Abstract
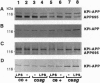
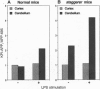
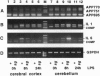
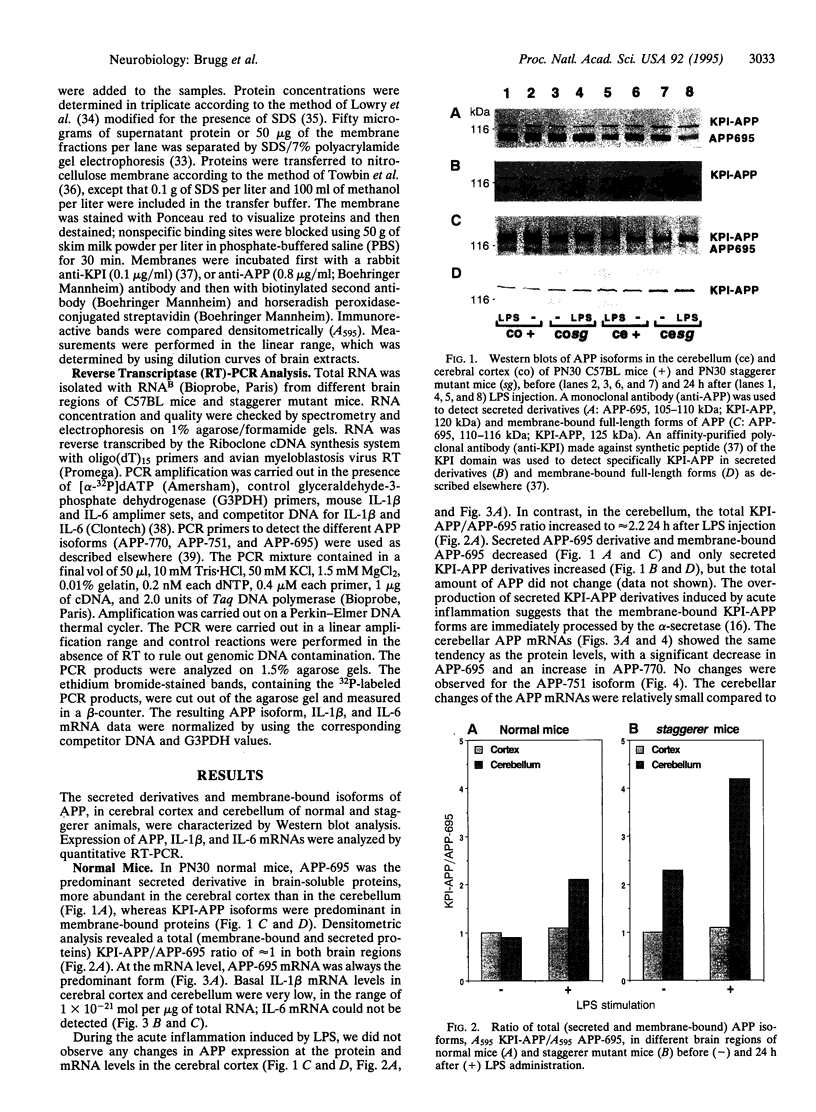
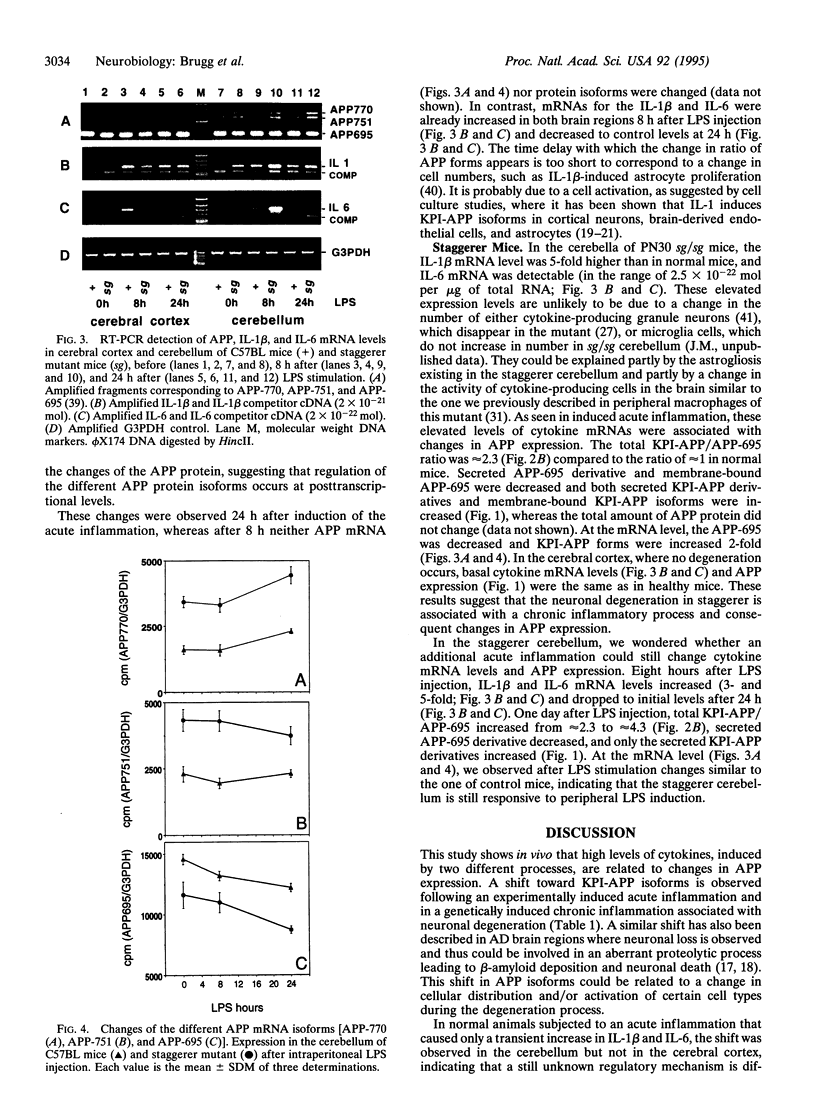
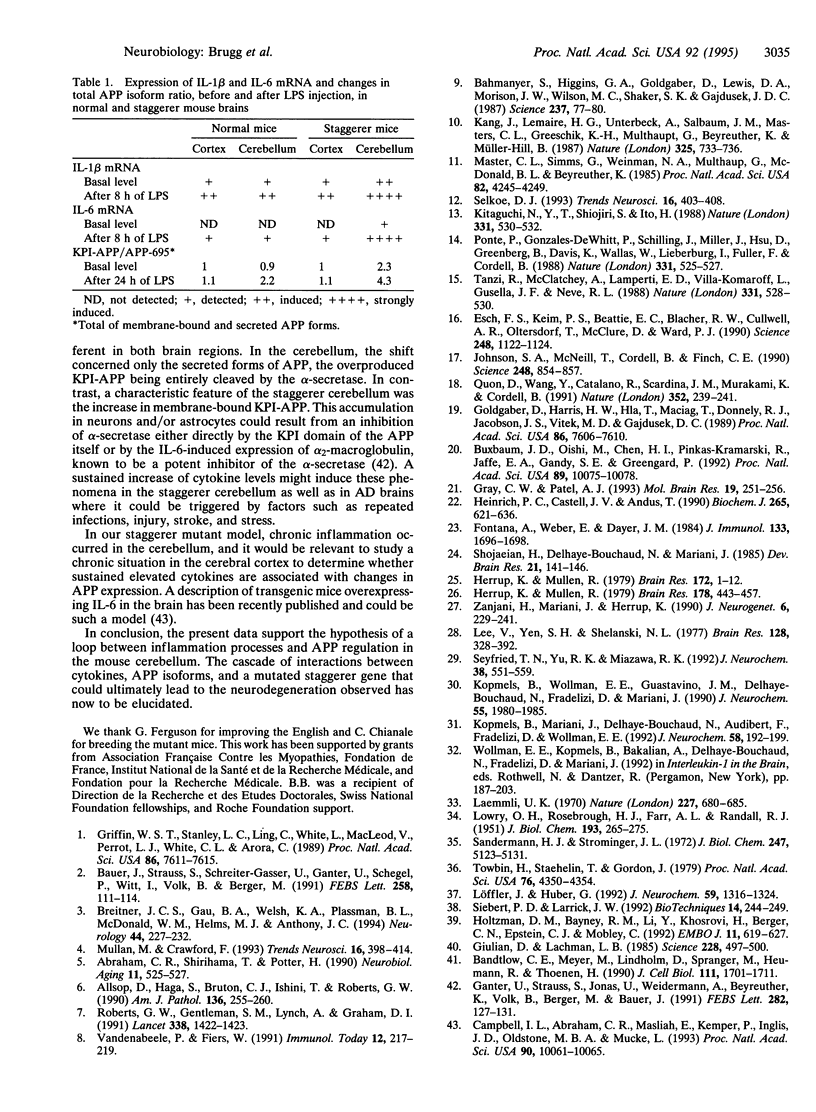
In Alzheimer disease, a combination of genetic predisposition and environmental factors may contribute to changes in beta-amyloid precursor protein (APP) expression, beta-amyloid peptide deposition, and neuronal loss. Factors such as head injury or acute infection that trigger inflammatory processes may play a crucial role in development of the disease. In the present in vivo study, we showed that, in mouse brain, peripheral stimulation with lipopolysaccharide (LPS) induced a transient increase in the inflammatory cytokine mRNAs (interleukin 1 beta and interleukin 6), followed by changes in expression of APP isoforms in the cerebellum but not in the cerebral cortex. These changes consisted of a decrease in the APP-695 and an increase in the Kunitz protease inhibitor-bearing isoforms (KPI-APP). In the cerebellum of the staggerer mouse mutant, where a severe loss of Purkinje and granule cells occurs, basal mRNA levels of these interleukins were elevated and an increase in the KPI-APP/APP-695 ratio compared to wild-type mice was observed. These abnormalities were further accentuated by LPS stimulation. This study shows that acute and chronic inflammatory processes play an important role in changes in APP expression possibly associated with neurodegeneration.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Haga S., Bruton C., Ishii T., Roberts G. W. Neurofibrillary tangles in some cases of dementia pugilistica share antigens with amyloid beta-protein of Alzheimer's disease. Am J Pathol. 1990 Feb;136(2):255–260. [PMC free article] [PubMed] [Google Scholar]
- Bahmanyar S., Higgins G. A., Goldgaber D., Lewis D. A., Morrison J. H., Wilson M. C., Shankar S. K., Gajdusek D. C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer's disease. Science. 1987 Jul 3;237(4810):77–80. doi: 10.1126/science.3299701. [DOI] [PubMed] [Google Scholar]
- Bandtlow C. E., Meyer M., Lindholm D., Spranger M., Heumann R., Thoenen H. Regional and cellular codistribution of interleukin 1 beta and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. J Cell Biol. 1990 Oct;111(4):1701–1711. doi: 10.1083/jcb.111.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Strauss S., Schreiter-Gasser U., Ganter U., Schlegel P., Witt I., Yolk B., Berger M. Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer's disease cortices. FEBS Lett. 1991 Jul 8;285(1):111–114. doi: 10.1016/0014-5793(91)80737-n. [DOI] [PubMed] [Google Scholar]
- Breitner J. C., Gau B. A., Welsh K. A., Plassman B. L., McDonald W. M., Helms M. J., Anthony J. C. Inverse association of anti-inflammatory treatments and Alzheimer's disease: initial results of a co-twin control study. Neurology. 1994 Feb;44(2):227–232. doi: 10.1212/wnl.44.2.227. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Oishi M., Chen H. I., Pinkas-Kramarski R., Jaffe E. A., Gandy S. E., Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Abraham C. R., Masliah E., Kemper P., Inglis J. D., Oldstone M. B., Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Fontana A., Weber E., Dayer J. M. Synthesis of interleukin 1/endogenous pyrogen in the brain of endotoxin-treated mice: a step in fever induction? J Immunol. 1984 Oct;133(4):1696–1698. [PubMed] [Google Scholar]
- Ganter U., Strauss S., Jonas U., Weidemann A., Beyreuther K., Volk B., Berger M., Bauer J. Alpha 2-macroglobulin synthesis in interleukin-6-stimulated human neuronal (SH-SY5Y neuroblastoma) cells. Potential significance for the processing of Alzheimer beta-amyloid precursor protein. FEBS Lett. 1991 Apr 22;282(1):127–131. doi: 10.1016/0014-5793(91)80460-k. [DOI] [PubMed] [Google Scholar]
- Giulian D., Lachman L. B. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985 Apr 26;228(4698):497–499. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Harris H. W., Hla T., Maciag T., Donnelly R. J., Jacobsen J. S., Vitek M. P., Gajdusek D. C. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. W., Patel A. J. Regulation of beta-amyloid precursor protein isoform mRNAs by transforming growth factor-beta 1 and interleukin-1 beta in astrocytes. Brain Res Mol Brain Res. 1993 Aug;19(3):251–256. doi: 10.1016/0169-328x(93)90037-p. [DOI] [PubMed] [Google Scholar]
- Griffin W. S., Stanley L. C., Ling C., White L., MacLeod V., Perrot L. J., White C. L., 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K., Mullen R. J. Regional variation and absence of large neurons in the cerebellum of the staggerer mouse. Brain Res. 1979 Aug 17;172(1):1–12. doi: 10.1016/0006-8993(79)90891-6. [DOI] [PubMed] [Google Scholar]
- Herrup K., Mullen R. J. Staggerer chimeras: intrinsic nature of Purkinje cell defects and implications for normal cerebellar development. Brain Res. 1979 Dec 14;178(2-3):443–457. doi: 10.1016/0006-8993(79)90705-4. [DOI] [PubMed] [Google Scholar]
- Holtzman D. M., Bayney R. M., Li Y. W., Khosrovi H., Berger C. N., Epstein C. J., Mobley W. C. Dysregulation of gene expression in mouse trisomy 16, an animal model of Down syndrome. EMBO J. 1992 Feb;11(2):619–627. doi: 10.1002/j.1460-2075.1992.tb05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. A., McNeill T., Cordell B., Finch C. E. Relation of neuronal APP-751/APP-695 mRNA ratio and neuritic plaque density in Alzheimer's disease. Science. 1990 May 18;248(4957):854–857. doi: 10.1126/science.2111579. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Kopmels B., Mariani J., Delhaye-Bouchaud N., Audibert F., Fradelizi D., Wollman E. E. Evidence for a hyperexcitability state of staggerer mutant mice macrophages. J Neurochem. 1992 Jan;58(1):192–199. doi: 10.1111/j.1471-4159.1992.tb09295.x. [DOI] [PubMed] [Google Scholar]
- Kopmels B., Wollman E. E., Guastavino J. M., Delhaye-Bouchaud N., Fradelizi D., Mariani J. Interleukin-1 hyperproduction by in vitro activated peripheral macrophages from cerebellar mutant mice. J Neurochem. 1990 Dec;55(6):1980–1985. doi: 10.1111/j.1471-4159.1990.tb05785.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee V., Yen S. H., Shelanski M. L. Biochemical correlates of astrocytic proliferation in the mutant Staggerer mouse. Brain Res. 1977 Jun 10;128(2):389–392. doi: 10.1016/0006-8993(77)91007-1. [DOI] [PubMed] [Google Scholar]
- Löffler J., Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J Neurochem. 1992 Oct;59(4):1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M., Crawford F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):398–403. doi: 10.1016/0166-2236(93)90007-9. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Quon D., Wang Y., Catalano R., Scardina J. M., Murakami K., Cordell B. Formation of beta-amyloid protein deposits in brains of transgenic mice. Nature. 1991 Jul 18;352(6332):239–241. doi: 10.1038/352239a0. [DOI] [PubMed] [Google Scholar]
- Roberts G. W., Gentleman S. M., Lynch A., Graham D. I. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991 Dec 7;338(8780):1422–1423. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Selkoe D. J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Yu R. K., Miyazawa N. Differential cellular enrichment of gangliosides in the mouse cerebellum: analysis using neurological mutants. J Neurochem. 1982 Feb;38(2):551–559. doi: 10.1111/j.1471-4159.1982.tb08662.x. [DOI] [PubMed] [Google Scholar]
- Shojaeian H., Delhaye-Bouchaud N., Mariani J. Decreased number of cells in the inferior olivary nucleus of the developing staggerer mouse. Brain Res. 1985 Jul;353(1):141–146. doi: 10.1016/0165-3806(85)90033-1. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. PCR MIMICS: competitive DNA fragments for use as internal standards in quantitative PCR. Biotechniques. 1993 Feb;14(2):244–249. [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P., Fiers W. Is amyloidogenesis during Alzheimer's disease due to an IL-1-/IL-6-mediated 'acute phase response' in the brain? Immunol Today. 1991 Jul;12(7):217–219. doi: 10.1016/0167-5699(91)90032-O. [DOI] [PubMed] [Google Scholar]
- Zanjani H. S., Mariani J., Herrup K. Cell loss in the inferior olive of the staggerer mutant mouse is an indirect effect of the gene. J Neurogenet. 1990 Aug;6(4):229–241. doi: 10.3109/01677069009107113. [DOI] [PubMed] [Google Scholar]