Abstract
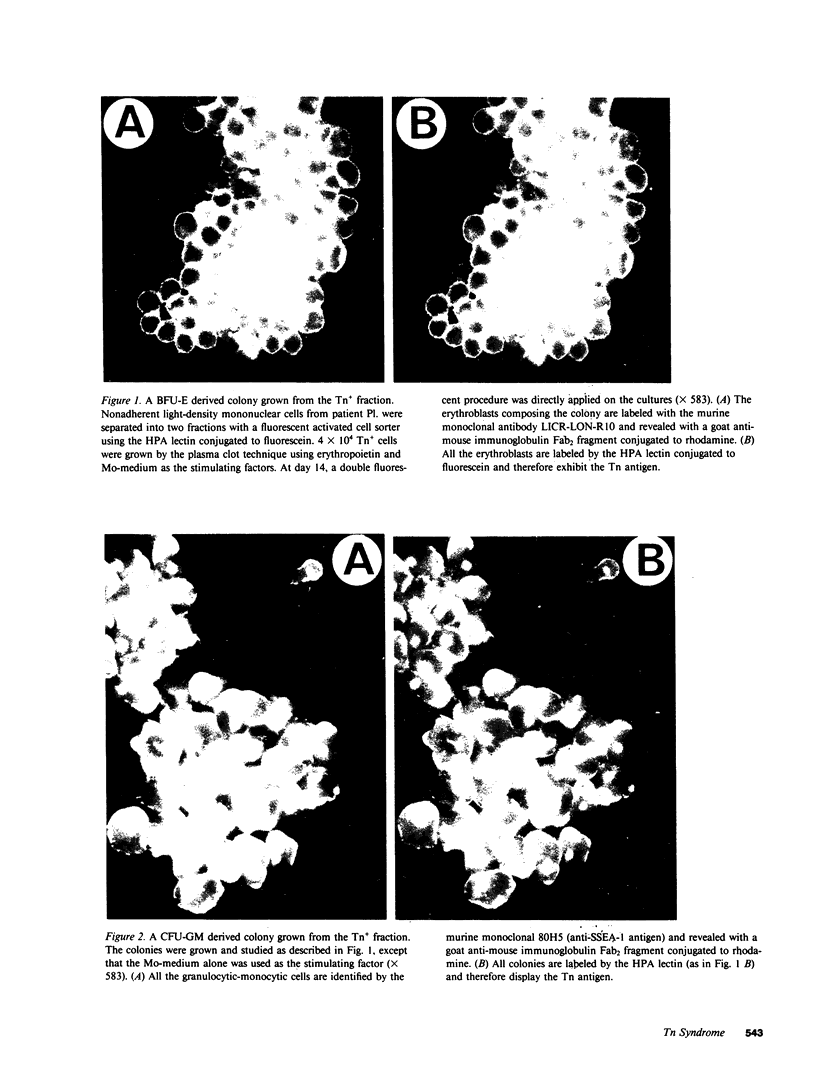
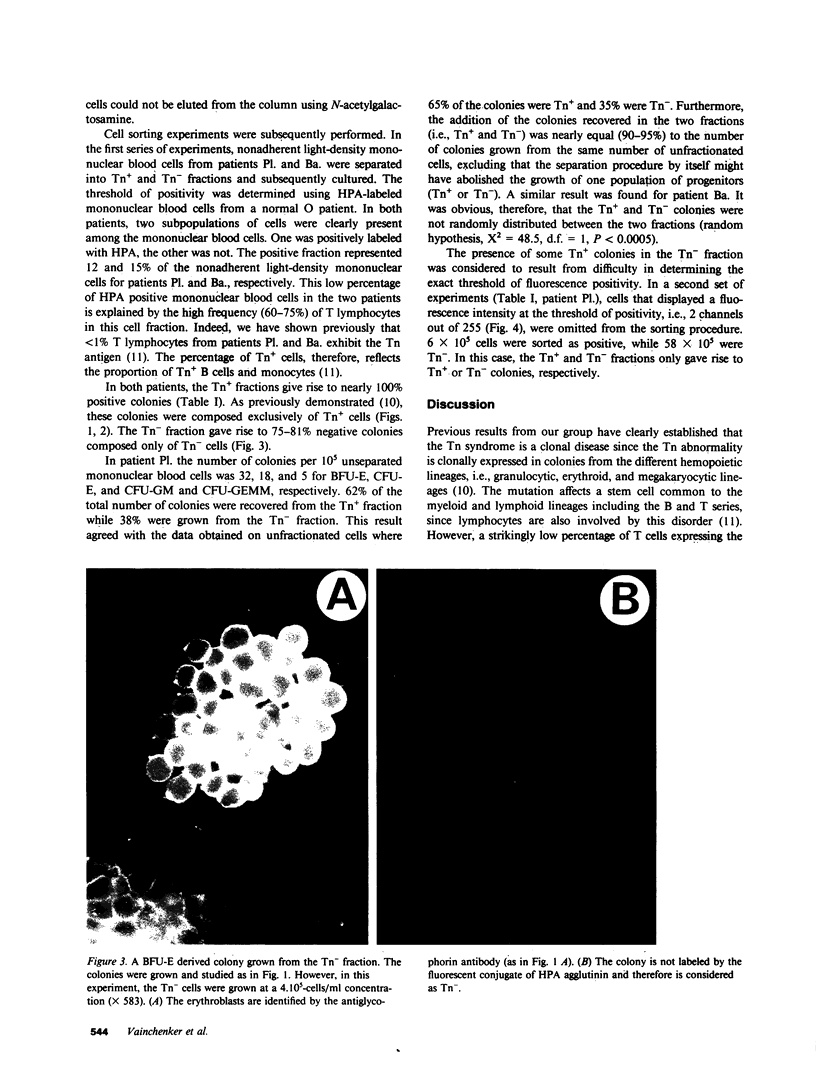
The Tn syndrome is an acquired clonal disorder characterized by the exposure of a normally hidden determinant, the Tn antigen, on the surface of human erythrocytes, platelets, granulocytes, and lymphocytes. Two distinct populations, Tn positive (Tn+) and Tn negative (Tn-), of mature hemopoietic cells are present in Tn patients. To determine whether the Tn antigen is already expressed on erythroid, myeloid, and pluripotent progenitors, light-density mononuclear blood cells from two patients with this syndrome were separated by fluorescent-activated cell sorting and by affinity chromatography into Tn+ and Tn- fractions, using their binding properties to Helix pomatia agglutinin (HPA). Burst-forming-unit erythroid (BFU-E), colony-forming-unit granulocyte/macrophage (CFU-GM), cells were assayed in plasma clot cultures. After 12-14 d of culture, colonies were studied by a double fluorescent labeling procedure. First, a fluorescein-conjugated HPA permitted evaluation of the presence or absence of the Tn antigen at the surface of the cells composing each colony, and second, the binding of a murine monoclonal antibody against either glycophorin A (LICR-LON-R10) or against a myeloid antigen (80H5), revealed by an indirect fluorescent procedure, was used to establish the erythroid or myeloid origin of each cell. The Tn+ fraction obtained by cell sorting gave rise to nearly 100% Tn+ colonies composed exclusively of cells bearing this antigen. The reverse was observed for the Tn- cell fraction. These results demonstrate that in the Tn syndrome, BFU-E, CFU-GM, and CFU-GEMM of the Tn+ clone express the Tn antigen at this early stage of differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aster R. H., Enright S. E. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: usefulness for the detection of platelet antibodies. J Clin Invest. 1969 Jul;48(7):1199–1210. doi: 10.1172/JCI106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin M. L., Barrasso C., Ridolfi R. L. Tn-polyagglutinability associated with acute myelomonocytic leukemia. Am J Clin Pathol. 1979 Dec;72(6):1024–1027. doi: 10.1093/ajcp/72.6.1024. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Shinton N. K., Wingham J. Persistent mixed-field polyagglutination. Br J Haematol. 1971 Oct;21(4):443–453. doi: 10.1111/j.1365-2141.1971.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Wingham J., Pippard M. J., Hoult J. G., Melikian V. Erythrocyte membrane modification in malignant diseases of myeloid and lymphoreticular tissues. I. Tn-polyagglutination in acute myelocytic leukaemia. Br J Haematol. 1976 Jun;33(2):289–294. doi: 10.1111/j.1365-2141.1976.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Wingham J. The M, N and NVg receptors of Tn-erythrocytes. Vox Sang. 1974 Feb;26(2):171–175. doi: 10.1111/j.1423-0410.1974.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Brouet J. C., Vainchenker W., Blanchard D., Testa U., Cartron J. P. The origin of human B and T cells from multipotent stem cells: a study of the Tn syndrome. Eur J Immunol. 1983 Apr;13(4):350–352. doi: 10.1002/eji.1830130416. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Andreu G., Cartron J., Bird G. W., Salmon C., Gerbal A. Demonstration of T-transferase deficiency in Tn-polyagglutinable blood samples. Eur J Biochem. 1978 Dec 1;92(1):111–119. doi: 10.1111/j.1432-1033.1978.tb12728.x. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Cartron J., Andreu G., Salmon C., Bird G. W. Selective deficiency of 3-beta-d-galactosyltransferase (T-transferase) in Tn-polyagglutinable erythrocytes. Lancet. 1978 Apr 22;1(8069):856–857. doi: 10.1016/s0140-6736(78)90197-6. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Nurden A. T. Galactosyltransferase and membrane glycoprotein abnormality in human platelets from Tn-syndrome donors. Nature. 1979 Dec 6;282(5739):621–623. doi: 10.1038/282621a0. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Bird G. W. Cryptic A-like receptor sites in human erythrocyte glycoproteins: proposed nature of Tn-antigen. Vox Sang. 1974;27(1):29–42. doi: 10.1111/j.1423-0410.1974.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Dessypris E. N., Clark D. A., McKee L. C., Jr, Krantz S. B. Increased sensitivity to complement or erythroid and myeloid progenitors in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1983 Sep 22;309(12):690–693. doi: 10.1056/NEJM198309223091202. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Monoclonal antibodies that bind to the human erythrocyte-membrane glycoproteins glycophorin A and Band 3 [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):334–335. doi: 10.1042/bst0080334. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Quan S. G., Lusis A. J. Production of erythroid-potentiating activity by a human T-lymphoblast cell line. Proc Natl Acad Sci U S A. 1980 Jan;77(1):593–596. doi: 10.1073/pnas.77.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi H. C., Thorpe S. J., Hounsell E. F., Rumpold H., Kraft D., Förster O., Feizi T. Marker of peripheral blood granulocytes and monocytes of man recognized by two monoclonal antibodies VEP8 and VEP9 involves the trisaccharide 3-fucosyl-N-acetyllactosamine. Eur J Immunol. 1983 Apr;13(4):306–312. doi: 10.1002/eji.1830130407. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Murphy L. A., Goldstein I. J., Etzler M. E. Carbohydrate binding specificity of four N-acetyl-D-galactosamine- "specific" lectins: Helix pomatia A hemagglutinin, soy bean agglutinin, lima bean lectin, and Dolichos biflorus lectin. Biochemistry. 1977 Jun 14;16(12):2750–2755. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- Mannoni P., Janowska-Wieczorek A., Turner A. R., McGann L., Turc J. M. Monoclonal antibodies against human granulocytes and myeloid differentiation antigens. Hum Immunol. 1982 Dec;5(4):309–323. doi: 10.1016/0198-8859(82)90022-2. [DOI] [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni S. B., Osunkoya B. O., Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970 Aug;36(2):145–152. [PubMed] [Google Scholar]
- Powell J. S., Fialkow P. J., Adamson J. W. Polycythemia vera: studies of hemopoiesis in continuous long-term culture of human marrow. J Cell Physiol Suppl. 1982;1:79–85. doi: 10.1002/jcp.1041130413. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieff C., Bicknell D., Caine G., Robinson J., Lam G., Greaves M. F. Changes in cell surface antigen expression during hemopoietic differentiation. Blood. 1982 Sep;60(3):703–713. [PubMed] [Google Scholar]
- Stern M., Rosse W. F. Two populations of granulocytes in paroxysmal nocturnal hemoglobinuria. Blood. 1979 May;53(5):928–934. [PubMed] [Google Scholar]
- Vainchenker W., Testa U., Deschamps J. F., Henri A., Titeux M., Breton-Gorius J., Rochant H., Lee D., Cartron J. P. Clonal expression of the Tn antigen in erythroid and granulocyte colonies and its application to determination of the clonality of the human megakaryocyte colony assay. J Clin Invest. 1982 May;69(5):1081–1091. doi: 10.1172/JCI110543. [DOI] [PMC free article] [PubMed] [Google Scholar]