SUMMARY
Expression of the immediate-early gene c-fos was used to test for different patterns of temporal lobe interactions when rats explore either novel or familiar objects. A new behavioural test of recognition memory was first devised to generate robust levels of novelty discrimination and to provide a matched control condition using familiar objects. Increased c-Fos activity was found in caudal, but not rostral portions, of perirhinal cortex (areas 35/36) and in area Te2 in rats showing object recognition i.e. preferential exploration of novel versus familiar objects. The findings are presented at a higher anatomical resolution than previous studies of immediate-early gene expression and object novelty and, crucially, provide the first analyses when animals are actively discriminating the novel objects. Novel versus familiar object comparisons also revealed altered c-Fos patterns in hippocampal subfields, with relative increases in CA3 and CA1 and decreases in the dentate gyrus. These hippocampal changes match those previously reported for the automatic coding of object-spatial associations. Additional analyses of the c-Fos data using structural equation modelling indicated the presence of pathways starting in the caudal perirhinal cortex that display a direction of effects from the entorhinal cortex to the CA1 field (temporo-ammonic) when presented with familiar objects, but switch to the engagement of the direct entorhinal cortex pathway to the dentate gyrus (perforant) with novel object discrimination. This entorhinal switch provides a potential route by which the rhinal cortex can moderate hippocampal processing, with a dynamic change from temporo-ammonic (familiar stimuli) to perforant pathway (novel stimuli) influences.
Keywords: area Te2, entorhinal cortex, hippocampus, immediate-early genes, perirhinal cortex, recognition memory
INTRODUCTION
There is much debate over how temporal lobe structures interact to support the learning and recognition of novel stimuli. While lesion studies in animals (monkeys and rats) repeatedly show that the perirhinal cortex is necessary for identifying visual novelty (Zola-Morgan et al., 1989; Meunier et al., 1993; Mumby & Pinel, 1994; Ennaceur et al., 1996), the nature of its functional links with the hippocampus remains highly contentious (Brown & Aggleton, 2001; Eichenbaum et al., 2007; Squire et al., 2007). One approach is to compare activity across multiple sites when rats are confronted with novel stimuli. An informative class of activity markers are immediate-early genes (IEGs), of which, c-fos, is arguably the marker of choice as its activity is consistently increased in the rat perirhinal cortex following exposure to novel visual stimuli (Zhu et al., 1995b; Zhu et al., 1996; Wan et al., 1999; Aggleton & Brown, 2005). c-fos imaging is functionally relevant given its close associations with neuronal plasticity and learning (Nikolaev et al., 1991; Herdegen & Leah, 1998; Tischmeyer & Grimm, 1999; Kasahara et al., 2001; Fleischmann et al., 2003). In particular, perirhinal c-Fos activity may be a critical requirement for effective, stable object recognition memory in rats (Seoane & Brown, 2007).
The present study introduced a new behavioural test of novelty recognition appropriate for activity imaging. In previous studies animals have been passively exposed to novel stimuli (Zhu et al., 1995b; Zhu et al., 1996; Wan et al., 1999). Consequently, with no behavioural evidence that the rats could distinguish novel from familiar stimuli it is difficult to interpret null results. Rats were accordingly trained on a new task that combines aspects of delayed nonmatching-to-sample (Mishkin & Delacour, 1975; Aggleton, 1985; Mumby et al., 1990) with spontaneous exploration (Ennaceur & Delacour, 1988). The goal was to devise a task in which multiple trials could be delivered to help yield a signal detectable by IEG imaging, while simultaneously generating clear behavioural measures of novelty discrimination.
The development of a more appropriate task also provided the opportunity to examine IEG expression at an enhanced degree of anatomical resolution. Target sites, therefore, included divisions of the perirhinal cortex (areas 35 and 36) that were further subdivided along their rostro-caudal axis to reflect changes in the patterns of their connectivity (Shi & Cassell, 1999; Furtak et al., 2007). These subdivisions were not distinguished in previous IEG studies. Additional attention was also given to the septo-temporal axis of the hippocampus given its functional heterogeneity (Bast, 2007).
The present study, therefore, sought to advance current understanding in three ways. First, a new behavioral task of novelty discrimination was developed. Second, parahippocampal/hippocampal interactions were examined at a higher anatomical resolution than previous IEG studies, so utilising one of the main advantages of the current method. Third, structural equation modelling was applied to the c-Fos results. These additional analyses made it possible to compare the derived direction of effects between structures within the temporal lobe when groups of rats explore either familiar or novel objects.
MATERIALS AND METHODS
Animals
Subjects were 20 naïve, male rats (Dark Agouti strain, Harlan, UK). The rats were 12-14 weeks old at the beginning of the experiment. Animals were food-deprived to 85% of their free-feeding body weight and were maintained at this level. Water was available ad libitum. Rats were housed in pairs under diurnal conditions (14 h light, 10 h dark), and testing occurred at a regular time during the light period. Animals were thoroughly habituated to handling before the study began. All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act (1986) and associated guidelines.
Apparatus
The animals were tested in a bow-tie shaped maze made of opaque Perspex (Figure 1A, Supplement Figure 1). The apparatus was 120 cm long, 50 cm wide and 50 cm high. Each end of the apparatus was triangular, the apices of which were joined by a narrow corridor (12 cm wide). There was an opaque guillotine door in the middle of the corridor that could be raised by the experimenter. The far wall of each triangle contained two recessed food wells, 3.5 cm in diameter and 2 cm deep. The food wells were separated by a short, opaque dividing wall, which protruded 15 cm from the middle of the end wall. These food wells were covered by objects in the experiment proper.
Figure 1.
A. Schematic of the Bow-tie maze, with dimensions in centimetres. Food wells shown in gray. B. General procedure showing the presentation order of the objects. All objects are rewarded (+). Arrow shows rats movements. Group Novel: Black print represents the novel objects and gray print the familiar objects. C. Spatial placement of a now familiar object compared to its previous trial location. Two possible configurations are possible: same (left) or displaced (right).
Objects
The study used 147 pairs of different junk objects with various shapes, textures, sizes, and colours. Each object was large enough to cover a circular food well (3.5 cm diameter) but light enough to be displaced (Supplemental Figure 1). Any object with an obvious scent was excluded. The objects were divided into seven sets of 21 pairs of objects.
Behavioural testing
Animals were then divided into two groups: Novel (n = 10) and Familiar (n = 10). Pairs of animals (one from Group Novel and one from Group Familiar) were housed together, behaviourally trained one immediately after the other, and then processed together for immunohistochemistry. The sole, critical difference between Group Novel and Group Familiar was the nature of the objects i.e. whether they were novel or familiar during later training and, most importantly, during the final session.
Pretraining
By the end of pretaining (7 days), all rats would run from one side of the maze to the other, and to displace an object covering a food well in order to reach food rewards. On day 1, pairs of rats were placed in the apparatus for 30 minutes where they explored the maze freely and ate sucrose pellets scattered on the floor and in the food wells (45mg; Noyes Purified Rodent Diet, Lancaster, NH, USA). On days 2 and 3, rats were pretrained singly in the maze for 20 minutes, where they were rewarded for shuttling between the two goal areas. From day 4, the central guillotine door was used to control the movement of the rat from one side of the maze to the other. From day 6, three pairs of different objects were introduced in the maze, which covered the food wells. Rats were rewarded for pushing these three objects to access the food rewards. These three pairs of objects were not used in the experiment proper.
General training protocol
In order to provide informative comparisons between Groups Novel and Familiar it was necessary to have the general training protocol identical for the two groups. Thus, for the both groups the training session began with a rat being placed on one side of the maze, where there was only one object (object A) covering a food well (Figure 1B). A single food pellet was placed in the well under each object before each trial. After one minute the central guillotine door was raised, and the rat ran to the opposite side of the maze. There, the rat had the free choice between object A, which was now familiar, and a novel object B (trial 1). Both objects A and B covered baited food wells that were concurrently available to the rat. As there were duplicates of each object the rat could not mark an object for the next trial (Figure 1B). After 1 min the guillotine door was raised (trial 2) to reveal object B (now familiar) and object C (novel). Once again, both object covered a single reward pellet. After 1 min the guillotine door was raised again (trial 3), and animals exposed to object C (now familiar) and object D (novel). Each session contained 20 such trials and, hence, used 21 sets of objects.
Throughout training all objects, both novel and familiar, covered a single reward. This feature ensured the rats’ continued approach to the objects, but did not affect the validity of the behavioural test of recognition as this relied on differential object exploration. Animals were video-recorded for the last three sessions.
Specific Behavioural Protocol for Group ‘Novel’
Animals received 12 training sessions over 6 days (2 sessions per day: morning and afternoon). For Group Novel, each trial consisted of being presented with two objects, one novel the other familiar because its duplicate had been the ‘novel’ object on the previous trial i.e. recently explored and so now familiar (see Figures 1, 2). The placement of the novel object varied from left to right according to a pseudorandom schedule. The pool of 126 different pairs of objects was exhausted after 6 sessions, and the complete pool was re-used over the next 6 sessions (Sessions 7-12), but the order and pairings of the individual objects changed from that used in Sessions 1-6.
Figure 2.
Specific procedure for Group Novel (left) and Group Familiar (right) during the penultimate session (top, session 12) and the final session (bottom, Test session). The letters α, β, γ represent a novel set of objects, whereas objects A, B, C are only new for Group Novel.
Specific Behavioural Protocol for Group ‘Familiar’
For Group Familiar, all aspects of the test procedure were identical to those used for Group Novel, with one critical difference: the same set of 21 objects was used for all 13 sessions, including the final ‘test’ session. This manipulation was designed to ensure that the rats were strongly familiarised with every individual object. The order of the objects changed from session to session (Figure 2). This extended training was designed to help the Group Familiar rats to become fully familiar with every object, and was not required to improve recognition by Group Novel.
Final Behavioural Test Session prior to c-Fos analysis
Training for Group Novel was identical to that described above except that a new set of 21 pairs of objects was used (Session 13) i.e. 21 novel sets of objects (see Figure 2). Training for Group Familiar was now identical to that for Group Novel as it used the very same set of 21 objects, but as these particular objects were the ones used throughout their previous 12 sessions the training protocol ensured that they were all highly familiar for just this group. Object order was identical for the two groups (Figure 2).
Analysis of behaviour
Exploration of an object was defined as directing the nose at a distance < 1 cm to the object and/or touching it with the nose or the paws. Turning around or sitting on the object was not counted. Analyses to determine if rats had learnt spatial attributes of the test objects were based on the exploration times when an object was shown the second time within a session, as the object could now be in the ‘same’ or a ‘displaced’ spatial configuration from the preceding trial with respect to the most salient room landmarks (Figure 1C).
c-Fos analysis
On the final test day, animals were processed using previously described immunohistochemical methods for c-Fos protein (Albasser et al., 2007) that recognise the potential limitations in this method (Fritschy, 2008). Ninety minutes after completing the test session, rats were deeply anaesthetized with sodium pentobarbital (60 mg/kg, Euthatal, Rhone Merieux, UK) and transcardially perfused with 0.1 M phosphate buffer saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS (PFA). This interval (90 min) was selected as it is within the typical period of peak production (between 90 and 120 mins) for c-Fos protein after a specific, initiating event (Bisler et al., 2002; Zangenehpour & Chaudhuri, 2002). The brains were removed and postfixed in PFA for 4 h and then transferred to 25% sucrose overnight at room temperature with continuous rotation.
Sections were cut at 40 μm on a freezing microtome in the coronal plane. One series (one-in-three sections) was collected in PBS. Sections were transferred to 10 mM citrate buffer (pH=6) dissolved in deionised H2O and incubated in a water bath at 70°C for 30 min. Endogenous peroxidase was blocked by incubating the sections in 0.3% hydrogen peroxide in PBST for 10 min, before rinsing several times with PBST. Sections were next incubated in PBST containing c-Fos rabbit polyclonal antibody (1:5000; Ab-5, Oncogene Science, UK), for 48 h at 4°C with periodic rotation. Sections were then washed with PBST and incubated for c-Fos in biotinylated goat anti-rabbit secondary antibody (diluted 1:200 in PBST; Vectastain, Vector Laboratories, Burlingame, USA) and 1.5% normal goat serum. Sections were then washed and processed with avidin-biotinylated horseradish peroxidase complex in PBST (Elite Kit, Vector Laboratories) for 1h at room temperature, again with constant rotation. Sections were washed again in PBST and then in 0.05 M Tris buffer. The reaction was then visualized using diaminobenzidine (DAB Substrate Kit, Vector Laboratories) and finally stopped by washing in cold PBS. Sections were mounted on gelatine-coated slides, dehydrated through a graded series of alcohols and coverslipped.
c-Fos positive cell counts
Estimates of c-Fos activated cells were made using an automated cell counting procedure. Images were viewed on a Leica DMRB microscope, photographed using an Olympus DP70 camera, and stored digitally. Stained nuclei were counted using the program analySIS^D (Soft-Imaging Systems; Olympus, UK). This program makes it possible to select and count cells automatically without experimenter bias (counts were conducted without knowledge of the group assignments). In order to derive accurate, absolute cell counts it is necessary to use stereological methods (Coggeshall and Leakan, 1996), but the goal of the present study was to compare relative numbers of activated cells across the two conditions. For this purpose, automated cell counting is appropriate when certain conditions are met e.g. no systematic changes in the volume or packing of the neurons in the two groups (Coggeshall & Lekan, 1996), along with random tissue sampling (Mura et al., 2004). Both conditions should be met in the present study.
Counts of labelled nuclei in each region of interest were determined by counting those nuclei (mean feret, a measure of particle size, of 4-20 μm) stained above an automatically determined threshold of grayscale intensity that was above background levels (software setting for histogram intensity phases = 3). The cortical counts were made in a frame area of 0.84 × 0.63 mm, which enabled all laminae to be included in one image. For the hippocampus, image montages of the dentate gyrus, CA3 and CA1 fields were created from coronal sections at the septal, intermediate, and temporal levels of the hippocampus (Figure 3).
Figure 3.
Coronal sections indicating regions of interest: anterior cingulate cortex (AC), areas 35 and 36 for the rostral, mid and caudal perirhinal (Prh) cortex, area Te2, primary auditory cortex (Audp), CA1, CA3, dentate gyrus (DG) for the septal, intermediate and temporal hippocampus, lateral entorhinal cortex (lEnt), medial entorhinal cortex (mEnt), infralimbic cortex (IL), prelimbic cortex (PL), retrosplenial dysgranular cortex (Rdg), retrosplenial granular a (Rga), retrosplenial granular b (Rgb), dorsal subiculum (d Sub), ventral subiculum (v Sub), primary visual cortex (Visp). The numbers refer to the distance (mm) from bregma according to the atlas of Paxinos and Watson (2005).
For all brain areas analysed, counts were taken from four consecutive immuno-reacted sections from each hemisphere i.e. sections that were 120 μm apart, as the tissue came from a one-in-three (40 μm) series. For statistical analyses of regional c-Fos, counts were first normalised according to thte matched pairs of animals (one Novel, one Familiar). This normalisation procedure reduces the impact of staining variability from batch to batch of animals. Normalisation involved dividing the mean number of activated neurons in a given animal for a given site by the combined mean of the two animals in each matched pair, and expressing this result as a percentage. Thus, all normalised scores across pairs sum to 100. These normalised data were then used for the initial statistical analyses of active cells, while absolute cell counts were used for the Pearson correlations that underline the structural equation modelling approach.
Regions of interest
Cytoarchitectonic sub-regions were identified from coronal sections. All of the regions sampled are depicted in Figure 3.
The perirhinal cortex nomenclature and borders were taken from Burwell (2001). The perirhinal cortex was subdivided into three sub-regions: rostral (from AP −2.76 to −3.84 relative to bregma), mid (AP −3.84 to −4.80), and caudal (from AP- 4.80 to −6.30 ). These borders correspond to those levels depicted by Paxinos and Watson (2005). These same three sub-regions were also divided into areas 35 and 36 (Burwell, 2001), which make up the perirhinal cortex. It should be noted that our rostral perirhinal measurements would include much of the caudal parietal insular cortex as described by Shi and Cassell (1999), who proposed a much more restricted perirhinal region than Burwell (2001). Counts were also taken from the adjacent visual association cortex area Te2, which has previously been implicated in novelty detection (Zhu et al., 1996; Wan et al., 1999) and is the visual region immediately dorsally to the caudal perirhinal cortex (Figure 3).
Cytoarchitectonic subfields within the hippocampal formation were subdivided into their septal (dorsal), intermediate, and temporal (ventral) parts (Bast, 2007; Bast et al., 2009). The septal hippocampus counts (dentate gyrus, CA3, and CA1) were obtained from sections near AP level −2.52 from bregma. Counts for the intermediate part (dentate gyrus, CA1, CA3) and the temporal pole of the hippocampus (CA1, CA3) were obtained from sections near AP level −4.8 from bregma. The intermediate/temporal hippocampal division corresponded to −5.0 dorso-ventrally from bregma (Paxinos & Watson, 2005). Adjacent regions included the dorsal and ventral subiculum (dSub, vSub; AP −4.92) and the lateral and medial entorhinal cortices (lEnt, mEnt; AP −4.92). The various layers of the entorhinal cortex could not be counted separately, as the relatively low level of c-Fos counts in this region, combined with the modest intensity of staining, meant that these distinctions could not be made with sufficient confidence.
Three frontal regions were also examined, the prelimbic (PL; AP +2.76), infralimbic (IL; AP +2.76), and anterior cingulate (AC; AP +0.24) cortices. More caudally, the retrosplenial cortex was examined. The retrosplenial cortex can be subdivided into granular b (Rgb), granular a (Rga), and dysgranular cortex (Rdg). Separate counts were made in all three sub-regions. Furthermore, the superficial (layer II and upper III) and deep (lower layer III to VI) layers of Rdg, Rgb, and Rga were counted separately as there is evidence of their differential involvement in learning tasks (Gabriel et al., 1983).
Finally, counts were also taken from two cortical areas: primary visual area (Visp) and primary auditory area (Audp). These two areas were used as control regions; any group differences in these regions might reflect different sensory demands across conditions despite attempts to match task demands.
Statistics
Regional c-fos activation
The c-Fos-positive cell counts were initially analysed in six separate regional groupings; 1) perirhinal cortex and area Te2, 2) hippocampal subfields, 3) entorhinal/subicular regions, 4) frontal cortex, 5), retrosplenial cortex and 6) primary sensory cortex. Normalised counts were analysed using a one between- (groups) × one within-subject (sub-regions) design. These groupings serve to reduce Type 1 errors. When there was a significant group × region interaction, simple effects were examined (Howell, 1982). For these multiple comparisons at the regional level, the significance level was further adjusted using the Modified Bonferonni test (Keppel, 1991). As a consequence, the probability level of ≤ 0.05 was taken as being statistically significant only when the number of sites (simple effects) to be compared did not exceed four. For the region including the perirhinal cortex and area Te2 (7 comparisons) and for the hippocampal subfields (8 comparisons) the significance level was adjusted to ≤ 0.029 and ≤ 0.025, respectively (Keppel, 1991). Bivariate correlations were calculated using the Pearson product-moment correlation coefficient for regional IEG activity and performance metrics.
Structural equation modelling
Structural equation modelling is an integrative approach to assess interrelationships among correlated (or uncorrelated) variables comprising an underlying theoretical structure. Structural equation models are multiple-equation regression models representing putative causal (and hence structural) relationships amoung a number of variables, some of which may affect one another mutually. As a consequence this approach provides a means to test for possible directions of effect.
In the present study, structural equation modelling of c-Fos counts offers a potentially useful way to help correlate the activity of the regions of interest to each other. In addition, the process makes it possible to test the direction of putative effects so allowing one to evaluate the feasibility of network (or model) dynamics (McIntosh & Gonzalez-Lima, 1991; Friston et al., 1993; Jenkins et al., 2003; Poirier et al., 2008). A starting assumption was that all models should conform to well-established patterns of connectivity between the regions of interest.
Using the same network, based on known temporal lobe connections, it was possible to compare statistical models derived for each group (Group Novel and Group Familiar). Path analyses of different network models could also be derived from the covariance matrices representing the relationships between the c-Fos counts in the various regions of interest. Data investigation used the specialized SEM analysis program Amos 6.0 (SPSS, Chicago). The term ‘fit’ refers to the ability of a model to reproduce the data i.e., usually the variance-covariance matrix.
The anatomical models considered six temporal lobe areas that have been at the heart of debates about the neural basis of visual recognition (Brown & Aggleton, 2001; Eichenbaum et al., 2007; Squire et al., 2007). Numerous fit indices exist, each being more or less sensitive to various model parameters (Fan et al., 1997; Hu & Bentler, 1998), and thus it is recommended to report several indices. These fit indices aim to test statistically the explanatory power of the models (similar to the F-test for an ANOVA). Three measures of goodness of fit are reported. The goodness of fit was first indicated by a nonsignificant chi-square (χ2), the only binary fit/no-fit decision of a model. Two additional measures provided an index of the degree of discrepancy of fit of a model to the data. The comparative fit index (CFI) is an index of the proportion of variance accounted, based on the comparison of a proposed model to an independent model, in which no regions are connected. Such independent models have the least fit, and a high index value means that the tested model is opposite to the independent model, i.e. thus exhibiting good fit. Alternatively, the root mean square error of approximation (RMSEA) provides an index of absolute fit by determining the average lack of fit per degree of freedom. A 90% confidence interval (CI) around the RMSEA demonstrates the precision of the estimate, and the RMSEA p value is for testing the null hypothesis that the population RMSEA is no greater than 0.05.
An important factor in choosing the CFI and RMSEA is that they are both also recommended for their good performance with small sample sizes (Fan et al., 1997; Hu & Bentler, 1998), thus each counter this limitation of the current study. In addition to a nonsignificant χ2 with a ratio of the χ2 to the degrees of freedom < 2, a good-fitting model that is a plausible representation of the underlying data structure was considered to either have a CFI ≥ 0.90-0.95 or RMSEA ≤ 0.05, good; < 0.08, acceptable (Tabachnik & Fidell, 1996)
The models to be tested were based on established connections within the medial temporal lobe e.g. perirhinal cortex with lateral entorhinal cortex, the tri-synaptic hippocampal pathway (dentate gyrus to CA3 to CA1), and the monosynaptic temporo-ammonic connections of the entorhinal cortex with the CA subfields (Steward & Scoville, 1976; Witter et al., 2000). Many models were then generated, taking into account a wide range of connections, even those connections that are thought to be very light (i.e from CA1 to perirhinal cortex, Furtak et al., 2007). However, most of these alternative models resulted in a low or very low fit.
Only the models with the best fit (Fan et al., 1997; Hu & Bentler, 1998), i.e. only “optimal models” are presented. While the focus on known, direct connections inevitably constrains the models to be tested, it helps to reduce Type 1 statistical errors and avoid the emergence of anatomically implausible models. In the case of the reciprocal projections between the lateral entorhinal cortex and CA1 as well as the perirhinal cortex (Witter et al., 2000), paths were tested in both directions, and the strongest one is presented. It is appreciated that these models are anatomically very simplistic and cannot capture the complex interplay that has been revealed between these sites (Van Strien et al., 2009). In addition, the squared multiple correlation (R2 or coefficient of determination) is also presented for each dependent brain region in the models. This value represents a measure of the proportion of the variance of the dependent variable that is explained by the independent variable(s). In the models a single-headed arrow represents a direct effect of one variable on another. A bidirectional (two-headed) arrow represents a covariance, between variables, that is not given causal interpretation. Attempts to integrate behavioural measures of recognition into the models derived for Group Novel are not presented as multiple models of similar fit could be generated i.e. no optimal class of model could be established.
RESULTS
Behavioural measures of object recognition
Initial analyses confirmed that Group Novel could discriminate novel from familiar objects.
Object Recognition - First choice
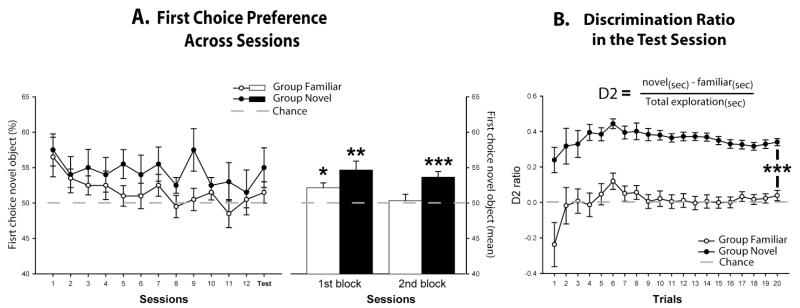
On each trial rats had a choice between a new object (Group Novel) or one not experienced since the previous session (Group Familiar) and the one just explored. The first choice preference (Figure 4A) was considered for all 13 sessions, with a score of over 50% showing that rats tended to explore the novel object first. The results are blocked into two sets: the first 6 sessions and the last 7 sessions (Figure 4B). Group Novel selected the novel object first significantly more times than Group Familiar selected the less familiar of the two objects (F(1,18) = 7.3, p < 0.05). No group × block interaction was found (F < 1). Subsequent analyses (one-sample t-test) demonstrated that the likelihood of exploring the novel object first was above chance for Group Novel in both blocks of sessions (Figure 4B; 1st block, t(9) = 3.7, p < 0.01; 2nd block, t(9) = 4.7, p < 0.001). In contrast, Group Familiar selected relative novelty above chance only during the 1st block of six sessions (Figure 4B;1st block, t(9) = 3.2, p < 0.05; 2nd block, t(9) < 1). These two different profiles of performance are to be expected as for Group Familiar the objects became increasingly familiar over sessions and, hence, less discriminable on the basis of recency.
Figure 4.
A. First choice preference of novel objects. The line plot graph shows the scores during the 13 sessions (left). The bar chart represents mean scores over the first set of six sessions (1st block) and the subsequent set of seven sessions (2nd block). Data are shown as mean ± SEM. A score significantly above 50 (one-sample t test) indicates first choice preference for the novel object. * p < 0.05, ** p < 0.01, *** p 0< 0.001 (right). B. Discrimination ratio D2 = [(novel(sec) -familiar(sec)) / total exploration(sec)] for final session. The D2 score is re-calculated after each trial as data from successive trials are accumulated. A score of zero reflects a failure to discriminate novel from familiar. Data shown are mean ± SEM. Group differences: *** p < 0.001.
Object Recognition - Exploration measures
Using differential object exploration two indices of object recognition are typically calculated (Ennaceur & Delacour, 1988). D1 is simply the difference in time spent exploring the novel and familiar objects, so a positive score indicates the recognition of novelty. D2 is the time spent exploring the novel object of a pair minus the time spent exploring the familiar object (i.e. D1), divided by the total time spent exploring both objects. The D2 index can, therefore, range between +1 and −1. This ratio measure is often preferable as it can partially compensate for differences in overall amounts of exploration. For Group Novel these two indices reflect novelty discrimination, while for Group Familiar they reflect recency discrimination.
The total amount of object exploration across the twenty trials in the final test session was greater for Group Novel than for Group Familiar (Group Novel: mean = 136.0 sec SEM = ±9.1; Group Familiar: mean = 102.4 sec, SEM = ±6.3; t(18) = 3.03, p < 0.01). The two groups also differed on their detection of novelty (or relative familiarity) using the D1 index (Group Novel: mean = 46.4 sec, SEM = ±3.5; Group Familiar: mean = 5.3 sec, SEM = ±3.3; t(18) = 8.87, p < 0.001).
In view of the group differences in overall exploration time, more detailed analyses focussed on the Discrimination Ratio, D2 (Ennaceur & Delacour, 1988). Figure 4B shows how the D2 index changed across the final test session as increasing numbers of trials were added to its calculation. As expected, Group Novel had significantly higher D2 final scores for this test session than Group Familiar (Group Novel: mean = 0.34, SEM = ±0.020; Group Familiar: mean = 0.039, SEM = ±0.029; t-test, t(18) = 8.53, p < 0.001; Figure 4B). Moreover, the D2 scores for Group Novel stabilised around 0.38-0.33 after only five trials (see Figure 4B). Group Novel showed clear object recognition in this final test session as they spent significantly more time exploring the novel object compared to the just seen (now familiar) object (one-sample t-test comparing D2 scores with a score of 0: Group Novel, t(9) = 16.92, p < 0.001). In contrast, Group Familiar failed to discriminate between the objects as they did not spend more time exploring the ‘new’ object (last seen on the previous session) compared to the ‘familiar’ object just seen on the previous trial (one-sample t-test; D2: Group Familiar, t(9) = 1.35, p = 0.21).
Object placement
On every trial both novel objects occupied new locations (as initial and repeat object exposures were at opposite ends of the maze), so reducing spatial influences. Even so, we tested for sensitivity to whether the familiar object was in the ‘same’ or ‘displaced’ arrangement with respect to the long axis of the maze (Figure 1C). There was a significant object placement effect; the two groups spent more time exploring an object when it was ‘displaced’ (F(1,18) = 4.5, p < 0.05). A group effect was also found (F(1,18) = 6.5, p < 0.05), as this difference was more pronounced in Group Familiar, but no interaction was found (F < 1). The larger effect in Group Familiar presumably arose because object exploration by Group Novel rats is predominantly controlled by object novelty, i.e. spatial effects would be overshadowed.
Immediate-early gene results
Perirhinal Cortex and area Te2
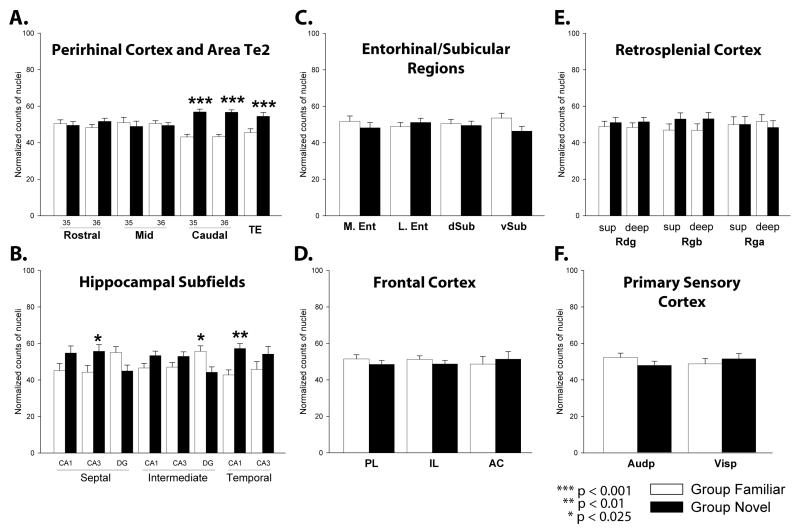
Exposure to novel as opposed to familiar objects (Group Novel versus Group Familiar) increased c-Fos counts in the perirhinal cortex and area Te2 (F(1,18) = 13.8, p < 0.01; Figure 5A). These c-Fos increases were not, however, uniform (group × sub-region interaction, F(6,108) = 7.4, p < 0.001), and only the caudal parts (c-Fos photomicrograph; Figure 6A) of perirhinal areas 35 and 36 showed significant c-Fos increases (simple effects; rostral: area 35, F < 1, area 36, F(1,126) = 1.6, p = 0.21; mid: area 35, F < 1, area 36, F < 1; caudal: area 35, F(1,126) = 25.4, p < 0.001, area 36, F(1,126) = 24.3, p < 0.001), along with area Te2 (F(1,126) = 10.6, p < 0.001).
Figure 5.
c-Fos levels following familiar (white) or novel (black) object exposure. A. Perirhinal cortex and Area Te2: areas 35 and 36 of the rostral, mid and caudal perirhinal cortex and area Te2. B. Hippocampal Subfields: dentate gyrus (DG), CA3, CA1, for septal, intermediate and temporal hippocampus. C. Entorhinal/Subicular Regions: lateral entorhinal (lEnt) cortex, medial entorhinal (mEnt) cortex, dorsal subiculum (d Sub) and ventral subiculum (v Sub). D. Frontal Cortex: prelimbic (PL), infralimbic (IL) and anterior cingulate (AC) cortices. E. Retrosplenial Cortex: superficial and deep layers of the dysgranular (Rdg) and granular (Rgb and Rga) retrosplenial cortex. F. Primary Sensory Cortex: primary auditory cortex (Audp), primary visual cortex (Vis). Normalized counts of c-Fos-positive cells are presented as mean ± SEM. Significance of group differences: * p < 0.025; ** p < 0.01; *** p < 0.001.
Figure 6.
Photomicrographs comparing c-Fos levels between Group Novel (left-hand side) and Group Familiar (right-hand side). A. Caudal perirhinal cortex (the numbers refer to areas 35 and 36). B. Septal hippocampus. C. Primary visual cortex. The photomicrographs are taken from behaviourally-matched pairs of animals where tissue was processed simultaneously to reduce variation. The brightfield photomicrographs of coronal sections show the comparable c-Fos levels in the primary auditory cortex, which contrasts, with the specific increase of c-Fos positive cells following novelty in the caudal perirhinal cortex. Scale bar, 100 μm.
Hippocampal Subfields
For the initial comparison, the three sets (septal, intermediate, temporal) of c-Fos counts for each subfield (CA1, CA3, dentate gyrus) were summed and compared. While there was no overall group effect (Novel versus Familiar, F(1,18) = 2.07, p = 0.17), there was a significant group × sub-region interaction (F(2,36) = 21.0, p < 0.001). Simple effect analyses confirmed overall increases in CA1 (F(1,54) = 9.58, p < 0.01) and CA3 (F(1,54) = 10.26, p < 0.01), but decreases in the dentate gyrus (F(1,54) = 8.62, p < 0.01) in Group Novel.
This same pattern of result was repeated when the septal, intermediate, and temporal counts were treated separately, as there was no overall group difference across the entire hippocampal formation (F(1,18) = 1.63, p = 0.22; Figure 5B), but there were consistent changes within specific subfields (group × subregion interaction F(7,126) = 8.36, p < 0.001). The same pattern of c-Fos changes (Figure 5B) was found for each of the three rostro-caudal levels (septal, intermediate, temporal) of the hippocampus i.e. Group Novel displayed a relative c-Fos increase in fields CA1 and CA3, but a decrease in the dentate gyrus (for septal hippocampus see Figure 6B). Subsequent analyses showed a significant increase in Group Novel c-Fos cell counts in septal CA3 (F(1,144) = 6.04, p < 0.025) and in temporal CA1 (F(1,144) = 9.70, p < 0.01). These increases contrasted with the significant decreases in c-Fos cell counts in Group Novel in the intermediate dentate gyrus (F(1,144) = 6.20, p < 0.025).
The lateral and medial entorhinal cortex project to different sites within the dentate gyrus (Van Strien et al., 2009), with the lateral entorhinal cortex preferentially terminating in the dorsal blade and the medial entorhinal cortex terminating in the ventral blade of the dentate gyrus. Additional c-Fos-positive cell counts were, therefore, made in these two blades of the dentate gyrus. Greater absolute numbers of c-Fos-positive cells were present in the dorsal blade compared to the ventral blade of the dentate gyrus for both Group Novel and Group Familiar (F(3,54) = 60.54, p < 0.001; Figure 6B).
Using normalised counts, it was found that there were decreased dentate gyrus c-Fos counts in Group Novel compared to Group Familiar (F(1,18) = 29.86, p < 0.001), but no interaction was found (F < 1) i.e. the reduction in Group Novel was present in a comparable degree in both the dorsal and ventral blades of the septal and intermediate dentate gyrus (simple effects; septal dorsal blade, F(1,72) = 16.38, p < 0.001; septal ventral blade, F(1,72) = 12,56 p < 0.001; intermediate dorsal blade, F(1,72) = 14.43, p < 0.001; intermediate ventral blade F(1,72) = 16.42, p < 0.001).
Entorhinal/Subicular Regions
No overall changes in c-Fos-positive cell counts were found in the four regions tested (medial and lateral entorhinal cortex, dorsal and ventral subiculum; group, F < 1; Figure 5C). Likewise, there was no group × sub-region interaction (F(3,54) = 1.7, p = 0.18).
Frontal and Retrosplenial Cortices
No significant c-Fos group differences (F < 1) were found for the three frontal regions (prelimbic, infralimbic, and anterior cingulate; Figure 5D) or for the three retrosplenial sub-regions (Rdg, Rgb, Rga, F < 1; Figure 5E) that were analysed. In neither case was there a group × sub-region interaction (both F < 1).
Primary Sensory Cortex
To help determine how well matched Group Novel was with its control Group Familiar, c-Fos counts were made in primary sensory areas. No group differences were found in the primary auditory and primary visual (group, F < 1, Figures 5F, 6C) cortices.
Correlations between regions of interest and recognition D2 ratio
In Table 1, Pearson correlations were calculated for all brain areas with D2. The only significant correlation was between c-Fos counts in CA3 and the degree of recency discrimination by the Group Familiar rats (r = 0.87, p < 0.001). This significant correlation within Group Familiar is consistent with evidence that the hippocampus is more critical for judgments of recency (relative novelty) than absolute novelty (Charles et al., 2004). Thus, while the discrimination performance of Group Novel was much higher than that of Group Familiar, the performance of the latter group was often still significantly above chance. This pattern of results shows that the Group Familiar rats could distinguish between just explored and previously explored objects.
Table 1.
Inter-region correlations of c-Fos counts and discrimination ratio (D2)
The bottom left diagonal matrix concerns data from Group Familiar (white background) while the top right diagonal matrix concerns data from Group Novel (pale grey background). r, Pearson coefficient, and correlations (two-tailed) that are significant at the 0.05(*) or 0.01(**) levels are shaded indarker grey. Abbreviations: caudPeri, caudal perirhinal cortex; lEnt, lateral entorhinal cortex; dentate gyrus, DG; CA3 and CA1.
| caudPeri | TE | lEnt | DG | CA3 | CA1 | D2 | |||
|---|---|---|---|---|---|---|---|---|---|
| caudPeri |
r
p |
.933** .001 |
.769** .009 |
.771** .009 |
.748* .013 |
.832** .003 |
−.416 .232 |
↑ Group Novel ↓ |
|
| TE |
r
p |
.961** .001 |
.878** .001 |
.861** .001 |
.758* .011 |
.781** .008 |
−.249 .488 |
||
| lEnt |
r
p |
.872** .001 |
.913** .001 |
.886** .001 |
.538 .108 |
.633* .050 |
.048 .895 |
||
| DG |
r
p |
.233 .517 |
.259 .470 |
.062 .865 |
.566 .088 |
.633* .049 |
.061 .868 |
||
| CA3 |
r
p |
.580 .079 |
.729* .017 |
.763* .010 |
.059 .872 |
.733* .016 |
−.605 .064 |
||
| CA1 |
r
p |
.720* .019 |
.676* .032 |
.661* .038 |
.424 .222 |
.582 .078 |
−.468 .173 |
||
| D2 |
r
p |
.353 .317 |
.487 .153 |
.440 .203 |
.196 .587 |
.867** .001 |
.560 .092 |
||
| ← Group Familiar → | |||||||||
Structural equation modelling
Models for Group Novel and Group Familiar were derived from the correlations across each brain region for the mean, absolute c-Fos counts (Table 1). Models were rejected if they were not based on established neuronal connectivity within the temporal lobe and if they did not have statistically significant pathways interlinking structures across the model.
Group Novel
Figure 7A shows the optimal model that matched our criteria. The Group Novel model was heavily dependent on a linear route via the perforant path and tri-synaptic circuit. Thus, the network essentially comprised caudal perirhinal ↔ area Te2 → lateral entorhinal → dentate gyrus → CA3 → CA1. All of these pathways were significant, and the model had good fit (χ2 = 11.01, df = 9, p = 0.28; CFI = 0.96; RMSEA = 0.16, CI = 0.001-0.42, p = 0.29). Reversing the direction of the paths between structures yielded poorer results (data not shown), with the exception of Te2 - perirhinal cortex, where no particular direction was optimal. The high covariance between caudal perirhinal cortex and area Te2 activity suggests that these regions are intimately inter-related, and that a pattern of reciprocal influence may best represent their interaction.
Figure 7.
Temporal lobe interactions derived from structural equation modelling. Notice the engagement of the tri-synaptic circuit induced by novelty. A. Optimal Group Novel model, B. Consequence of trying to fit Group Familiar data into the optimal model of Group Novel (used for stacked comparison between groups) and C. Optimal Group Familiar model. The strength of the causal influences is depicted in the diagrams in black. The squared multiple correlations (R 2) are in grey. Sites depicted: area Te2, dentate gyrus (DG), CA1, CA3, lateral entorhinal cortex (lEnt), caudal perirhinal cortex (caudPrh). *p < 0.025; ***p< 0.001.
Group Familiar
While the initial cortical elements of the optimal model for Group Familiar (caudal perirhinal ↔ area Te2 → lateral entorhinal) were the same as those for Group Novel, the model changed radically thereafter (Figure 7C). The tri-synaptic path was now disengaged as the influences of the lateral entorhinal cortex on dentate gyrus, of dentate gyrus on CA3, and of CA3 on CA1 were no longer involved. In the model with the best fit across all three indices only four structures were involved (Figure 7C) as the dentate gyrus and CA3 were removed. The direction of effect was now directly from the lateral entorhinal cortex to CA1 i.e. the temporo-ammonic projection was now engaged rather than the perforant pathway. The resulting model for Group Familiar (Figure 7C) had good fit (χ2 = 1.8, df = 3, p = 0.62; CFI = 1.00; RMSEA < 0.001, CI = 0.001-0.46, p = 0.63).
It should be added that a model incorporating CA3 could also be derived for Group Familiar. In this model, which is identical in all other respects to that depicted in Figure 7C, there are parallel routes from lateral entorhinal cortex to CA3 and CA1, both of which are significant (lEnt - CA1: strength path = 0.76 p < 0.01; lEnt - CA3: strength path = 0.66, p <0.001). This model has an acceptable level of fit for most indices (χ2 = 10.3, df = 6, p = 0.12; CFI = 0.92; RMSEA = 0.281, CI = 0.001-0.57, p = 0.12), but there is no significant pathway from CA3 to CA1. Importantly, this alternate model still highlights the dominance of the temporo-ammonic pathway over the perforant pathway.
Group Novel versus Group Familiar
The c-Fos results for Group Familiar were next set within the optimal model derived for Group Novel (Figure 7B). Not surprisingly, Group Familiar exhibited poor fit to this model (χ2 = 27.9, df = 9, p = 0.001; CFI = 0.67; RMSEA = 0.48, CI = 0.28-0.69, p < 0.001). Because both groups were tested on the same model it was possible to compare them formally by stacking Group Novel and Group Familiar (see Figure 7A, B and Table 2). When both groups were combined the resultant model exhibited poorer fit on all measures (χ2 = 38.7, df = 18, p = 0.003; CFI = 0.81), consistent with the deleterious impact of Group Familiar. Overall comparison of the unconstrained stacked model and another model, in which each path was simultaneously constrained (structural weights) to be equivalent between the conditions, revealed that the overall structure of the two groups was significantly different (χ 2 = 11.77, df = 5, p = 0.038). This group difference was tested by constraining those individual paths exhibiting a relatively substantial difference in strength (≥ 0.05). Group differences included the reduction in the effective linear connectivity of the lateral entorhinal cortex to the dentate gyrus (χ2 = 8.59, df = 2, p = 0.014).
Table 2.
Results of the multiple-sample structural equation model analyses in which stacked groups Novel and Familiar were evaluated with specific path constraints; df, degrees of freedom; lEnt, lateral entorhinal cortex; DG, dentate gyrus. Statistical significance < 0.05 level (*) is indicated in bold with a grey background.
|
Model fit
|
Model compafrisons
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Stacked model constraints | χ 2 | df | p | CFI | RMSEA | df | χ 2 | p |
| Unconstrained | 38.87 | 18 | 0.003 | 0.81 | 0.25 | |||
| All paths | 50.64 | 23 | 0.001 | 0.75 | 0.26 | 5 | 11.77 | 0.038* |
| lEnt - DG | 47.45 | 20 | 0.001 | 0.75 | 0.28 | 2 | 8.59 | 0.014* |
| DG - CA3 | 41.80 | 20 | 0.003 | 0.80 | 0.25 | 2 | 2.93 | 0.23 |
| CA3 - CA1 | 39.42 | 20 | 0.006 | 0.83 | 0.23 | 2 | 0.55 | 0.76 |
| lEnt - CA1 | 39.08 | 20 | 0.007 | 0.83 | 0.23 | 2 | 0.22 | 0.90 |
DISCUSSION
Using a new behavioural protocol, rats were shown novel objects and compared to those shown identical, yet familiar objects. The active discrimination of novel from familiar objects was strongly associated with c-Fos increases in perirhinal cortex and area Te2. While previous studies have reported c-Fos increases in these areas when rats are exposed to novel objects, this is the first study where the rats’ behaviour simultaneously confirms their ability to distinguish novel from familiar. A more complex pattern of c-Fos changes occurred in the hippocampus as there was no overall activity change with novelty, yet different subfields either increased (CA1, CA3) or decreased (dentate gyrus) activity levels. Structural equation modelling highlighted the difference in patterns of c-Fos activation within the temporal lobe for novel versus familiar stimuli. Especially striking was the switch in parahippocampal – hippocampal effective connectivity from the temporo-ammonic pathway in Group Familiar to the perforant (dentate gyrus) pathway in Group Novel. An important caveat is that like most imaging findings, the results are correlative and so it is misleading to draw causal inferences. It should, however, be noted that there is evidence from antisense infusions to believe that c-Fos activity in the perirhinal cortex is a critical requirement for effective, stable object recognition memory in rats (Seoane & Brown, 2007). Thus, the present study used a marker that may have a causal role for long-term recognition memory, though the study could only examine recognition memory over short retention periods in Group Novel. This same temporal mismatch may help to explain the lack of any direct correlations between perirhinal c-Fos-positive cells counts and individual levels of recognition (D2 ratio).
Standard spontaneous exploration tasks typically comprise one trial (two objects) per session. For the goals of the present study this feature would leave the research prone to null results if cellular correlates of novelty for a given object are restricted to a small subpopulation of cells or if the signal itself is small. Also, the very small number of stimuli used in the standard task can introduce biases associated with specific objects. The present study, therefore, used a new behavioural protocol that combined features of delayed nonmatching-to-sample, e.g. multiple trials, with spontaneous recognition. Very clear exploration differences emerged in every Group Novel animal for novel versus familiar objects, indicative of the robustness of this new test and its potential for examining the neural basis of recognition memory. One side-effect of these high levels of discrimination in Group Novel was that although all objects were baited, to help match total exploration times between the two groups, Group Familiar still displayed less overall exploration. This difference could provide a potential confound and so it is important to note that the behavioural analyses focussed on the D2 ratio, which corrects for activity differences. Furthermore, the c-Fos counts in the two control areas (primary auditory and primary visual cortices) failed to show any evidence of a group difference, in contrast to the selective patterns of change found elsewhere. The implication is that the qualitatively different patterns of c-Fos expression found across the two groups reflected the novel versus familiar nature of the test stimuli. That the objects had become very familiar to the rats in Group Familiar by their re-occurrence in every session was demonstrated by the failure of this group, on the final test day, to discriminate between an object that had just been explored and one that had not been seen since the previous day (Figure 4B).
Exposure to familiar stimuli was associated with a relative decrease of c-Fos activity in the perirhinal cortex. This pattern has parallels with the decreases in neuronal activity (Brown & Aggleton, 2001) and the decreases in the BOLD signal (Henson et al., 2003; Gonsalves et al., 2005; Montaldi et al., 2006) found, respectively, in the perirhinal region of monkeys and people when presented with familiar stimuli. Within the perirhinal cortex, caudal areas 35 and 36 appeared to show the clearest distinction between novel and familiar objects, as measured by c-Fos. While previous IEG studies have also found changed c-Fos counts in caudal perirhinal cortex associated with familiarity (Zhu et al., 1996; Wan et al., 1999), none has tested for rostro-caudal differences. Similarly, while electrophysiological recordings in rats indicate that caudal areas 35 and 36 can signal novelty (Zhu et al., 1995a), subregions within these areas have not been compared. For similar reasons the present findings accord with studies disrupting recognition following perirhinal cortex infusions of e.g. lidocaine hydrochloride (Winters & Bussey, 2005) or a lentiviral suspension (Griffiths et al., 2008), as all such studies have primarily targeted caudal perirhinal cortex. The most likely explanation for this prior focus on caudal perirhinal cortex is that it is a reaction to the uncertainty over the placement of the rostral border of this area (Shi & Cassell, 1999; Burwell, 2001).
The present finding that caudal perirhinal cortex (both areas 35 and 36) has particular importance for identifying object novelty agrees with the recent finding of a significant correlation between object recognition deficits and the amount of perirhinal tissue loss in the caudal, but not the mid or rostral portions of areas 35 and 36 (Albasser et al., 2009). This convergent evidence for a rostro-caudal functional difference has anatomical support as it is the caudal perirhinal cortex that is most closely linked to visual inputs (Furtak et al., 2007), including those from area Te2 (Shi & Cassell, 1999) and from the occipital cortex (Furtak et al., 2007). The inputs from the dorsal (especially midline) thalamus also preferentially target caudal perirhinal cortex (Furtak et al., 2007). Caudal perirhinal cortex is also noteworthy as it is a major source of projections to the other parts of the perirhinal cortex, along with the projections to the postrhinal and entorhinal cortices (Furtak et al., 2007). Area Te2 consistently shows c-Fos increases with visual novelty (Zhu et al., 1996; Wan et al., 1999) and is thought to function in tandem with caudal perirhinal cortex (Zhu et al., 1995a).
In addition to the c-Fos increases in the caudal perirhinal cortex, hippocampal c-Fos changes were also associated with novel object exploration. These c-Fos changes in the hippocampus (increases and decreases) are notable as they suggest a complex pattern of hippocampal activity changes that is potentially involved in the detection of novel objects i.e. for object recognition. This latter proposal is, however, highly contentious (Aggleton & Brown, 1999; Mumby, 2001; Squire et al., 2007). It is, therefore, helpful to note that all previous c-Fos studies that passively exposed rats to sets of novel objects (versus familiar objects) have found no hippocampal activity changes (Zhu et al., 1995b; Zhu et al., 1996; 1997; Wan et al., 1999; Warburton et al., 2003; Wan et al., 2004; Aggleton & Brown, 2005). For this reason, the present study stands out as an exception.
In view of the consistent null results in previous studies i.e. no c-Fos hippocampal activity change with novel visual stimuli (Zhu et al., 1995b; Zhu et al., 1996; 1997; Wan et al., 1999; Warburton et al., 2003; Wan et al., 2004; Aggleton & Brown, 2005), it is important to consider any procedural differences with the present study. Probably the most notable difference is that the rats in the present study could actively investigate the objects in their various locations. It is known that rats automatically learn the spatial disposition of objects explored in an arena (Poucet, 1989; Dix & Aggleton, 1999), and evidence for spontaneous spatial learning was found in the present study. It is, therefore, possible that this automatic spatial learning element contributed to the hippocampal activity changes. This interpretation receives further support from studies reporting that the spatial re-arrangement of familiar room cues (Jenkins et al., 2004), as well as the introduction of novel spatial cues (Vann et al., 2000; Van Elzakker et al., 2008), consistently changes hippocampal c-Fos activity. Particularly striking support for this view comes from the fact that the hippocampal results in the present study (increased c-Fos in CA1 and CA3, decreased c-Fos in dentate gyrus) precisely match the c-Fos patterns elicited by the passive presentation of novel spatial arrays of highly familiar visual stimuli (Wan et al., 1999). Indeed, the similarity in these patterns points to a specific mode of hippocampal function when novel object-spatial conjunctions are detected and acquired. Consequently, the hippocampal IEG activations in the present study may well include spatial processes that are inherent in any study involving object investigation. At the same time, the results in the current study cannot preclude a role in novelty detection.
There remains much debate over how the perirhinal cortex and hippocampus contribute to recognition memory (Brown & Aggleton, 2001; Eichenbaum et al., 2007; Squire et al., 2007). Recent rat studies, based on receiver operating characteristics, point to different but complementary roles for the perirhinal cortex and hippocampus (Fortin et al., 2004). The perirhinal cortex can detect stimulus novelty while the hippocampus helps detect changes in associative aspects of the stimuli, e.g. ‘where’ and ‘when’. This model readily fits the present findings as it has already been noted that the present hippocampal activations in Group Novel match patterns associated with learning spatial dispositions (‘where’) of stimuli (Wan et al., 1999; Vann et al., 2000). A potential criticism of this account is, however, that both the Novel and Familiar groups showed spatial disposition learning, and so differential hippocampal activation might not initially be predicted, i.e. the groups should be similar. A possible account for this particular aspect of the findings is suggested by the ‘gatekeeper’ hypothesis of perirhinal function (Fernandez & Tendolkar, 2006). This model, largely derived from in-depth recordings in the human temporal lobe, states that the rhinal cortex serves as a ‘gatekeeper’ for memory via its ability to regulate hippocampal activity (Fernandez & Tendolkar, 2006). In this model, information is serially processed via the rhinal cortices, which are ideally placed to make rapid familiarity/novelty discriminations before deeper encoding has begun. This information is then used to regulate encoding and retrieval operations by the hippocampal formation. The rhinal cortex is thought to provide a number of rapid, high-level operations (repetition suppression, encoding, integration, processing) supporting both item recognition and memory encoding (Fernandez & Tendolkar, 2006). Consequently, when a new item is perceived, a large number of rhinal neurons is required to process this item, leading to the feeling of novelty, effective encoding and the effective transfer of information to the hippocampus for further encoding. In contrast, when a familiar item is detected, smaller numbers of rhinal neurons are needed to process this item, leading to less vigorous encoding, reduced information transfer to the hippocampus, and a feeling of familiarity. As a consequence, familiar stimuli initiate fewer hippocampal resources.
The present findings can be fitted into this general framework, although they also indicate a number of refinements. The first is that novel stimuli need not simply initiate more hippocampal resources per se, rather they differentially change activity (upwards or downwards) in the various subfields. The second, related refinement emerges from the structural equation modelling. Here, the optimal models again underline the qualitative differences in the patterns of temporal connectivity evoked by novelty versus familiarity. While novel stimuli engaged the tri-synaptic path via the perforant pathway (lateral entorhinal → dentate gyrus → CA3 → CA1), familiar stimuli (recency) engaged the temporo-ammonic route (lateral entorhinal directly to CA1). This temporal direction of effects in Group Novel (rhinal cortex to hippocampus) matches that found with in-depth electrodes in human patients learning word lists (Fernandez et al., 1999). The overall decrease in c-Fos counts in the dentate gyrus in Group Novel may, at first sight, appear in conflict with this account, but the correlations across regions were positive so that those rats with the highest increase in perirhinal c-Fos counts tended to be those rats with the highest dentate gyrus counts (i.e. the smallest c-Fos decreases in the dentate gyrus). This relationship supports the model of the perirhinal cortex ‘driving’ the dentate gyrus response in Group Novel (but not in Group Familiar, where there is no correlation). At the same time, evidence for a switch in the dominant routes to the hippocampus proper refines the ‘gatekeeper’ hypothesis of perirhinal cortex function (Fernandez & Tendolkar, 2006). It should, of course, be noted that such analyses can only present a highly simplified account of the multitude of potential medial temporal interconnections (Van Strien et al., 2009). Such limitations do not, however, detract from one the core findings of the present study: that qualitatively different patterns of network effects were found in Groups Novel and Familiar.
Evidence for the reproducibility of our network findings comes from related immediate-early gene studies. In a comparison of rats in either the early or late stages of learning a radial-arm maze task (Poirier et al., 2008), a strikingly similar shift in network patterns emerged from an analyses of Zif268 expression. Once again the pattern involved a loss of dentate gyrus influence combined with an increased reliance of CA1 on its direct temporo-ammonic input, with a related CA1/CA3 uncoupling, when spatial learning involved increasingly familiar room cues (Poirier et al., 2008). Similarly, ‘place’ versus ‘landmark’ learning in a water maze resulted in qualitatively different patterns of c-Fos activation between perirhinal cortex, lateral entorhinal cortex and dentate gyrus, with only new place learning engaging all three in a linear model (Jenkins et al., 2003). The emergence of these similar network patterns across different studies reveals a general switch in hippocampal function from a temporo-ammonic mode to a temporo-dentate mode as behaviour involves increasingly unfamiliar cues or novel spatial arrangements (Poirier et al., 2008; see also Van Elzakker et al., 2008). Such an engagement of the perforant pathway route would then enable the hippocampal learning of novel object attributes (Hunsaker et al., 2007; Staresina & Davachi, 2008) including learning their spatial or temporal context (Wan et al., 1999; Hoge & Kesner, 2007).
Supplementary Material
A. Photograph of a rat performing the object recognition task in the bow-tie maze. B. Photograph of a selection of objects used in the test session. Scale bar is 8 cm.
Acknowledgements
This work was supported by the Wellcome Trust [WT087855]. We wish to thank M.W. Brown for his helpful comments, and Gilles Freund for his scoring software.
REFERENCES
- Aggleton JP. One-trial object recognition by rats. Q J Exp Psychol. 1985;37B:279–294. [Google Scholar]
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Davies M, Futter JE, Aggleton JP. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci. 2009;123:115–124. doi: 10.1037/a0013829. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Warburton EC, Aggleton JP. Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. Eur J Neurosci. 2007;26:1254–1266. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–281. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- Bast T, Wilson IA, Witter MP, Morris RG. From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol. 2009;7:e1000089. doi: 10.1371/journal.pbio.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K, Staiger JF. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J Chem Neuroanat. 2002;23:187–198. doi: 10.1016/s0891-0618(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. J Comp Neurol. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci. 2004;24:2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fan X, Wang L, Thompson B. The effects of Sample Size, Estimation Methods, and Model Specification on SEM Fit Indices. Paper presented at the annual meeting of American Educational Research Association; New York, NY. 1997. [Google Scholar]
- Fernandez G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dumpelmann M, Van Roost D, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Tendolkar I. The rhinal cortex: ‘gatekeeper’ of the declarative memory system. Trends Cogn Sci. 2006;10:358–362. doi: 10.1016/j.tics.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur J Neurosci. 2008;28:2365–2370. doi: 10.1111/j.1460-9568.2008.06552.x. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17:709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci. 1983;97:675–696. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J, Brown MW, Warburton EC, Bashir ZI. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–194. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. Duxberry Press; Belmont, California: 1982. [Google Scholar]
- Hu L, Bentler P. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–453. [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Amin E, Harold GT, Pearce JM, Aggleton JP. Distinct patterns of hippocampal formation activity associated with different spatial tasks: a Fos imaging study in rats. Exp Brain Res. 2003;151:514–523. doi: 10.1007/s00221-003-1499-0. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Amin E, Pearce JM, Brown MW, Aggleton JP. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: a c-fos expression study. Neuroscience. 2004;124:43–52. doi: 10.1016/j.neuroscience.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Activation of calcium/calmodulin-dependent protein kinase IV in long term potentiation in the rat hippocampal CA1 region. J Biol Chem. 2001;276:24044–24050. doi: 10.1074/jbc.M100247200. [DOI] [PubMed] [Google Scholar]
- Keppel . Design and analysis: A researcher’s handbook. Third edition Prentice Hall; New Jersey: 1991. [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. J Exp Psychol Anim Behav Process. 1975;1:326–334. doi: 10.1037//0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JPJ, Wood ER. Nonreccuring-items delayed nonmatching-to-sample in rats: A new paradigm for testing nonspatial working memory. Psychobiology. 1990;18:321–326. [Google Scholar]
- Mura A, Murphy CA, Feldon J, Jongen-Relo AL. The use of stereological counting methods to assess immediate early gene immunoreactivity. Brain Res. 2004;1009:120–128. doi: 10.1016/j.brainres.2004.02.054. [DOI] [PubMed] [Google Scholar]
- Nikolaev E, Tischmeyer W, Krug M, Matthies H, Kaczmarek L. c-fos protooncogene expression in rat hippocampus and entorhinal cortex following tetanic stimulation of the perforant path. Brain Res. 1991;560:346–349. doi: 10.1016/0006-8993(91)91257-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fifth edition Academic; San Diego: 2005. [Google Scholar]
- Poirier GL, Amin E, Aggleton JP. Qualitatively different hippocampal subfield engagement emerges with mastery of a spatial memory task by rats. J Neurosci. 2008;28:1034–1045. doi: 10.1523/JNEUROSCI.4607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A, Brown MW. Interfering with Fos expression impairs recognition memory in rats. British Neurosci. Assocn. Abstracts. 2007;19:39.05. [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. J Comp Neurol. 1999;406:299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using multivariate statistics. Third edition Harper Collins; New York: 1996. [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cellular and Molecular Life Sciences. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15:899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 2000;20:2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Warburton EC, Zhu XO, Koder TJ, Park Y, Aggleton JP, Cho K, Bashir ZI, Brown MW. Benzodiazepine impairment of perirhinal cortical plasticity and recognition memory. Eur J Neurosci. 2004;20:2214–2224. doi: 10.1111/j.1460-9568.2004.03688.x. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Zangenehpour S, Chaudhuri A. Differential induction and decay curves of c-fos and zif268 revealed through dual activity maps. Brain Res Mol Brain Res. 2002;109:221–225. doi: 10.1016/s0169-328x(02)00556-9. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, Aggleton JP. Neuronal signalling of information important to visual recognition memory in rat rhinal and neighbouring cortices. Eur J Neurosci. 1995a;7:753–765. doi: 10.1111/j.1460-9568.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Zhu XO, Brown MW, McCabe BJ, Aggleton JP. Effects of the novelty or familiarity of visual stimuli on the expression of the immediate early gene c-fos in rat brain. Neuroscience. 1995b;69:821–829. doi: 10.1016/0306-4522(95)00320-i. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Mapping visual recognition memory through expression of the immediate early gene c-fos. Neuroreport. 1996;7:1871–1875. doi: 10.1097/00001756-199607290-00037. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Differential activation of the rat hippocampus and perirhinal cortex by novel visual stimuli and a novel environment. Neurosci Lett. 1997;229:141–143. doi: 10.1016/s0304-3940(97)00437-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Photograph of a rat performing the object recognition task in the bow-tie maze. B. Photograph of a selection of objects used in the test session. Scale bar is 8 cm.