RNA was extracted from 6 independent biological replicates. Error bars represent standard errors of the means (SEM). Asterisks represent different P value thresholds as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Importantly, the effect of Muc5AC on C. albicans physiology is not limited to this type of mucin but was also observed for two other mucins: Muc2 from pig intestinal mucus and Muc5B from human saliva (Fig. 1B). This indicates that mucins have a general effect on C. albicans that likely extends across all mucosal surfaces. Due to the abundant availability of Muc5AC, the remainder of the experiments in this study were performed using this mucin.
At first sight, the mucin-induced morphology resembles opaque cells, which are the mating competent form of C. albicans (12) that have reduced virulence in systemic infection models (13). Since the ability of C. albicans to switch to the opaque state is controlled by genes at the mating type-like (MTL) locus, such that only MTLa or MTLα strains can undergo the transition to the opaque form, we first tested whether mucins would induce our starting MTLa/α heterozygous strains to become homozygous MTLa or MTLα mating-competent cells. Using PCR with primers specific to the MTL locus, we found that mucin-exposed cells remained heterozygous at the MTL locus (n = 100). To further probe their identity, the mucin-exposed cells were assayed for three features that are indicative of opaque cells: the abilities to form opaque colonies on agar plates (14), mate in the presence of the opposite mating type (15, 16), and form mating protrusions in the presence of mating pheromone (17). Our data show that the mucin-exposed cells were incapable of these three traits: they did not form opaque colonies, were not mating competent, and did not form mating protrusions in the presence of mating α-pheromone (see Table S1 in the supplemental material). Moreover, exposure of wor1Δ/Δ cells (which cannot form opaque cells) to mucins also induced the oval-shaped morphology (Fig. S2), suggesting that the mucin-dependent morphology can develop independently of the master regulator of opaque status. As one further control, we repeated the white-opaque switching, quantitative mating, and pheromone response assays using mating competent MTLa or MTLα cells and found that mucins do not affect these opaque processes in mating competent strains (Table S1). Taken together, these results indicate that exposure to mucins suppresses the formation of hyphae while inducing the formation of a novel phenotype that superficially resembles the opaque cell type but is distinct in its physiological responses.
Mucins downregulate virulence-associated genes in C. albicans.
To better characterize the mucin-induced morphological change, we carried out transcriptional profiling experiments. A wild-type C. albicans strain (SC5314) was grown for 8 h in RPMI at 37°C in the presence and absence of 0.5% mucins. We performed quantitative PCR (qPCR) (Fig. 1C) to measure the expression levels of selected virulence genes (listed in Table S2 in the supplemental material), including those associated with adhesion (18), biofilm formation (19), and secreted proteinases (20). TAF145, a general transcription factor TFIID subunit (21), did not change expression in the presence of mucins and was used as a reference for calibration. The results of this experiment show that 7 of the 16 genes tested (Fig. 1C, P values indicated by asterisks) were downregulated by more than 1.5-fold in the presence of mucins (P < 0.05) as determined by a two-tailed, unpaired t test comparing the cycle threshold (ΔCT) values of samples with and without mucins.
Mucins suppress C. albicans transition to the filamentous state.
To understand in more detail the effect of mucins on C. albicans physiology, we monitored the C. albicans strain HGFP3, which expresses green fluorescent protein (GFP) when the hyphal-specific gene HWP1 is transcribed, during growth in RPMI with or without 0.5% natively purified mucins. To test whether the effects are simply a response to an increased viscosity or osmotic stress, we also subjected the cells to 0.5% industrially purified mucins (Sigma-Aldrich), 0.5% methylcellulose, or 1 M sorbitol. Industrially purified mucins are proteolytically processed and, as a result, have lost the gel-forming capacity characteristic of native mucins (22, 23). The polysaccharide methylcellulose is commonly used to mimic the viscosity of a mucus environment (24, 25). Sorbitol at high concentrations can induce osmotic stress; testing this condition is informative because the osmotic stress pathway is linked to hyphal formation (26). Our data show that in the no-polymer control, as well as in the presence of methylcellulose or 1 M sorbitol, the majority of cells formed hyphae, as indicated both by the elongated structures and the presence of GFP fluorescence (Fig. 2A, left; see also Fig. S3A in the supplemental material). In contrast, in the presence of native mucins, hyphal formation was nearly completely suppressed. Industrial mucins also inhibited hyphal formation, although not as effectively as the native mucins; a proportion of cells remained in the hyphal form. These results suggest that specific biochemical attributes present in the mucins suppress hyphal formation. Natively purified mucins are obtained by a relatively mild purification procedure to preserve their structure, and it is formally possible that contaminants contribute to the regulation of the C. albicans phenotype. To exclude this possibility, we also tested mucins purified with CsCl, a more stringent condition which removes the majority of associated proteins and lipids from the mucins. We observed the same suppression of hyphal formation as in the presence of natively purified mucins (Fig. S4), indicating that this effect is due to the mucins and not to any associated factors.
FIG 2 .
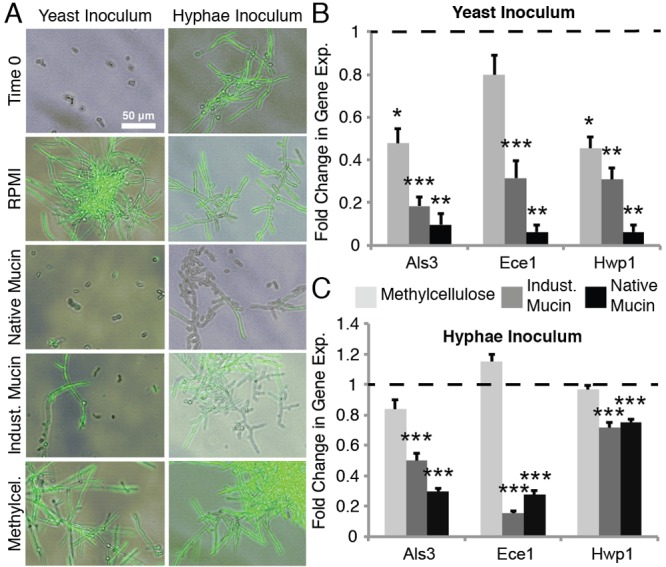
Mucins suppress hyphal growth from both yeast and hyphal cells. Fluorescence microscopy images overlaid on phase-contrast images (A) and quantitative PCR of the expression of hyphal-specific genes from yeast (B) or hyphae (C) after incubation for 8 h in RPMI alone or with 0.5% methylcellulose, 0.5% native mucins, or 0.5% industrially purified mucins. The strain used, HGFP3, expresses GFP only when cells are true hyphae. RNA was extracted from 6 independent biological replicates. Error bars represent SEM.
The ability of mucins to suppress filamentation was also studied at the level of gene expression. Using qPCR, we analyzed the expression levels of the hypha-specific genes ALS3, ECE-1, and HWP1 under four medium conditions: medium without polymers and with native mucins, industrial mucins, and methylcellulose. For all three genes, native mucins mediated the strongest downregulation (Fig. 2B). Of note is that this mucin-induced downregulation of hypha-specific genes was also observed in an alternative culture medium, yeast extract-peptone-dextrose (YPD) plus fetal bovine serum (FBS) (see Fig. S5 in the supplemental material), indicating that the effects of mucins are not dependent on a specific medium condition.
We next tested the effects of mucins on preformed hyphae. Hyphae formed in the absence of mucins were inoculated into RPMI with and without 0.5% mucins or 0.5% methylcellulose. Our data show that newly formed cells, which bud off from the hyphae, were predominantly in the yeast form after exposure to mucins (Fig. 2A, right). In comparison, hyphae in the absence of mucins continued to produce hyphae. Hyphal cells inoculated into medium containing methylcellulose also continued to produce more hyphal cells. These results were verified by qPCR, which showed that native mucins have the strongest capacity to suppress hyphal formation (Fig. 2C). We note that the levels of expression of the gene HWP1 showed a lower degree of suppression (yet still significant; P < 0.001) when hyphae were added as the starting culture than when yeast cells were added (−1.3-fold change in expression with hyphal inoculum versus −15.9-fold change for yeast). Thus, in addition to inhibiting the growth of hyphae in yeast form cells, we can also conclude that the hypha-suppressive capacity of mucins is strong enough to downregulate the responsible pathway in existing hyphae.
Mucins suppress surface adhesion of C. albicans
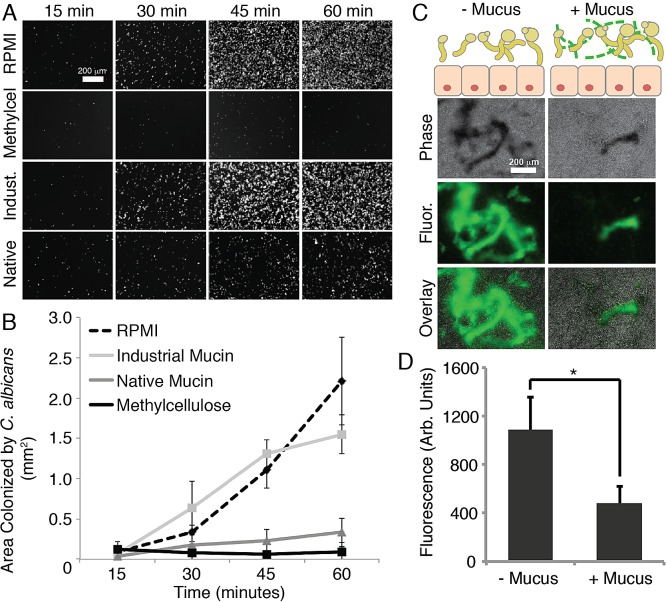
A critical, early step of infection by C. albicans is its attachment to a solid surface. From the results shown in Fig. 1C, we observed that genes involved in cell adhesion (ALS1 and ALS3) were downregulated in the presence of mucins. Moreover, we know that mucins may physically trap certain particles and cells, thereby preventing their association with an underlying surface. Hence, we tested whether medium containing gel-forming mucins also decreases the surface attachment of C. albicans. We analyzed the ability of C. albicans to colonize two different surfaces in the presence of mucins: abiotic polystyrene, which is often used in the context of biofilm formation assays, and human epithelial mucus-secreting cells. For adhesion to polystyrene, a suspension of yeast cells was inoculated into polystyrene 96-well plates containing RPMI without or with 0.5% native mucins, industrially purified mucins, or methylcellulose. The phase-contrast microscopy images and quantification results in Fig. 3A and B show that native mucins significantly reduced cell attachment to polystyrene. This effect was detectable as early as 30 min and became stronger over the course of the hour (unpaired two-tailed t test; P < 0.05). Methylcellulose was similarly effective in suppressing cell surface attachment (Fig. 3A). For comparison, industrially purified mucins provided no significant protection from surface attachment. Native mucins and methylcellulose both increase the viscosity of the medium, and hence, this parameter could be responsible for the observed antiadhesion effect. To test this, we subjected the yeast to medium containing 0.5% polyethylene glycol (PEG), which is often used as an antifouling coating (27). Our data show that PEG was not able to decrease surface attachment to the same degree as mucins and methylcellulose (see Fig. S3B in the supplemental material), suggesting that increased viscosity alone is not sufficient to protect a surface from C. albicans attachment.
FIG 3 .
Mucins reduce attachment of C. albicans to polystyrene and mucus-secreting human cells. (A) Fluorescence microscopy images of 96-well polystyrene plates after incubation with C. albicans under different medium conditions (RPMI alone or with 0.5% methylcellulose, 0.5% industrially purified mucin, or 0.5% native mucin). Images were obtained every 15 min, after removal of nonadherent cells. (B) Quantification of attachment to the polystyrene plates. Error bars represent standard deviations of the results from 3 replicates. (C) Diagram illustrating the experimental setup of the epithelial infection assay, and fluorescence and phase-contrast microscopy images of C. albicans SC5314 stained with calcofluor white after 2 h of incubation with HT29-MTX human mucus-secreting cells. (D) Quantification of C. albicans attachment to HT29-MTX cells. Error bars represent standard deviations of the results from 3 replicates.
We next infected human mucus-secreting cells with C. albicans to determine the effects of mucus on attachment to a living surface. C. albicans typically resides in the intestine as a commensal, but this environment can also be the starting point for systemic dissemination (28). A simple in vitro model for the study of C. albicans interactions with mucus-coated epithelial cells of the intestines makes use of HT29-MTX cells derived from human colorectal adenocarcinoma cells, which secrete native-like mucus when growing in culture (29). The mucus layer can be removed from the secreting cells with N-acetylcysteine (NAC), allowing for a comparison of cell adhesion with and without native mucus. To determine the effects of mucus on the attachment of C. albicans to human mucus-secreting epithelial cells, a suspension of C. albicans was exposed to cells that were lined with an intact mucus layer or to cells from which the mucus had been removed. After 2 h, the unbound C. albicans cells were removed by washing the epithelial cells with phosphate-buffered saline. The microscopy images and data in Fig. 3C and D show that significantly more C. albicans cells attached to the unprotected epithelial cells than to cells that had been shielded with a layer of mucus. This suggests that the mucus lining of epithelial cells provides an efficient protection against the attachment of C. albicans.
Mucins decrease C. albicans biofilm formation.
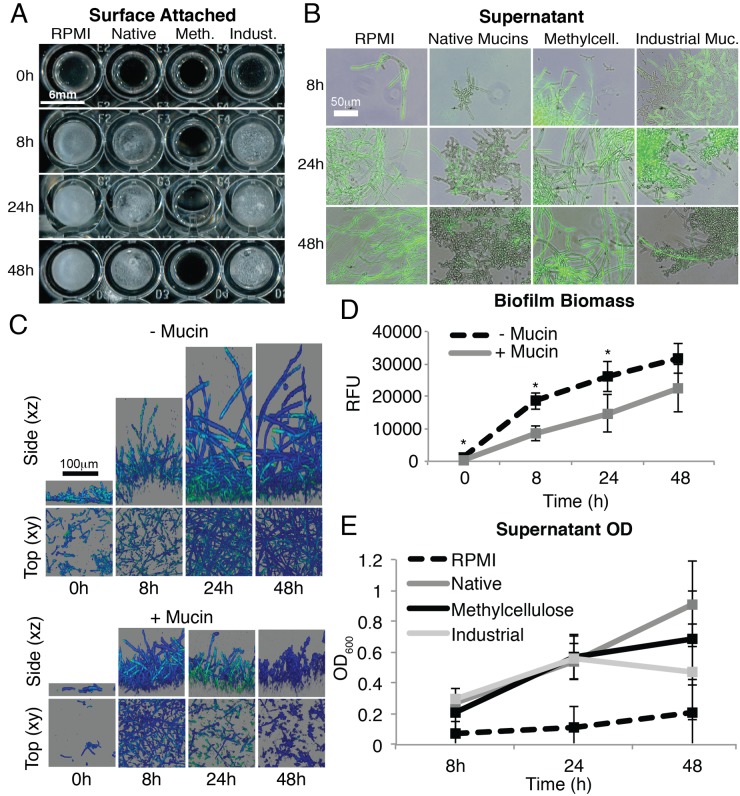
C. albicans readily forms surface-attached biofilms on both abiotic and biotic surfaces. Because biofilm formation relies on attachment, filamentation, and cell-cell interactions (30, 31), we tested whether mucins inhibit biofilm formation by C. albicans. We used a standard biofilm assay in which a polystyrene surface is immersed in a yeast culture (in RPMI) to allow attachment of the cells and, after 90 min, washed to remove nonadherent cells. Then, the surface is submerged in fresh, cell-free medium to follow the development of the initially attached cells into a biofilm over 8, 24, and 48 h. We performed this assay in the presence of native mucins and, for comparison, with methylcellulose and industrial mucins.
To evaluate the effect of the individual polymers on C. albicans surface attachment, we removed the supernatant after the time points indicated above (8, 24, and 48 h) and analyzed the resulting biofilms (Fig. 4). Surface attachment was visibly reduced in the presence of native mucins and even more effectively prevented in the presence of methylcellulose (Fig. 4A). Confocal xy and xz sections of the biofilms show that both the surface coverage (xy images) and thickness (xz scan) of the biofilm are reduced in the presence of mucins (Fig. 4C). Moreover, a smaller percentage of cells in the mucin-treated biofilms form hyphae within the thin biofilms, confirming the previous observation from the experiment whose results are shown in Fig. 2. Finally, quantification of the biofilm biomass shows a significant reduction in the presence of mucins (Fig. 4D). These data suggest that both mucins and methylcellulose have the capacity to suppress, or destabilize, the attachment of C. albicans to an underlying polystyrene surface over a relatively long period of time. In an experiment whose results are shown in Fig. S1 in the supplemental material, we showed that the cells continued to grow strongly in the presence of both mucins and methylcellulose, indicating that the reduced number of attached cells is not due to toxicity of the polymers.
FIG 4 .
Mucins reduce biofilm formation of C. albicans. Biofilms were grown using strain HGFP3 that produces GFP upon transcription from a hyphal-specific promoter. (A) Macroscopic view of biofilms grown in the presence or absence of mucins or methylcellulose. (B) Fluorescence microscopy images overlaid on phase-contrast images of C. albicans found in biofilm supernatants. (C) Confocal images of biofilms grown in the presence or absence of mucins. (D and E) Quantification of biofilm biomass (D) and of C. albicans found in biofilm supernatants (E). RFU, relative fluorescence units. Error bars show SD. P values indicated by asterisks are <0.05.
If cell proliferation is not suppressed by the polymers and, yet, a reduction of biomass is observed on the surface, one would expect that a significant proportion of the population has been shifted to the supernatant. This could occur if cells are released from the biofilm and continue to propagate in the planktonic phase. Indeed, the results in Fig. 4E show that the supernatant from biofilms in the presence of mucins or methylcellulose contained higher numbers of cells than the supernatants from biofilms growing without mucins. This experiment also reveals another important point: in the presence of mucins, the nonattached cells are largely devoid of filaments and, therefore, presumably are in the less invasive form (Fig. 4B). In contrast, the cells in the supernatant of methylcellulose cultures were all highly filamentous. Together, these data suggest that both mucins and methylcellulose can suppress the formation and maturation of biofilms by reducing attachment of the cells to an underlying surface, possibly by preventing stable maintenance of cells within the biofilm. However, mucins have a distinct effect: they render nearly the entire population devoid of filaments.
DISCUSSION
This work shows that the mucin Muc5AC, which is expressed in the stomach and in the lungs, can induce the downregulation of several virulence traits in C. albicans, both at the level of gene expression and phenotype. These include the suppression of both filamentous growth and the formation of surface-attached biofilms. Studies with other types of mucins (Fig. 1B) (32, 33) suggest that the ability of mucins to manage microbial virulence may be a general mechanism that is present on all mucosal surfaces as part of the innate mucosal immune system.
How might mucins prevent the transition to the hyphal form? Mucins, but not the other tested polymers, were capable of blocking this transition, suggesting that specific, mucin-associated glycans might be involved in this process. Consistent with this idea, glucose, maltose, and galactose in solution can all influence the formation of hyphae in C. albicans (34). The identity of the mucin glycan moieties that are recognized by C. albicans, as well as the receptors and pathways in the yeast that are affected by these sugars, are currently unknown; their identification may suggest new valuable strategies for preventing or recovering C. albicans infections of mucosal surfaces.
Preventing biofilm formation on materials exposed to living organisms presents a vexing engineering challenge; the results obtained for mucus hydrogels may provide some interesting new strategies. Our experiments show that mucins and methylcellulose are both effective in suppressing C. albicans surface attachment. Both polymers appear to reduce the ability for initial surface attachment; moreover, they render newly formed cells less capable of stably integrating into an emerging biofilm. How these polymers work to suppress surface attachment and even whether they function by the same mechanisms are open questions. Despite their superficial resemblance in surface protection, mucins and methylcellulose have different effects on the surrounding C. albicans cell population. With methylcellulose, the vast majority of cells remain in the filamentous form, while a cell population exposed to mucins remains largely devoid of filaments. There is good experimental evidence that the ability to form filaments is required for C. albicans virulence (35). Therefore, we have shown that the native mucins in the body are capable of providing a dual mechanism for virulence control, suppressing both the yeast-hyphal transition and surface attachment.
The ability of mucins to suppress virulence traits is not specific for C. albicans but appears to apply also to a range of other microorganisms, including the bacteria Helicobacter pylori and Pseudomonas aeruginosa (7, 8) and, also, certain viruses, such as HIV and influenza virus (10, 11). Understanding the mechanism of the mucin-Candida host-pathogen interaction could direct treatment strategies for regulating the healthy microbiota and also shed light on the molecular origins of increased susceptibility to microbial disease.
MATERIALS AND METHODS
C. albicans strains and media.
Strains were maintained on YPD agar (2% Bacto peptone, 2% glucose, 1% yeast extract, 2% agar) and grown at 30°C. Single colonies were inoculated into YPD broth and grown with shaking overnight at 30°C prior to each experiment.
The experiments were performed using either Gibco RPMI 1640 medium (catalog number 31800-089; Life Technologies) buffered with 165 mM morpholinepropanesulfonic acid (MOPS) and supplemented with 0.2% NaHCO3 and 2% glucose or YPD medium with 10% fetal bovine serum (FBS). Medium with 0.5% methylcellulose (15 centipoise [cP]; Sigma) was prepared from a 5% stock solution by dilution in RPMI. Type II mucin from porcine stomach (Sigma) was dialyzed in a Spectra/Por Float-A-Lyzer G2 dialysis tube with a 100-kDa molecular mass cutoff, followed by lyophilization. Industrial PGM and native PGM were dissolved in RPMI and gently vortexed at 4°C overnight.
The C. albicans yeast strains used in this study were SC5314 and HGFP3. Strain HGFP3 (36) was constructed by inserting the GFP gene next to the promoter of HWP1, a gene encoding a hyphal cell wall protein, and was provided by E. Mylonakis (Massachusetts General Hospital, Boston, MA) with the permission of P. Sundstrom.
Mucin purification.
The mucins were natively purified to preserve their properties, as opposed to industrially purified mucins which do not form gels in solution (23, 22). Porcine gastric mucins (PGM) were purified from fresh pig stomachs as previously described (37). Briefly, the mucus layer was isolated from pig stomachs and solubilized in sodium chloride buffer containing protease inhibitors to prevent mucin degradation and sodium azide to prevent bacterial proliferation. Insoluble components were removed via centrifugation, and the mucins were isolated using gel filtration chromatography on a Sepharose column (CL2B). The mucins were then concentrated and lyophilized. As a control to ensure that there were no contaminants in the mucin preparation, mucins prepared by CsCl gradient centrifugation (as described in references 38 and 39) were compared to those prepared without this step.
Extraction of RNA and cDNA synthesis.
One milliliter of RPMI or 0.5% PGM in RPMI in a culture tube was inoculated with 10 µl of an overnight culture of strain SC5314 and incubated at 37°C and 180 rpm for 8 h. RNA was extracted using the Epicentre MasterPure yeast RNA purification kit and treated with Sigma-Aldrich AMPD1 amplification-grade DNase I. Five hundred nanograms of RNA per sample was used to generate cDNA using the Invitrogen SuperScript III first-strand synthesis system. cDNA samples were stored at −80°C until use.
Quantitative PCR.
All primers were obtained from Sigma and analyzed for efficiency before use in experiments. Efficiency was calculated by performing qPCR with serial 1:10 dilutions of genomic DNA. Bio-Rad iQ SYBR green supermix was used for qPCRs. Experiments were performed in a Roche LightCycler 480 II machine with the following run protocol: 95°C for 3 min and then 40 cycles of 95°C for 10 s, 58°C for 30 s, and 72°C for 30 s. Cycle threshold (CT) values were obtained and used for analysis. Fold changes were calculated using the ΔΔCT method in comparison to the results for the reference gene TAF145.
Filamentation assay.
One hundred microliters each of RPMI, 0.5% methylcellulose medium, 0.5% industrial PGM medium, and 0.5% native PGM medium were inoculated with the strain HGFP3 as yeast form cells or hyphae in a 96-well plate. One microliter of an overnight culture was used as the source for yeast form cells. For hyphae, an overnight culture was diluted 1:100 into YPD plus 10% FBS, which stimulates the transition to hyphae, and grown to an optical density at 600nm (OD600) of 0.5. The hyphae were centrifuged and then resuspended to an OD600 of 5, and 10-µl amounts were added to media under the aforementioned conditions. The cells were incubated at 37°C with shaking at 180 rpm for 8 h. Adherent cells were scraped off of the surface, and samples were pipetted vigorously to break up aggregates. A 15-µl amount of each sample was placed on a microscope slide for visualization. Slides were imaged with a Zeiss Observer Z1 inverted fluorescence microscope with a Zeiss Plan-Apochromat 20× objective lens using phase-contrast and fluorescein isothiocyanate (FITC) (excitation at 475 nm and emission at 530 nm).
Polystyrene attachment assay.
Polystyrene 96-well plates were inoculated with 100 µl of RPMI, 0.5% methylcellulose medium, 0.5% industrial PGM medium, and 0.5% native PGM medium containing yeast form cells from the strain SC5314. The plates were incubated statically at 37°C. Every 15 min, a time point sample was taken by washing the wells with 200 µl of phosphate-buffered saline (PBS) twice, followed by the addition of 100 µl of PBS. After 1 h, 1 µl of 1 mg/ml calcofluor white solution was added to each well. The samples were imaged similarly to the description above using a 10× objective (excitation at 365 nm and emission at 445 nm). The experiment was performed in triplicate with 5 pictures taken of each well. The images were analyzed in ImageJ as follows: each image was converted to 8-bit and the contrast was enhanced (0.4% saturated pixels). The image was then thresholded to create a binary image. The image was then analyzed using the Analyze Particles tool to measure the surface area covered by cells. The surface area measurements of the 15 images for each condition and time point were averaged.
Attachment to human mucus-secreting colorectal cells (HT29-MTX).
HT29-MTX mucus-secreting cells reliably secrete a thick, homogeneous layer of mucus as soon as 7 days postconfluence. The cells were grown in a 24-well plate. At 2 weeks postconfluence, the cells were treated with 10 mM N-acetylcysteine, which cleaves disulfide bonds between mucins (40), for 30 min to remove the adherent mucus layer or with PBS as a control. For infection, C. albicans strain SC5314 was diluted from an overnight culture into Dulbecco modified Eagle medium to an OD600 of 0.5. Five hundred microliters of C. albicans was added on top of the HT29-MTX cells and incubated statically at 37°C for 2 h. After 2 h, the medium was removed from the wells, which were subsequently washed twice with 500 µl of PBS. The remaining C. albicans cells were stained with calcofluor white and analyzed using a Zeiss Observer Z1 inverted fluorescence microscope and a plate reader (excitation at 355 nm and emission at 460 nm).
The HT29-MTX cells were derived from HT-29 cells (ATCC HTB-38) as described previously (29). HT-29 cells were derived from an anonymous donor.
Biofilm formation assay and visualization.
Biofilms were grown on either of two surfaces: in a 96-well plate (for macroscopic views and quantification) or on 8-mm-diameter silicone circles (for confocal imaging). Before the experiment, the silicone circles were washed with water and autoclaved. The surfaces were incubated in adult bovine serum overnight with shaking at 37°C. The next day, the surfaces were washed in PBS and submerged in a C. albicans cell suspension with an OD600 of 0.5 in RPMI with or without 0.5% mucins. The samples were incubated at 37°C with shaking at 180 rpm for 90 min to facilitate attachment of yeast cells to the surface. Nonadherent cells were washed away with PBS, and the samples were subsequently submerged in fresh RPMI with or without 0.5% mucins, 0.5% industrial mucins, or 0.5% methylcellulose. The biofilms were allowed to grow with shaking (180 rpm) at 37°C for the times indicated in Fig. 4. For biomass quantification, the biofilms were stained with 20 µg/ml calcofluor white in PBS for 10 min and analyzed in a SpectraMax M3 plate reader (excitation at 355 nm and emission at 460 nm). Biofilm supernatant quantification was performed in the same plate reader (absorbance at 600 nm). For confocal imaging, the biofilms were submerged in 6 ml of 20 µg/ml calcofluor white and stained for 10 min. The biofilms were imaged using a photo scanner or a Zeiss LSM 700 upright confocal microscope. Planktonic cells were placed on microscope slides and imaged using a Zeiss wide-field fluorescence microscope.
SUPPLEMENTAL MATERIAL
Supplementary methods. Download
(A) Growth curve of C. albicans with or without mucins in YPD at 30°C. In this case, the growth rates were roughly the same. (B) Growth curve of C. albicans with or without mucins in RPMI at 30°C. Here, the cells grown in mucins increased in optical density faster than those grown without mucins due to decreased hyphal formation and flocking of cells in mucins. Download
The mucin-induced morphological state is distinct from the opaque state. Phase-contrast images compare the mucin-induced morphology of the wild type (WT) to that of the wor1Δ/Δ strain, which is locked in the white phase. Download
Effects of osmotic stress and viscosity on virulence traits. (A) Fluorescence micrographs overlaid onto phase-contrast images of C. albicans grown in RPMI without or with 0.5% mucin, 0.5% methylcellulose, or 1 M sorbitol (an osmotic stress inducer). (B) Quantification of attachment to the polystyrene plates in the presence of RPMI without or with 0.5% PEG (a viscous polymer), 0.5% methylcellulose, or 0.5% native mucins. The colonized area of each frame was measured. The experiment was performed in triplicate with 5 pictures of each well taken at each time point. Error bars represent standard deviations. Download
Native and CsCl-purified mucins suppress hyphal formation of C. albicans. Fluorescence microscopy images are overlaid on phase-contrast images of C. albicans HGFP3 after incubation for 8 h in RPMI alone or with 0.5% native mucins or 0.5% CsCl-purified mucins. Download
Mucins suppress hyphal formation in YPD plus FBS. A filamentation assay was performed as previously described for RPMI in YPD plus FBS, a different hypha-inducing medium. Download
Analysis of opaque state attributes upon exposure to mucins. C. albicans cells that were either homozygous or heterozygous at the mating locus were assayed for their ability to switch from white to opaque, respond to alpha factor, and mate after exposure to mucins.
List of virulence genes analyzed using qPCR.
ACKNOWLEDGMENTS
We thank Paula Sundstrum and Eleftherios Mylonakis for strain HGFP3, Suzanne Noble for the wor1Δ/Δ strain SN1064, Richard Bennett for strains RBY717a/a, RBY731a/a, RBY1177, and RBY1180, and Thécla Lesuffleur for the HT29-MTX cell line. We also thank Bradley Turner for performing CsCl gradient centrifugation. We are grateful to Gerry Fink and Dawn Thompson for helpful comments and advice.
This work was supported by CEHS pilot project grant number P30-ES002109 (K.R. and N.L.K.), the MIT/NIGMS Biotechnology Training Program, grant number 5T32GM008334-24 (N.L.K.), Burroughs Wellcome Fund 2012 Collaborative Research travel grant (C.J.N.), National Institutes of Health grant K99AI100896 (C.J.N.), and National Institutes of Health grant R01 AI083311 (A.D.J.).
Footnotes
Citation Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K. 2014. Mucins suppress virulence traits of Candida albicans. mBio 5(6):e01911-14. doi:10.1128/mBio.01911-14.
REFERENCES
- 1. Sudbery P, Gow N, Berman J. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol. 12:317–324. 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2. Calderone RA, Fonzi WA. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327–335. 10.1016/S0966-842X(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 3. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193. 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspõllu A, Väin E, Saarma I, Salumets A, Donders GG, Metsis M. 2013. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS One 8:e54379. 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. 2010. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6:e1000713. 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scanlan PD, Marchesi JR. 2008. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2:1183–1193. 10.1038/ismej.2008.76. [DOI] [PubMed] [Google Scholar]
- 7. Lindén SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, Sutton P, McGuckin MA. 2009. MUC1 Limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 5:e1000617. 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caldara M, Friedlander RS, Kavanaugh NL, Aizenberg J, Foster KR, Ribbeck K. 2012. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 22:2325–2330. 10.1016/j.cub.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83–90. 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergey EJ, Cho MI, Blumberg BM, Hammarskjöld ML, Rekosh D, Epstein LG, Levine MJ. 1994. Interaction of HIV-1 and human salivary mucins. J. Acquir. Immune Defic. Syndr. 7:995–1002. [PubMed] [Google Scholar]
- 11. Couceiro JN, Paulson JC, Baum LG. 1993. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 29:155–165. 10.1016/0168-1702(93)90056-S. [DOI] [PubMed] [Google Scholar]
- 12. Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. 1999. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 67:6652–6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson JM, Soll DR. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169:5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie J, Tao L, Nobile CJ, Tong Y, Guan G, Sun Y, Cao C, Hernday AD, Johnson AD, Zhang L, Bai FY, Huang G. 2013. White-opaque switching in natural MTLa/α isolates of Candida albicans: evolutionary implications for roles in host adaptation, pathogenesis, and sex. PLoS Biol. 11:e1001525. 10.1371/journal.pbio.1001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller MG, Johnson AD. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302. 10.1016/S0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 17. Bennett RJ, Uhl MA, Miller MG, Johnson AD. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 23:8189–8201. 10.1128/MCB.23.22.8189-8201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoyer LL, Green CB, Oh SH, Zhao X. 2008. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med. Mycol. 46:1–15. 10.1080/13693780701435317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. 2012. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148:126–138. 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naglik JR, Challacombe SJ, Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400–428. 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gangloff YG, Romier C, Thuault S, Werten S, Davidson I. 2001. The histone fold is a key structural motif of transcription factor TFIID. Trends Biochem. Sci. 26:250–257. 10.1016/S0968-0004(00)01741-2. [DOI] [PubMed] [Google Scholar]
- 22. Crater JS, Carrier RL. 2010. Barrier properties of gastrointestinal mucus to nanoparticle transport. Macromol. Biosci. 10:1473–1483. 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 23. Kocevar-Nared J, Kristl J, Smid-Korbar J. 1997. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials 18:677–681. 10.1016/S0142-9612(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 24. Worku ML, Sidebotham RL, Baron JH, Misiewicz JJ, Logan RP, Keshavarz T, Karim QN. 1999. Motility of Helicobacter pylori in a viscous environment. Eur. J. Gastroenterol. Hepatol. 11:1143–1150. 10.1097/00042737-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 25. Smith DJ, Gaffney EA, Gadêlha H, Kapur N, Kirkman-Brown JC. 2009. Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity. Cell Motil. Cytoskeleton 66:220–236. 10.1002/cm.20345. [DOI] [PubMed] [Google Scholar]
- 26. Alonso-Monge R, Navarro-García F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sánchez M, Nombela C. 1999. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee I, Pangule RC, Kane RS. 2011. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23:690–718. 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 28. Nucci M, Anaissie E. 2001. Revisiting the source of candidemia: skin or gut? Clin. Infect. Dis. 33:1959–1967. 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 29. Lesuffleur T, Porchet N, Aubert JP, Swallow D, Gum JR, Kim YS, Real FX, Zweibaum A. 1993. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 106 (Pt 3):771–783. [DOI] [PubMed] [Google Scholar]
- 30. Baillie GS, Douglas LJ. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671–679. 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- 31. Ramage G, VandeWalle K, López-Ribot JL, Wickes BL. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214:95–100. 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- 32. Ogasawara A, Komaki N, Akai H, Hori K, Watanabe H, Watanabe T, Mikami T, Matsumoto T. 2007. Hyphal formation of Candida albicans is inhibited by salivary mucin. Biol. Pharm. Bull. 30:284–286. 10.1248/bpb.30.284. [DOI] [PubMed] [Google Scholar]
- 33. Butrus SI, Klotz SA. 1986. Blocking Candida adherence to contact lenses. Curr. Eye Res. 5:745–750. 10.3109/02713688609000015. [DOI] [PubMed] [Google Scholar]
- 34. Maidan MM, Thevelein JM, Van Dijck P. 2005. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem. Soc. Trans. 33:291–293. 10.1042/BST0330291. [DOI] [PubMed] [Google Scholar]
- 35. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053–1060. 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staab JF, Bahn Y-S, Sundstrom P. 2003. Integrative, multifunctional plasmids for hypha-specific or constitutive expression of green fluorescent protein in Candida albicans. Microbiology 149(Pt 10):2977–2986. 10.1099/mic.0.26445-0. [DOI] [PubMed] [Google Scholar]
- 37. Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K. 2012. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 13:1724–1732. 10.1021/bm3001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith BF, LaMont JT. 1984. Hydrophobic binding properties of bovine gallbladder mucin. J. Biol. Chem. 259:12170–12177. [PubMed] [Google Scholar]
- 39. Gong DH, Turner B, Bhaskar KR, LaMont JT. 1990. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am. J. Physiol. 259:G681–G686. [DOI] [PubMed] [Google Scholar]
- 40. Sheffner AL. 1963. The reduction in vitro in viscosity of mucoprotein solutions by a new mucolytic agent, N-acetyl-l-cysteine. Ann. N. Y. Acad. Sci. 106:298–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods. Download
(A) Growth curve of C. albicans with or without mucins in YPD at 30°C. In this case, the growth rates were roughly the same. (B) Growth curve of C. albicans with or without mucins in RPMI at 30°C. Here, the cells grown in mucins increased in optical density faster than those grown without mucins due to decreased hyphal formation and flocking of cells in mucins. Download
The mucin-induced morphological state is distinct from the opaque state. Phase-contrast images compare the mucin-induced morphology of the wild type (WT) to that of the wor1Δ/Δ strain, which is locked in the white phase. Download
Effects of osmotic stress and viscosity on virulence traits. (A) Fluorescence micrographs overlaid onto phase-contrast images of C. albicans grown in RPMI without or with 0.5% mucin, 0.5% methylcellulose, or 1 M sorbitol (an osmotic stress inducer). (B) Quantification of attachment to the polystyrene plates in the presence of RPMI without or with 0.5% PEG (a viscous polymer), 0.5% methylcellulose, or 0.5% native mucins. The colonized area of each frame was measured. The experiment was performed in triplicate with 5 pictures of each well taken at each time point. Error bars represent standard deviations. Download
Native and CsCl-purified mucins suppress hyphal formation of C. albicans. Fluorescence microscopy images are overlaid on phase-contrast images of C. albicans HGFP3 after incubation for 8 h in RPMI alone or with 0.5% native mucins or 0.5% CsCl-purified mucins. Download
Mucins suppress hyphal formation in YPD plus FBS. A filamentation assay was performed as previously described for RPMI in YPD plus FBS, a different hypha-inducing medium. Download
Analysis of opaque state attributes upon exposure to mucins. C. albicans cells that were either homozygous or heterozygous at the mating locus were assayed for their ability to switch from white to opaque, respond to alpha factor, and mate after exposure to mucins.
List of virulence genes analyzed using qPCR.