Abstract
Objectives
This study sought to examine the prevalence and predictors of pulmonary regurgitation (PR) following balloon dilation (BD) for pulmonary stenosis (PS) and to investigate its impact on ventricular volume and function, and exercise tolerance.
Background
Balloon pulmonary valvuloplasty relieves PS but can cause late PR. The sequelae of isolated PR are not well understood.
Methods
Patients were at least 7 years of age and 5 years removed from BD, and had no other form of congenital heart disease or significant residual PS. Cardiac magnetic resonance imaging and exercise testing were performed prospectively to quantify PR fraction, ventricular volumes and function, and exercise capacity.
Results
Forty-one patients underwent testing a median of 13.1 years after BD. The median PR fraction was 10%; 14 patients (34%) had PR fraction >15%; 7 (17%) had PR >30%. PR fraction was associated with age at dilation (ln-transformed, R = −0.47, p = 0.002) and balloon:annulus ratio (R = 0.57, p < 0.001). The mean right ventricular (RV) end-diastolic volume z-score was 1.8 ± 1.9; RV dilation (z-score ≥2) was present in 14/35 patients (40%). PR fraction correlated closely with indexed RV end-diastolic volume (R = 0.79, p < 0.001) and modestly with RV ejection fraction (R = 0.50, p < 0.001). Overall, peak oxygen consumption (Vo2) (% predicted) was below average (92 ± 17%, p = 0.006). Patients with PR fraction >15% had significantly lower peak Vo2 than those with less PR (85 ± 17% vs. 96 ± 16%, p = 0.03).
Conclusions
Mild PR and RV dilation are common in the long term following BD. A PR fraction >15% is associated with lower peak Vo2, suggesting that isolated PR and consequent RV dilation are related to impaired exercise cardiopulmonary function.
Keywords: pulmonary regurgitation, exercise function, balloon valvuloplasty, pulmonary stenosis, right ventricular dilation
Little is known about the pathophysiology of chronic isolated pulmonary regurgitation (PR). Congenital pulmonary valve incompetence is extremely rare, with most published reports consisting of individual cases (1, 2 and 3). In the largest published series, Shimazaki et al. (4) described long-term outcomes of 72 patients with this condition. Symptoms of heart failure were uncommon at 20 years (6%), but the likelihood of developing them increased dramatically over time, and premature death occurred in 3 symptomatic patients. A growing number of children and adults are subjected to long-term PR, including those with repaired conotruncal lesions as well as patients with valvar pulmonary stenosis (PS) following balloon dilation (BD). In the context of this expanding population, the need to understand the impact of chronic PR has become increasingly important.
In this study, we evaluated patients previously treated for uncomplicated valvar PS with percutaneous BD in an effort to gain insight into the late physiologic effects of chronic, isolated PR. Percutaneous balloon pulmonary valvuloplasty was introduced in 1982 (5) and subsequently became the first-line therapy for valvar PS at most centers. In the years since, a number of reports have detailed medium- and long-term outcomes of this therapy. With rare exceptions (6), however, the primary focus of these reports has been freedom from recurrence of valvar stenosis (7, 8, 9, 10 and 11). In the present study, we used cardiac magnetic resonance (CMR) imaging and exercise testing to investigate the prevalence and physiologic impact of long-term PR after balloon pulmonary valvuloplasty for congenital valvar PS. Our primary goal was to determine whether chronic PR is associated with compromised exercise cardiopulmonary function in the absence of confounding factors (such as surgical ventriculotomy, long-standing pre-procedural cyanosis, or exposure to cardiopulmonary bypass). A secondary goal was to assess the relationships between demographic and procedural factors at valvuloplasty and late PR, and between PR and ventricular size and function.
Methods
Patients
Subjects with valvar PS having undergone BD of the pulmonary valve were identified from the database of the cardiovascular program at Children’s Hospital Boston. Inclusion criteria were: history of valvar PS treated with percutaneous balloon valvuloplasty; managed at our institution; age ≥7 years at the time of enrollment; and at least a 5-year interval between the pulmonary valve dilation procedure and enrollment. Patients were excluded if they had significant residual PS (≥30 mm Hg gradient on last available echo or CMR evaluation); other congenital heart disease, including significant atrial septal defect; or any history of cardiac surgery. Eligible patients were contacted by mail with an informational packet describing the study, followed by a series of up to 3 telephone calls. Enrolled patients were prospectively studied with CMR imaging and exercise testing. This study was performed according to a protocol approved by the Committee for Clinical Investigation at Children’s Hospital Boston. All study subjects provided written informed consent.
Catheterization and balloon valvuloplasty
Balloon pulmonary valvuloplasty was performed as previously described, with a target balloon:annulus diameter ratio (BAR) of 1.2 to 1.4 (12). Catheterization reports from the pulmonary valve dilation procedure were reviewed. Recorded data included: age at catheterization, initial right ventricular (RV) pressure, the ratio of RV to systemic arterial or ventricular pressure, the diameter of the largest balloon used for valve dilation at maximal inflation, and the pulmonary valve annulus diameter measured during systole (5). The BAR was calculated as the ratio of these 2 diameters. For patients with data missing from the catheterization report, measurements were performed by direct review of the angiograms by 1 of the authors (D.B.M.). In the event of multiple catheterizations with valve dilation, the largest BAR and the age at the first catheterization procedure were used for analysis. The pulmonary valve annulus diameter z-score was calculated relative to body surface area from published normative data obtained at our institution (13).
CMR imaging
CMR was performed using a 1.5-Tesla scanner (Achieva, Philips Medical Systems, Best, the Netherlands) and radiofrequency coils selected according to body size. All examinations were performed without sedation. Biventricular dimensions and function were assessed as previously described (14). Briefly, electrocardiogram-gated breath-held steady-state free precession imaging was performed in the ventricular short-axis plane with 12 to 14 equidistant slices completely covering both ventricles. Flow measurements were performed in the proximal main pulmonary artery with a retrospectively gated velocity-encoded cine MR pulse sequence during free breathing. CMR data were analyzed using commercially available software packages (QMASS version 6.2.3 and QFLOW version 4.1.7, Medis, Leiden, the Netherlands). Left ventricular (LV) and RV end-diastolic (maximal) and end-systolic (minimal) volumes, mass at end diastole, stroke volume, and ejection fraction (EF) were measured as previously described (15). For all patients, ventricular volumes were indexed to body surface area. RV and LV enddiastolic volume z-scores were calculated for males and females in whom published normative data are available (body surface area >1.25 m2 and 1.0 m2, respectively) (15). An RV end-diastolic volume z-score ≥2 signified at least mild dilation. PR volume was measured as the amount of retrograde flow in the main pulmonary artery per cardiac cycle, and was indexed to body surface area to yield a PR volume index (milliliters/square meter/beat); the PR fraction was calculated as the retrograde flow divided by the antegrade flow in the main pulmonary artery, expressed as a percentage (16). Tricuspid regurgitation (TR) fraction, expressed as a percentage, was quantified as: (100 × TR volume)/TR inflow, where TR volume = (RV stroke volume − LV stroke volume) − PR volume; and TR inflow = RV stroke volume − PR volume.
Exercise testing
Exercise testing was performed with a progressive symptom-limited protocol using a cycle (17) or treadmill (18) ergometer. Electrocardiographic monitoring and breath-by-breath expiratory gas analysis were performed using a CardiO2 exercise testing system (Medical Graphics Corp., Minneapolis, Minnesota). Cuff blood pressure measurements and 12-lead electrocardiograms were obtained at 2- to 3-min intervals during exercise, at peak exercise, and at 1, 3, and 5 min after exercise. Oxygen saturation was also monitored by pulse oximetry throughout the study. Immediately prior to each exercise test, spirometric measurements of forced vital capacity and volume of air exhaled in the first second of forced expiration were obtained. The exercise testing system was calibrated according to manufacturer specifications prior to each test.
Peak exercise parameters were expressed as the percentage of the predicted value for age, sex, and size. Normal data were taken from the following sources: age ≤17 years, Cooper and Weiler-Ravell (19); age >18 years, Jones (20). Outcome parameters included the percentage of predicted values for peak oxygen consumption (Vo2), peak work rate, peak heart rate, and O2 pulse (a measure of forward stroke volume) (20); the slope of the minute ventilation:carbon dioxide production ratio (VE/VCO2, an index of the efficiency of gas exchange during exercise [17]); and spirometric measurements. For peak Vo2, peak work rate, O2 pulse, and heart rate, a subnormal value was defined as <85% of the predicted value, in accordance with the 2003 American Thoracic Society/American College of Chest Physicians Statement on Cardiopulmonary Exercise Testing; normal VE/VCO2 was <29 (21 and 22).
Data analysis
The primary hypothesis was that higher PR fraction would be associated with lower percent predicted peak Vo2. The analysis was organized according to 3 outcome categories: PR fraction, ventricular volumes and function, and exercise function. The first outcome was PR fraction, which was considered a procedural outcome and was treated both as a continuous variable and dichotomized around 15% on the basis of receiver-operator curve analysis for percent predicted peak Vo2. Demographic and procedural factors analyzed for association with PR fraction included age at the time of valvuloplasty (ln-transformed and dichotomized around 1 year), pre-intervention RV pressure and RV:systemic pressure ratio, and BAR (continuous and dichotomized around 1.4, the upper limit of the usual target BAR range). For the second category of outcomes, associations between PR and ventricular volumes and function were assessed. For the third category of outcomes, relationships between the primary outcome (percent predicted peak Vo2) and CMR (PR fraction and volume, RV and LV volumes and function) and demographic (age at intervention and follow-up, body mass index, gender) factors were assessed. Procedural variables were not included in the analysis of exercise function based on the rationale that any potential association between the procedure and exercise function should be reflected in cardiac physiology. Linear regression was used to assess the relationship between continuous variables, independent-samples t test to compare normally distributed continuous variables between groups, Wilcoxon rank sum test to compare continuous variables with a skewed distribution, and Fisher exact test to compare categorical variables. Exercise functional parameters were also compared with average (mean percent predicted value = 100%) using 1-sample t test. z-Scores were compared with normal (value = 0) using a 1-sample t test. SPSS (version 16, SPSS Inc., Chicago, Illinois) was used for statistical analysis.
Results
Demographic and catheterization data
Recruitment letters were sent to the last available address for 183 potentially eligible patients identified in the cardiology database. Of these, 81 were contacted by telephone (conversation or voice mail message); 41 enrolled in the study. The median age at BD in these 41 patients was 2.9 months (1 day to 27 years), and 28 (68%) were under 1 year; 18 patients (44%) were male. In 37 patients, balloon pulmonary valvuloplasty was performed at Children’s Hospital Boston; in the other 4 patients, the procedure was performed elsewhere, and care was subsequently transferred to our institution. Balloon valvuloplasty was performed between 1986 and 1990 in 9 patients (22%), 1991 and 1999 in 27 (66%), and in 2000 or later in 5 (12%). At the time of intervention, the median pulmonary annulus diameter z-score was −1.0 (−3.4 to 1.7), and the median BAR was 1.3 (1.0 to 2.0). BAR was inversely related to pulmonary valve z-score (n = 34, R = 0.67, p < 0.001). Additional data are summarized in Table 1.
Table 1.
Catheterization, CMR, and Exercise Data
| Value | p Value | ||
|---|---|---|---|
| Mean | Median | ||
| Catheterization (n = 41) | |||
| Age at balloon dilation (yrs) | 2.3 ± 5.5 | 0.2 (0 to 26.7) | |
| Pulmonary annulus (mm) | 10.6 ± 4.6 | 9.1 (4.0 to 21.7) | |
| Pulmonary annulus z-score | −0.8 ± 1.2 | −1.0 (−3.4 to 1.7) | |
| Balloon size (mm) | 13.3 ± 5.4 | 12 (6.0 to 30.0) | |
| Balloon to annulus ratio | 1.3 ± 0.2 | 1.3 (1.0 to 2.0) | |
| RV:systemic pressure ratio (%) | 111.2 ± 40.6 | 110.2 (44.2 to 206.9) | |
| CMR imaging (n = 41) | |||
| PR percent | 14 ± 13 | 10 (1 to 45) | |
| PR volume (indexed, ml/m2) | 8.5 ± 8.6 | 5.3 (0.5 to 30.2) | |
| RV end-diastolic volume (indexed, ml/m2) | 103 ± 28 | 100 (63 to 205) | |
| RV end-diastolic volume z-score (n = 35) | 1.8 ± 1.9 | 1.5 (−0.2 to 8.4) | |
| RV stroke volume (indexed, ml/m2) | 61.5 ± 14.3 | 56.7 (40.8 to 104.3) | |
| RV EF% | 60 ± 7 | 61 (46 to 77) | |
| LV end-diastolic volume (indexed, ml/m2) | 80.8 ± 12.6 | 77.9 (52.5 to 108.5) | |
| LV end-diastolic volume z-score (n = 35) | 0.1 ± 1.0 | −0.1 (−2.3 to 2.3) | |
| LV stroke volume (indexed, ml/m2) | 50.9 ± 8.5 | 49.2 (33.6 to 70.0) | |
| LV EF% | 64 ± 6 | 63 (55 to 78) | |
| Exercise (n = 40) | |||
| Peak Vo2 (% predicted) | 92 ± 17 | 90 (61 to 126) | 0.006 |
| Peak work rate (% predicted) | 95 ± 22 | 93 (60 to 149) | 0.14 |
| O2 pulse (% predicted) | 97 ± 17 | 96 (60 to 128) | 0.66 |
| Peak HR (% predicted) | 96 ± 7 | 97 (76 to 113) | <0.001 |
| FVC (% predicted) | 101 ± 13 | 99 (68 to 125) | 0.54 |
| FEV1 (% predicted) | 100 ± 15 | 100 (64 to 132) | 0.85 |
p values for the exercise data refer to percentage predicted values compared with an average value of 100% (1- sample t test).
CMR = cardiac magnetic resonance; EF = ejection fraction; FEV1 = forced expiratory volume (1 s); FVC = forced vital capacity; HR = heart rate; LV = left ventricular; RV = right ventricular; Vo2 = oxygen consumption.
Four patients underwent repeat BD, 3 within 1 year of the initial procedure and 1 who underwent the first procedure during infancy and the second 4 years later.
Follow-up
The median age at testing was 14.4 years (7.7 to 47.2 years), and the median time interval from balloon valvuloplasty was 13.1 years (6.2 to 22.9 years). In 37 patients, CMR and exercise testing were performed on the same day; the longest duration between the 2 tests in any patient was 3 months. One patient did not consent to exercise testing. Three patients had TR fraction ≥20%.
PR
The median PR fraction was 10% (1% to 45%), and the median PR volume index was 5.3 ml/m2/beat (0.5 to 30.2 ml/m2/beat). There was a very close correlation between PR volume index and PR fraction (R = 0.98, p < 0.001). Fourteen patients (34%) had a PR fraction >15%; 7 (10%) had a PR fraction >30%; and 2 (4.9%) had a PR fraction >40%.
Among demographic and procedural factors, larger BAR (R = 0.36, p < 0.001) and younger age (In-transformed) at intervention (R = −0.47, p = 0.002) were associated with higher PR fraction (Fig. 1). PR fraction was not related to duration between valvuloplasty and subsequent testing (p = 0.91), year of intervention (p = 0.96), or the degree of RV hypertension prior to valve dilation (p = 0.10).
Figure 1.
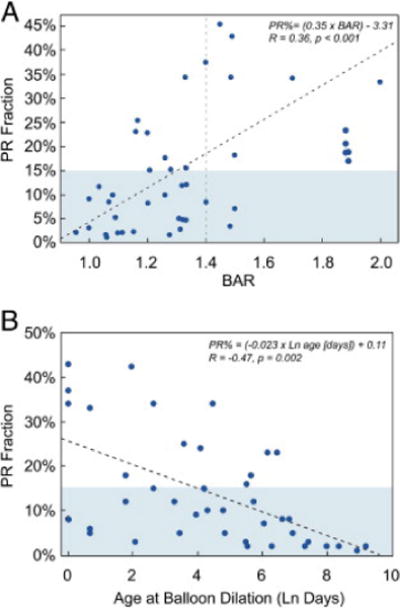
Factors at Catheterization and PR Fraction
(A) Balloon:annulus ratio and (B) ln (age at catheterization) versus pulmonary regurgitation (PR) fraction. PR fraction ≤15% is shaded. In the regression equations, PR% is expressed as a fraction.
The mean BAR was higher in the 14 patients with a PR fraction >15% than in those with a PR fraction ≤15% (1.42 ± 0.23 vs. 1.21 ± 0.17, p < 0.008). BAR ≥1.4 was associated with a significantly higher risk of PR fraction >15% (odds ratio: 4.5 [1.4 to 14.5], p = 0.01); the median PR fraction among patients with a BAR ≥1.4 was 26%. A PR fraction >15% was significantly more common in patients who underwent valvuloplasty at <1 year of age (12 of 26 patients) than those who were ≥1 year of age (2 of 15; odds ratio: 1.7 [1.1 to 2.5], p = 0.03).
Ventricular volume and function
The mean RV end-diastolic volume z-score (n = 35) was 1.8 ± 1.9 (p < 0.001 compared with z-score = 0). The median value was 1.5 (range −0.2 to 8.4). z-scores were unavailable in 6 patients due to small body surface area. In 14 patients (40%), RV dilation was present (z-score ≥2); Figure 2. In 1 (3%), it was severe (z-score ≥6); the BAR for this patient was 1.45. Median values for RV and LV EF were 61% (46 to 77%) and 63% (55% to 78%), respectively. Other CMR data are summarized in Table 1.
Figure 2.
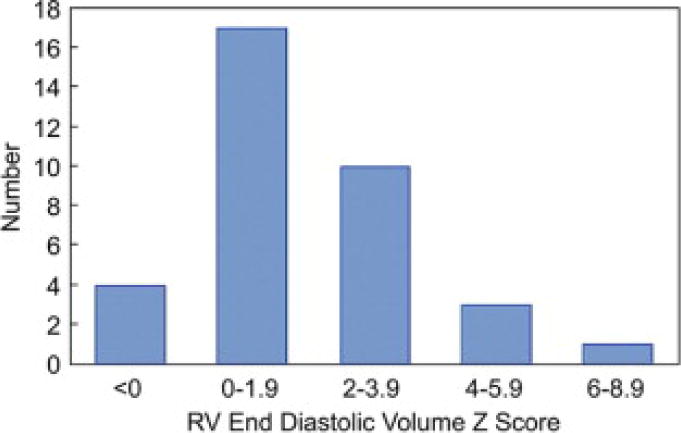
Prevalence of RV Dilation Distribution of right ventricular (RV) end-diastolic volume z -score.
By linear regression, PR fraction correlated well with indexed RV end-diastolic volume (R = 0.79, p < 0.001) (Fig. 3). It correlated inversely, and less well, with RV EF (R = −0.50, p = 0.001) (Fig. 3). The RV EF was modestly and inversely related to the pre-intervention RV to systemic pressure ratio (R = −0.43, p = 0.006). The PR fraction was not significantly associated with LV factors such as EF, indexed end-diastolic volume, or indexed stroke volume.
Figure 3.

PR Fraction and RV Parameters on CMR Imaging
PR fraction versus (A) RV end diastolic volume (indexed) (RVEDVi) and (B) RV ejection fraction (EF). In the regression equations, PR% is expressed as a fraction. CMR = cardiac magnetic resonance; other abbreviations as in Figure 1 and Figure 2.
Exercise function
On exercise testing with metabolic analysis using cycle ergometry (n = 34) or treadmill (n = 6), study subjects had peak Vo2 and heart rate (% predicted) that were modestly, but significantly, lower than average (Table 1). Of the 13 patients with subnormal peak Vo2, 9 (69%) had a subnormal O2 pulse, suggesting an insufficient stroke volume response to exercise; only 1 patient had a subnormal heart rate. Mean VE/VCO2 slope was 27 ± 4.
None of the demographic or continuous CMR variables correlated with the percentage of predicted peak VO2or peak work. Body mass index correlated modestly with the VE/VCO2 slope (R = −0.41, p = 0.009), but not with other exercise outcomes. When PR fraction was dichotomized, peak Vo2 was found to be significantly higher in patients with a PR fraction ≤15% than in those in whom it was >15% (96 ± 16% vs. 85 ± 17%, p = 0.03) (Fig. 4). There was a trend towards a higher percentage of predicted peak work in patients with a PR fraction ≤15% as well (99 ± 20% vs. 86 ± 24%, p = 0.06) (Fig. 4).
Figure 4.
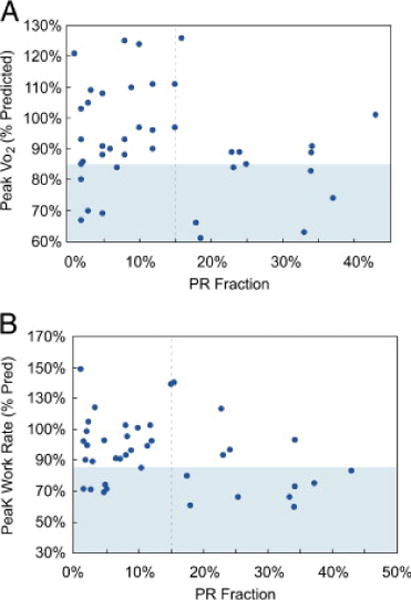
PR Fraction and Exercise Parameters
Pulmonary regurgitation (PR) fraction versus percent predicted values of (A) peak oxygen consumption (Vo2) and (B) peak work. Subnormal ranges for exercise parameters, as defined in the text, are shaded.
Discussion
In this study, we prospectively evaluated 41 patients who had undergone balloon valvuloplasty for valvar PS a median of 13 years earlier, with minimal residual outflow tract obstruction, using CMR imaging and exercise testing. This allowed us both to characterize the prevalence of PR and its risk factors and to assess the long-term impact of PR on cardiac and exercise function. In this cohort, the degree of PR and RV dilation ranged markedly, but severe PR and RV dilation were very uncommon. Larger BAR and younger age at intervention were associated with higher PR fraction, which in turn was strongly associated with RV dilation and modestly with decreased RV function. Overall, exercise cardiopulmonary function in this group was modestly below average, perhaps as a consequence of impaired ability to augment stroke volume. Patients with a PR fraction >15% had significantly lower peak Vo2 than those with less PR. No significant association was found between these variables using linear regression analysis, although this may have been due at least in part to the limited number of enrolled patients.
Some degree of RV dilation in this group was common, but was usually mild. The correlation between PR and indexed RV end-diastolic volume was fairly close, suggesting that RV compliance does not vary widely over the range of RV volumes seen in this group. In general, RV EF was normal, although there was a modest inverse relationship with PR fraction; this suggests that isolated PR and consequent RV dilation may adversely impact RV function. LV volume and function were also normal, and there was no evidence of an inverse relationship between PR fraction or RV volume/function and LV stroke volume or EF. This provides evidence that, with modest degrees of PR and RV dilation, there is unlikely to be significant ventricular interaction. As a group, no relationship was seen between time from valvuloplasty and PR fraction. Changes in this value over time for a particular individual, however, were not specifically addressed.
These findings add to a growing body of literature on the effects of chronic PR (23, 24 and 25). They are unique, however, in that the impact of PR in this patient population was studied in isolation, absent the many confounders present in other populations of patients with PR, such as those with repaired tetralogy of Fallot (26, 27, 28, 29 and 30). Exercise data from our patients differ from those reported for patients with repaired tetralogy of Fallot in at least 2 respects. First, in contrast to the mildly reduced peak Vo2 observed in our patients, peak Vo2 is often substantially diminished in patients with post-operative tetralogy of Fallot (31). Second, whereas subjects in our series on average had normal values of VE/VCO2 slope, patients with tetralogy of Fallot frequently have elevations of this value (32). In tetralogy patients, these abnormalities are probably the result of ventilation/perfusion mismatch secondary to pulmonary artery stenoses, which may further exacerbate PR and RV volume load during exercise. Such stenoses would be unexpected in the patients in this series, and a more normal VE/VCO2 slope was generally preserved.
In addition to offering insight into the pathophysiology of chronic isolated PR, this study provides important long-term data on the outcomes of balloon pulmonary valvuloplasty, which has become the standard of care for treatment of congenital valvar PS. The exclusion of patients with associated anomalies or significant residual PS limits our ability to generalize these findings to the broader population of patients who undergo BD for PS. Previous studies, however, have shown that residual or recurrent PS is relatively uncommon, and that valvar PS is usually an isolated lesion (33 and 34). The study design also allowed the possibility of recruitment bias, although there is no obvious means by which this would dramatically alter our findings. Thus, although these factors must be taken into account and could in principle distort assessment of prevalence, we anticipate that our findings are generally representative.
The target BAR for patients undergoing balloon pulmonary valvuloplasty, 1.2 to 1.4, was based on experimental studies in which a BAR of 1.5 or higher was associated with a greater risk of damage to the RV outflow tract (35), later substantiated in early human studies (12). In the current study, the finding that significant PR was uncommon in patients dilated with a BAR <1.4 provides support for the appropriateness of 1.2 to 1.4 as a target BAR range. In this cohort, patients with a smaller pulmonary valve z-score were more likely to undergo dilation with a larger BAR, and several patients were treated with a BAR >1.4. In patients with recalcitrant obstruction, the tradeoff between using a larger balloon in an effort to relieve PS compared with the increased risk of complications and PR is important to consider and deserves further study.
Study limitations
The power of our study and strength of its conclusions regarding the relationship between exercise outcomes and PR fraction would have benefited from a larger sample size or a higher burden of PR among enrolled patients. Although an analysis of the effect of pre-intervention pressure load on long-term outcomes was included in this study, the impact of the duration of the load was not specifically assessed, and bears further evaluation. Our findings may be confounded by the coexistence of TR, another cause of RV volume loading, with PR. However, the impact of TR was probably minimal in this cohort, given its low prevalence. The low percentage of eligible patients who enrolled may introduce bias and, as discussed above, complicates accurate estimation of the overall prevalence of PR and RV dilation. However, this factor is unlikely to have an important effect on the study’s primary aims.
Conclusions
Percutaneous pulmonary valvuloplasty yields a substantial long-term prevalence of PR and mild RV dilation. In this series, 34% of patients had a PR fraction >15% and 40% had an RV end-diastolic volume z-score ≥2. Severe PR or RV dilation, however, was very uncommon. Of the 41 patients, only 2 had PR fraction >40% and 1 had RV end-diastolic z-score ≥6. The BAR in each of these cases was >1.4. PR was associated closely with RV dilation and modestly with RV function. No significant relationship was found in linear regression analysis between PR fraction and exercise capacity. The cohort with PR fraction >15%, however, had significantly lower peak Vo2 than the group with PR fraction ≤15%. This finding provides evidence that significant PR, in the absence of confounding cardiac comorbidities, is associated with reduced exercise tolerance.
Acknowledgments
The authors are grateful to Erin Chong for her assistance during the recruitment phase of this project.
Funding was provided by the Ed Marram and Karen Carpenter Fund; by the Faye and Karen Sinclair Research Fund; and the NIH Loan Repayment Program. This work was also supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- BAR
balloo: nannulus ratio
- BD
balloon dilation
- CMR
cardiac magnetic resonance
- EF
ejection fraction
- LV
left ventricle/ventricular
- PR
pulmonary regurgitation
- PS
pulmonary stenosis
- RV
right ventricle/ventricular
- TR
tricuspid regurgitation
- Ve/Vco2
ratio of minute ventilation: carbon dioxide production
- Vo2
oxygen consumption
Footnotes
Dr. Lock acts as an investigator for Medtronic, Inc., and receives royalties on products sold by Cook and NMT. Dr. McElhinney acts in a consultant and investigator role for Medtronic Inc.
References
- 1.Price BO. Isolated incompetence of the pulmonic valve. Circulation. 1961;23:596–602. doi: 10.1161/01.cir.23.4.596. [DOI] [PubMed] [Google Scholar]
- 2.Tanabe Y, Takahashi M, Kuwano H, et al. Long-term fate of isolated congenital absent pulmonary valve. Am Heart J. 1992;124:526–529. doi: 10.1016/0002-8703(92)90627-8. [DOI] [PubMed] [Google Scholar]
- 3.Spigelman, Wright JS. Isolated congenital pulmonary incompetence: case report and literature review. Aust N Z J Surg. 1984;54:177–181. doi: 10.1111/j.1445-2197.1984.tb06710.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimazaki Y, Blackstone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. Thorac Cardiovasc Surg. 1984;32:257–259. doi: 10.1055/s-2007-1023399. [DOI] [PubMed] [Google Scholar]
- 5.Kan JS, White RI, Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982;307:540–542. doi: 10.1056/NEJM198208263070907. [DOI] [PubMed] [Google Scholar]
- 6.Poon LKH, Menahem S. Pulmonary regurgitation after percutaneous balloon valvoplasty for isolated pulmonary valvar stenosis in childhood. Cardiol Young. 2003;13:444–450. [PubMed] [Google Scholar]
- 7.Peterson C, Schilthuis JJ, Dodge-Khatami A, Hitchcock JF, Meijboom EJ, Bennink GB. Comparative long-term results of surgery versus balloon valvuloplasty for pulmonary valve stenosis in infants and children. Ann Thorac Surg. 2003;76:1078–1082. doi: 10.1016/s0003-4975(03)00678-7. [DOI] [PubMed] [Google Scholar]
- 8.Rao PS, Galal O, Patnana M, Buck SH, Wilson AD. Results of three to 10 year follow up of balloon dilatation of the pulmonary valve. Heart. 1998;80:591–595. doi: 10.1136/hrt.80.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garty Y, Veldtman G, Lee K, Benson L. Late outcomes after pulmonary valve balloon dilatation in neonates, infants and children. J Invasive Cardiol. 2005;17:318–322. [PubMed] [Google Scholar]
- 10.Fawzy ME, Hassan W, Fadel BM, et al. Long-term results (up to 17 years) of pulmonary balloon valvuloplasty in adults and its effects on concomitant severe infundibular stenosis and tricuspid regurgitation. Am Heart J. 2007;153:433–438. doi: 10.1016/j.ahj.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Santoro G, Formigari R, Pasquini L, De Zorzi A, Ballerini L. Pulmonary valvuloplasty in childhood: the immediate results and long-term follow-up. G Ital Cardiol. 1995;25:139–147. [PubMed] [Google Scholar]
- 12.Radtke W, Keane JF, Fellows KE, Lang P, Lock JE. Percutaneous balloon valvotomy of congenital pulmonary stenosis using oversized balloons. J Am Coll Cardiol. 1986;8:909–915. doi: 10.1016/s0735-1097(86)80434-x. [DOI] [PubMed] [Google Scholar]
- 13.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 14.Samyn MM, Powell AJ, Garg R, Sena L, Geva T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of Fallot: right ventricular outflow tract patch vs. conduit repair. J Magn Reson Imaging. 2007;26:934–940. doi: 10.1002/jmri.21094. [DOI] [PubMed] [Google Scholar]
- 15.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 16.Rebergen SA, Chin JG, Ottenkamp J, van der Wall EE, de Roos A. Pulmonary regurgitation in the late postoperative follow-up of tetralogy of Fallot: Volumetric quantitation by nuclear magnetic resonance velocity mapping. Circulation. 1993;88:2257–2266. doi: 10.1161/01.cir.88.5.2257. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman K. Principles of Exercise Testing & Interpretation: Including Pathophysiology and Clinical Applications. 3. Lippincott Williams & Wilkins; Philadelphia, PA: 1999. [Google Scholar]
- 18.Cumming GR, Everatt D, Hastman L. Bruce treadmill test in children: normal values in a clinic population. Am J Cardiol. 1978;41:69–75. doi: 10.1016/0002-9149(78)90134-0. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DM, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 20.Jones NL. Clinical Exercise Testing. 4. Saunders; Philadelphia, PA: 1997. [Google Scholar]
- 21.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Heart. 2007;93:1285–1292. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ATS/ACCP. Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 23.Lurz P, Nordmeyer J, Muthurangu V, et al. Comparison of bare metal stenting and percutaneous pulmonary valve implantation for treatment of right ventricular outflow tract obstruction: use of an x-ray/magnetic resonance hybrid laboratory for acute physiological assessment. Circulation. 2009;119:2995–3001. doi: 10.1161/CIRCULATIONAHA.108.836312. [DOI] [PubMed] [Google Scholar]
- 24.Bouzas B, Kilner PJ, Gatzoulis MA. Pulmonary regurgitation: not a benign lesion. Eur Heart J. 2005;26:433–439. doi: 10.1093/eurheartj/ehi091. [DOI] [PubMed] [Google Scholar]
- 25.Marx GR, Hicks RW, Allen HD, Goldberg SJ. Noninvasive assessment of hemodynamic responses to exercise in pulmonary regurgitation after operations to correct pulmonary outflow obstruction. Am J Cardiol. 1988;61:595–601. doi: 10.1016/0002-9149(88)90771-0. [DOI] [PubMed] [Google Scholar]
- 26.Meadows J, Powell AJ, Geva T, Dorfman A, Gauvreau K, Rhodes J. Cardiac magnetic resonance imaging correlates of exercise capacity in patients with surgically repaired tetralogy of Fallot. Am J Cardiol. 2007;100:1446–1450. doi: 10.1016/j.amjcard.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 27.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghez O, Tsang VT, Frigiola A, et al. Right ventricular outflow tract reconstruction for pulmonary regurgitation after repair of tetralogy of Fallot: Preliminary results. Eur J Cardiothorac Surg. 2007;31:654–658. doi: 10.1016/j.ejcts.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–1675. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 30.Warner KG, O’Brien PK, Rhodes J, Kaur A, Robinson DA, Payne DD. Expanding the indications for pulmonary valve replacement after repair of tetralogy of Fallot. Ann Thorac Surg. 2003;76:1066–1071. doi: 10.1016/s0003-4975(03)00748-3. [DOI] [PubMed] [Google Scholar]
- 31.Wessel HU, Cunningham WJ, Paul MH, Bastanier CK, Muster AJ, Idriss FS. Exercise performance in tetralogy of Fallot after intracardiac repair. J Thorac Cardiovasc Surg. 1980;80:582–593. [PubMed] [Google Scholar]
- 32.Sutton NJ, Peng L, Lock JE, et al. Effect of pulmonary artery angioplasty on exercise function after repair of tetralogy of Fallot. Am Heart J. 2008;155:182–186. doi: 10.1016/j.ahj.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 33.McCrindle BW. Independent predictors of long-term results after balloon pulmonary valvuloplasty: Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators. Circulation. 1994;89:1751–1759. doi: 10.1161/01.cir.89.4.1751. [DOI] [PubMed] [Google Scholar]
- 34.Rao PS. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc Interv. 2007;69:747–763. doi: 10.1002/ccd.20982. [DOI] [PubMed] [Google Scholar]
- 35.Ring JC, Kulik TJ, Burke BA, Lock JE. Morphologic changes induced by dilation of the pulmonary valve annulus with overlarge balloons in normal newborn lambs. Am J Cardiol. 1985;55:210–214. doi: 10.1016/0002-9149(85)90330-3. [DOI] [PubMed] [Google Scholar]


