Abstract
Objectives:
Age-related arterial stiffening and reduction of arterial elasticity are attenuated in individuals with high levels of cardiorespiratory fitness. Viscosity is another mechanical characteristic of the arterial wall; however, the effects of age and cardiorespiratory fitness have not been determined. We examined the associations among age, cardiorespiratory fitness and carotid arterial wall viscosity.
Methods:
A total of 111 healthy men, aged 25–39 years (young) and 40–64 years (middle-aged), were divided into either cardiorespiratory fit or unfit groups on the basis of peak oxygen uptake. The common carotid artery was measured noninvasively by tonometry and automatic tracking of B-mode images to obtain instantaneous pressure and diameter hysteresis loops, and we calculated the effective compliance, isobaric compliance and viscosity index.
Results:
In the middle-aged men, the viscosity index was larger in the unfit group than in the fit group (2533 vs. 2018 mmHg·s/mm, respectively: P < 0.05), but this was not the case in the young men. In addition, effective and isobaric compliance were increased, and viscosity index was increased with advancing age, but these parameters were unaffected by cardiorespiratory fitness level.
Conclusion:
These results suggest that the wall viscosity in the central artery is increased with advancing age and that the age-associated increase in wall viscosity may be attenuated in cardiorespiratory fit men.
Keywords: ageing, arterial compliance, arterial viscoelasticity, cardiopulmonary fitness, exercise
INTRODUCTION
The central elastic arteries are viscoelastic tubes, the principal functions of which are to act as conduits and to buffer flow pulsatile energy imposed by cardiac contractions. Although purely elastic materials permit all the stored energy to be restored during the unloading phase, arteries are not purely elastic and exhibit marked viscous behaviour. Arterial wall viscosity is a source of energy dissipation, considering viscosity as an energy-dissipating phenomenon during mechanical transduction (conversion of cardiac pulsatile energy into arterial elastic energy) [1–4]. Therefore, the arterial mechanical characteristics include both elastic and viscous properties. However, arterial elastic properties, such as dynamic arterial compliance, beta-stiffness and pulse wave velocity, are often used to assess vascular health (i.e. the effects of ageing, cardiovascular diseases, habitual exercise, cardiorespiratory fitness status and so on) [5–8], and viscous properties have been considered less important in the assessment of vascular function.
Although arterial stiffening develops with advancing age [7], left ventricular contraction remains unchanged [9], which suggests that stiffening arteries with reduced elasticity cannot flexibly receive the steady pulsatile energy imposed by cardiac contractions. Thereby, in arteries stiffening with advancing age, because pulsatile energy produced by cardiac contraction is converted to less elastic energy, energy dissipation during mechanical transduction may be elevated with ageing. Furthermore, age-related arterial stiffening is attenuated in individuals with high cardiorespiratory fitness [7,8]. This suggests that inefficient elastic contraction in the vessel wall occurring with advancing age is attenuated in cardiorespiratory fit individuals. Hence, when efficient elastic contraction in the arterial wall is maintained by good cardiorespiratory fitness, the age-related increase in energy dissipation may also be attenuated.
Previous studies indicated that wall viscosity in the central artery is positively associated with sympathetic-adrenergic tone [10] and intima–media thickness (IMT) [11]. Although sympathetic nerve activity and IMT develop with advancing age, these age-related increases are attenuated by higher cardiorespiratory fitness level [12,13]. Accordingly, we hypothesized that wall viscosity may be increased with advancing age, and the age-related increase in wall viscosity may be attenuated in cardiorespiratory fit individuals. To test this hypothesis, we determined the associations among age, cardiorespiratory fitness and carotid wall viscosity using a cross-sectional study design.
MATERIALS AND METHODS
Individuals and ethical approval
A total of 111 healthy men aged 25–39 years (young) and aged 40–64 years (middle-aged) participated in the present study (Table 1). The individuals were recruited from the local community around the National Institute of Health Nutrition. None of the individuals were regularly engaged in endurance and/or weight training exercise. Individuals who were taking medications, had ever used anabolic steroids or who had significant carotid intima–media thickening (<1.1 mm), plaque formation and/or other characteristics of atherosclerosis [ankle-brachial index (ABI) <0.9] were excluded from the study. The purpose, procedures and risks of the study were explained to each individual, all of whom provided written informed consent before participating in the study, which was approved by the Human Research Committee of the National Institute of Health and Nutrition. This study was carried out in accordance with the guidelines of the Declaration of Helsinki.
TABLE 1.
Individual characteristics
| Young | Middle-aged | ||||||
| Fit | Unfit | Fit | Unfit | Age effect | Fitness effect | Age × Fitness | |
| n | 18 | 22 | 34 | 37 | |||
| Age (years) | 32.3 ± 0.8 | 34.7 ± 0.7 | 50.7 ± 1.3 | 52.2 ± 1.3 | <0.0001 | NS | NS |
| Height (cm) | 171.8 ± 1.0 | 173.7 ± 1.0 | 168.9 ± 1.1 | 169.2 ± 1.2 | <0.01 | NS | NS |
| Body weight (kg) | 70.2 ± 2.2 | 71.1 ± 1.7 | 66.4 ± 1.5 | 68.6 ± 1.3 | NS | NS | NS |
| BMI (kg/m2) | 23.8 ± 0.7 | 23.6 ± 0.5 | 23.2 ± 0.4 | 23.9 ± 0.3 | NS | NS | NS |
| %Fat (%) | 19.3 ± 1.5 | 19.6 ± 0.8 | 19.2 ± 0.6 | 22.2 ± 0.6 | NS | <0.05 | NS |
| Total cholesterol (mg/dl) | 183 ± 6 | 192 ± 7 | 202 ± 4 | 202 ± 5 | <0.05 | NS | NS |
| HDL cholesterol (mg/dl) | 54 ± 3 | 52 ± 2 | 61 ± 2 | 52 ± 2 | NS | NS | NS |
| Plasma glucose (mg/dl) | 90 ± 2 | 89 ± 1 | 91 ± 2 | 93 ± 1 | NS | NS | NS |
| Triglycerides (mg/dl) | 86 ± 11 | 105 ± 13 | 103 ± 7 | 120 ± 8 | NS | NS | NS |
| Peak heart rate (bpm) | 193 ± 3 | 187 ± 2 | 178 ± 2 | 173 ± 2 | <0.0001 | <0.05 | NS |
| VO2 peak (l/min) | 3.00 ± 0.11 | 2.42 ± 0.09 | 2.56 ± 0.08 | 1.97 ± 0.05 | <0.0001 | <0.0001 | NS |
| VO2peak/body weight (ml/kg per min) | 42.9 ± 1.2 | 33.9 ± 0.7 | 38.6 ± 0.9 | 28.7 ± 0.5 | <0.0001 | <0.0001 | NS |
Data are means ± SEM. HDL, high-density lipoprotein; n, no. of individuals; VO2peak, peak oxygen uptake.
To assess the effects of cardiorespiratory fitness on viscoelasticity in the common carotid artery, the individuals were divided into either cardiorespiratory fit or unfit groups on the basis of the mean value of peak oxygen uptake (VO2peak) every 10 years of age.
The individuals abstained from caffeine and fasted for at least 4 h (12 h overnight fast was used to determine carotid arterial viscoelasticity, compliance and stiffness) before they were tested. The individuals also abstained from heavy exercise for at least 24 h to avoid the immediate (acute) effects of exercise. All measurements were carried out under comfortable laboratory conditions between 0900 and 1200 h. The fitness assessment was performed after the other tests.
Carotid arterial diameter waveform
Carotid arterial diameter waveform was measured using a B-mode ultrasound device (Vivid i; GE Medical Systems, Milwaukee, Wisconsin, USA) equipped with a 7.5-MHz probe. A longitudinal image of the cephalic portion of the right common carotid artery was acquired 1–2 cm proximal to the carotid bulb in the supine position. Sequences of images from at least 25 cardiac cycles were obtained to determine the instantaneous carotid arterial diameter waveform. ECG was synchronized with the sequence of carotid arterial images to average carotid diameter waveforms and to determine hysteresis loop diameter-pressure in the carotid artery. Images of the carotid artery acquired at 78.3 Hz were transferred to a personal computer, digitized into 636 × 434 pixels with 256 levels of gray and analysed off-line with auto-tracking system software (MoveTr2D; Library Corporation, Tokyo, Japan). The pattern matching system of this analysis software was used to track the edges of far and near walls in the carotid artery in a frame-to-frame manner. The files containing selected digitized sequence of images in the carotid artery were imported into this software. Each sequential digitized image was individually examined by software, and the subsequent measurements were stored in a data file that could be imported into spreadsheets, such as Microsoft Excel, for later analysis. First, the centres of square markers were set manually on the far (blood-intima interface; >50 points) and near (media-adventitia interface; >50 points) vessel walls in these images. Each pair of markers was placed vertically to the vessel wall. At least 50 diameters per image were stored in the virtual memory system of this software. The markers can be identified using an edge detection technique on the basis of determination of a threshold in the optical field. This threshold can be set manually or automatically, and the pixels with values exceeding this threshold are regarded as the foreground and the other pixels are regarded as the background. The threshold can be set to 8-bit gray field for digital images. Every group of connected pixels, the values of which are within the foreground, make up the identified edge and are regarded as the target, and the coordinates of the wall edge of each target can be obtained. The two-dimensional movement of each target can be measured automatically by this software. That is, arterial diameter was identified by the distance between central points of square markers on the far and near walls of each central point partner. This automatic method for calculating arterial diameter was strongly correlated with the traditional method [14] (diastole r = 0.917 and systole r = 0.906).
Intima–media thickness
Carotid IMT was measured from the images obtained using a B-mode ultrasound device (Vivid i; GE Medical Systems). Ultrasound images were analysed using image analysis software (ImageJ 1.42; NIH, Bethesda, Maryland, USA). At least 10 measurements of IMT were taken at each segment, and the mean values were used for analysis, as described previously [15].
Carotid arterial pressure waveform
The carotid arterial pressure wave was measured at the same site as the diameter wave but after echographic recording, using a multielement tonometry sensor, consisting of 15 pressure-sensitive small elements aligned side by side, which was coupled to the device (Colin PWV/ABI; Colin Medical Technology, Komaki, Japan). The carotid tonometry sensor is compact and lightweight, and can be easily attached around the neck. The sensor elements located manually at the centre of the carotid artery can be identified by screening the pulse pressure levels of the 15 elements, provided that the sensor element size is sufficiently small compared with the vessel diameter. The quality of the carotid pulse wave and the downward force were checked visually by carotid compression tonography. To average at least 25 cardiac cycles and to determine hysteresis loop diameter-pressure in the carotid artery, carotid arterial pressure waveform and ECG were recorded simultaneously in the supine position. Both carotid arterial pressure and ECG waveform were sampled at 1000 Hz by connecting each device to a personal computer using an analog/digital converter (PowerLab; AD Instruments, Bella Vista, New South Wales, Australia). As the baseline levels of carotid blood pressure are subjected to hold-down force, the pressure signal obtained by tonometry was calibrated by equating the carotid mean and DBP to the brachial artery values [16].
Arterial blood pressure at rest
Chronic levels of arterial blood pressure at rest were measured with a semi-automated device (Form PWV/ABI; Colin Medical Technology) over the brachial and dorsalis pedis artery. Recordings were made in triplicate with individuals in the supine position as described previously [17].
Blood samples
Blood samples were taken after an overnight fast of at least 10 h to determine fasting glucose levels and fasting total cholesterol, high-density lipoprotein (HDL) cholesterol and triglyceride levels from serum samples with enzymatic techniques [7,18].
Cardiorespiratory fitness
Cardiorespiratory fitness, assessed from VO2peak, was measured by incremental cycle exercise test using a cycle ergometer (Ergomedic 828E Test Cycle; Monark, Varberg, Sweden). The incremental cycle exercise began at a work rate of 30 or 60 W for women and 60, 90 or 120 W for men (60 rpm), and power output was increased by 15 W/min until the individuals could not maintain the fixed pedalling frequency. The individuals were encouraged during the ergometer test to exercise at the level of maximum intensity. The heart rate and rating of perceived exertion (RPE) were monitored minute by minute during exercise. VO2 was monitored during the last 30 s of each increase in work rate after the RPE reached 18. RPE was obtained using a modified Borg scale [19]. VO2 was measured by the Douglas bag method. The highest value of VO2 during the exercise test was designated as VO2peak. As the test requires incremental cycle exercise to exhaustion, individuals were allowed to determine whether they were willing to participate in the test [18,20].
Body composition
Body composition was determined by dual-energy X-ray absorptiometry (DEXA) (model DPX-IQ; Lunar Radiation, Madison, Wisconsin, USA) with the individuals in the supine position. Measurement of fat mass using DEXA has been well validated against other standards [21].
Pressure-dependent analysis of diameter, compliance and stiffness
As described above, during both carotid arterial diameter and pressure measurements, the spikes corresponding to the R-wave of the ECG were acquired and stored along with the diameter and pressure signals. A computerized procedure was used to determine the pressure–diameter hysteresis loop [22,23] and to calculate the purely elastic pressure–diameter relationship using an original system called Kaiseki KENTA-KUN (Fig. 1, Software: Visual Basic). Both pressure and diameter waveforms were identified according to the R-wave of the ECG. Each cardiac cycle, both for pressure and diameter, was interpolated in time to obtain the same number of data points. Each cardiac cycle from the pressure and diameter signals (a total of at least 25 beats) was resampled to 256 samples per beat, and then the average pressure and diameter beat were calculated. The resampling procedure was carried out by interpolation using the time array as input, with the original number of samples corresponding to pressure or diameter. The output was a new time alignment containing 256 samples with the same time period. The pressure–diameter hysteresis loop was then obtained by x–y composition of pressure and diameter waveforms. When the pressure–diameter hysteresis loop was analysed, we synchronized the foot (end diastole) of pressure vs. time with that of diameter vs. time. Such a loop involved elastic and viscous components in its area. To obtain the purely elastic pressure–diameter relationship, we transformed real pressure (Preal) into elastic pressure (Pelastic) using a first-order differential equation [24], which characterizes the viscoelastic behaviour of the arterial wall. 
FIGURE 1.
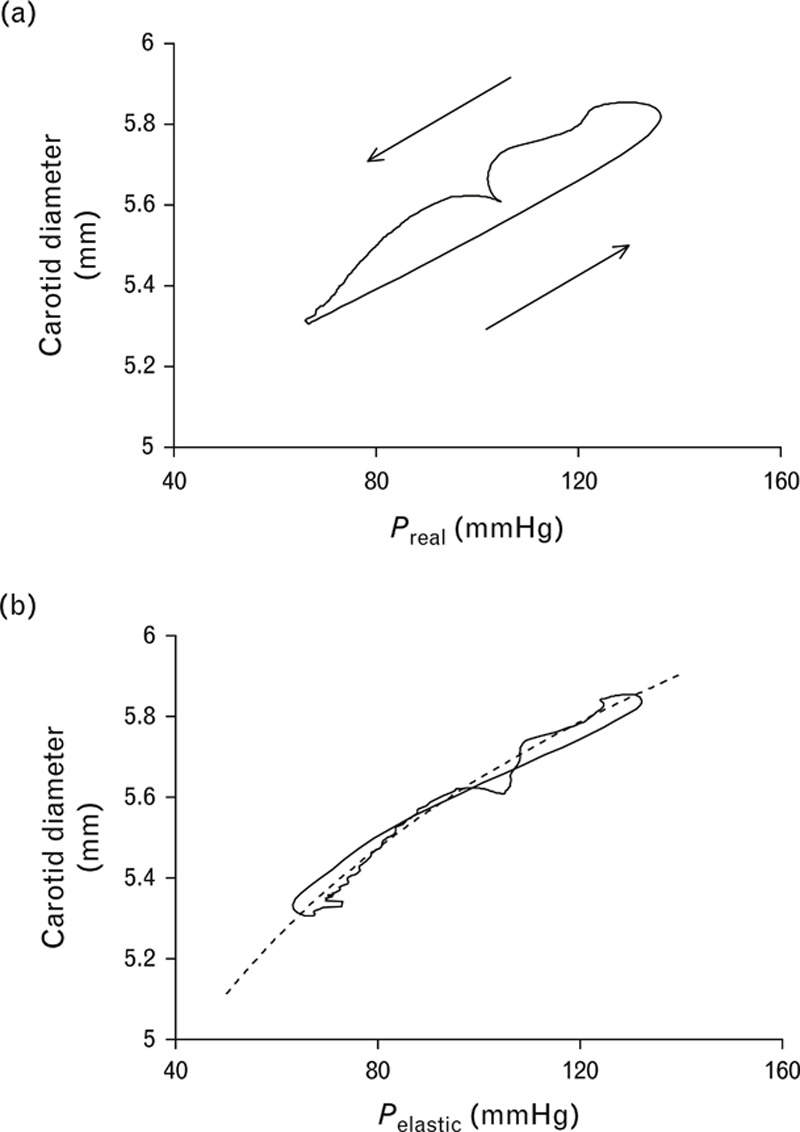
Procedure for calculation of sampled carotid arterial diameter and pressure waveforms from one individual. (a) Hysteresis curve composed by Preal and vessel diameter, (b) curve fitting to D = α+β•ln Pelastic, by hysteresis elimination procedure. The dotted curve is Eq. (2) in (b).
where η is the viscosity index and dD/dt is the first derivative of the diameter with respect to time. The value of η was increased by iteration to obtain reduction of the hysteresis loop area until it reached a minimum value that maintained the clockwise course of the loop.
The purely elastic relationship obtained by the hysteresis elimination procedure was fitted by a logarithmic model previously applied to the description of the elastic properties of large arteries and transformed into a diameter–pressure curve according to the formula expressing the diameter (D) as a function of Pelastic with two constants, α and β, determined by the fitting procedure, according to the procedure described previously by Armentano et al.[11,16]. In the 
In the present study, the purely elastic relationship after the hysteresis elimination procedure was strongly predicted by Eq. (2) (r = 0.972–0.998). The compliance–pressure curve was calculated by deriving Eq. (2) with respect to pressure (dD/dPelastic). The stiffness–pressure curve was calculated by deriving Eq. (2) with respect to pressure (Pelastic/dD). The diameter–pressure, compliance–pressure and stiffness–pressure curves were represented over the same range of blood pressure, from 50 to 150 mmHg.
Elastic parameters, viscosity index, dynamic compliance and beta-stiffness index
The compliance–pressure and stiffness–pressure curves allowed us to calculate effective values of compliance and stiffness corresponding to the prevailing mean blood pressure of each individual. The curves also allowed us to calculate isobaric values of compliance and stiffness corresponding to the same standard pressure in all individuals. This standard pressure was chosen arbitrarily as the arithmetic average of mean arterial pressure of all individuals (92.15 mmHg).
The wall viscosity index was computed according to Eq. (1) by incrementally increasing the η coefficient until the minimum area of the hysteresis loop was obtained. The viscosity index was the η value corresponding to the minimal hysteresis loop area.
The dynamic arterial compliance and the beta-stiffness index were measured as common arterial function [6,7,14,15,17,25]. The beta-stiffness index provides an index of dynamic arterial compliance adjusted for distending pressure [26]. The dynamic arterial compliance and the beta-stiffness index were calculated using the following equations:
and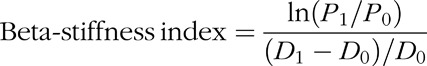
where D1 and D0 were maximal and minimal diameters, and P1 and P0 were the highest and lowest blood pressures, respectively.
Statistical analyses
Statistical analyses were performed with the statistical software StatView (SAS Institute, Cary, North Carolina, USA). Two-way analysis of variance (ANOVA) (age × cardiorespiratory fitness) was used to compare continuous variables. When a significant F-value was obtained, Scheffe's posthoc test was used to identify significant differences among mean values. All data are reported as means ± SEM. For all comparisons, a P value of less than 0.05 was taken to indicate statistical significance.
RESULTS
Individual characteristics are summarized in Table 1. There were no significant differences in body weight, BMI, percentage body fat, HDL-cholesterol, plasma glucose or triglyceride among all four groups. All fitness parameters were decreased by advancing age, and both absolute and relative VO2peak values were affected by cardiorespiratory fitness.
Cardiovascular indices are summarized in Table 2. Although ANOVA revealed no interactions in all parameters, resting heart rate, brachial pulse pressure and carotid diastolic diameter were affected by cardiorespiratory fitness. Moreover, brachial SBP, DBP and mean blood pressure and carotid IMT parameters were affected by advancing age.
TABLE 2.
Cardiovascular indices
| Young | Middle-aged | ||||||
| Fit | Unfit | Fit | Unfit | Age effect | Fitness effect | Age × Fitness | |
| Resting heart rate (bpm) | 60 ± 1 | 66 ± 2 | 60 ± 2 | 64 ± 2 | NS | <0.01 | NS |
| Brachial SBP (mmHg) | 116 ± 2 | 115 ± 2 | 123 ± 2 | 124 ± 2 | <0.001 | NS | NS |
| Brachial DBP (mmHg) | 67 ± 1 | 71 ± 2 | 79 ± 2 | 81 ± 2 | <0.0001 | NS | NS |
| Brachial pulse pressure (mmHg) | 50 ± 1 | 44 ± 1 | 45 ± 2 | 44 ± 1 | NS | <0.05 | NS |
| Brachial mean BP (mmHg) | 84 ± 2 | 87 ± 2 | 95 ± 2 | 96 ± 2 | <0.0001 | NS | NS |
| Carotid SBP (mmHg) | 110 ± 4 | 108 ± 2 | 118 ± 3 | 117 ± 3 | <0.01 | NS | NS |
| Carotid pulse pressure (mmHg) | 43 ± 3 | 38 ± 2 | 39 ± 2 | 37 ± 2 | NS | NS | NS |
| Carotid systolic diameter (mm) | 6.58 ± 0.09 | 6.68 ± 0.09 | 6.67 ± 0.10 | 6.89 ± 0.12 | NS | NS | NS |
| Carotid diastolic diameter (mm) | 6.07 ± 0.09 | 6.21 ± 0.09 | 6.31 ± 0.10 | 6.55 ± 0.11 | NS | <0.01 | NS |
| Carotid mean diameter (mm) | 6.33 ± 0.09 | 6.45 ± 0.10 | 6.49 ± 0.10 | 6.73 ± 0.12 | NS | NS | NS |
| IMT (mm) | 0.55 ± 0.02 | 0.58 ± 0.02 | 0.66 ± 0.02 | 0.66 ± 0.02 | <0.0001 | NS | NS |
| IMT/carotid diastolic diameter (%) | 9.1 ± 0.3 | 9.4 ± 0.3 | 10.5 ± 0.3 | 10.1 ± 0.3 | <0.01 | NS | NS |
Data are means ± SEM. BP, blood pressure; IMT, intima–media thicknes.
The diameter–pressure, compliance–pressure and stiffness–pressure relationships in age and cardiorespiratory fit categories are shown in Figs. 2 and 3, respectively. In men of both age groups, each of the three lines overlapped between the cardiorespiratory fit and unfit groups (Fig. 2). In the unfit group, the compliance-pressure curve in the middle-aged men was shifted towards lower values and the stiffness-pressure relationship was shifted towards higher values with respect to those in the young men, but not in the fit group (Fig. 3). Indeed, alpha and beta values of Eq. (2) were affected by age using two-way ANOVA (Table 3).
FIGURE 2.
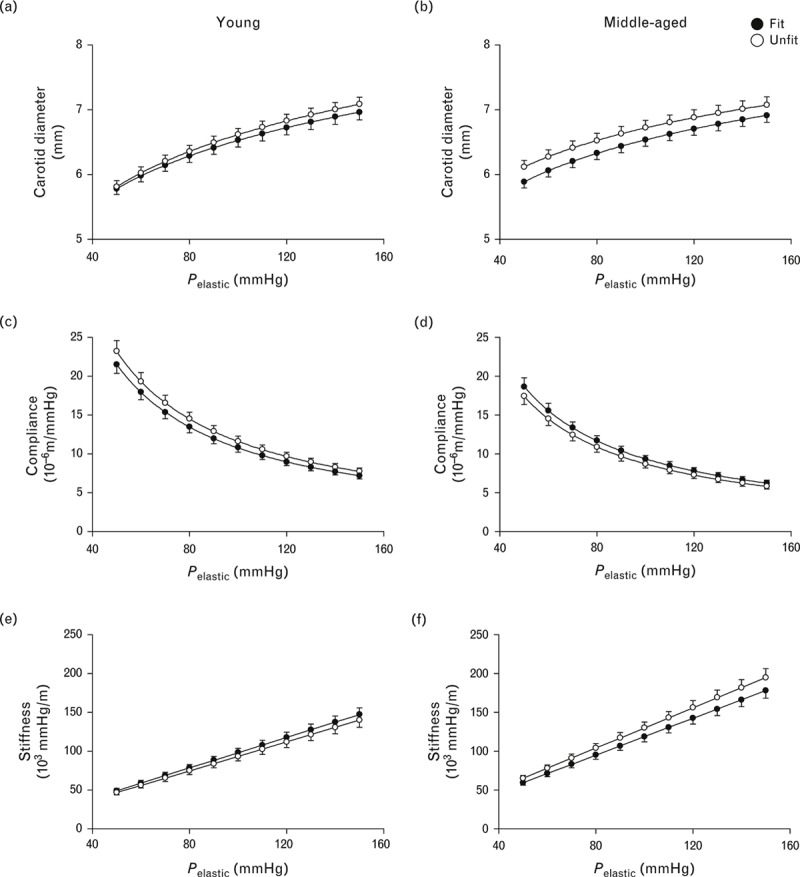
Line graphs showing comparisons of diameter–pressure (a and b), compliance–pressure (c and d) and stiffness–pressure (e and f) curves over a pressure range from 50 to 150 mmHg between fit and unfit groups in young (left) and middle-aged men (right). These graphs were calculated using the values of alpha and beta in Table 3. Data are means ± SEM.
FIGURE 3.
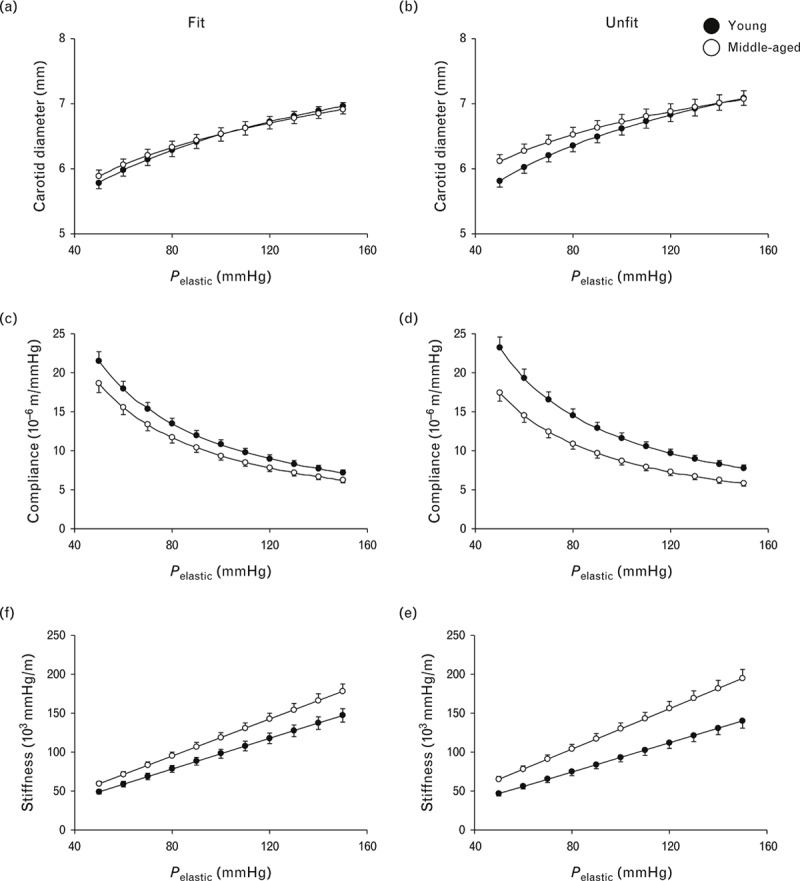
Line graphs showing diameter–pressure (a and b), compliance–pressure (c and d) and stiffness–pressure (e and f) curves over a pressure range from 50 to 150 mmHg between young and middle–aged men in cardiorespiratory fit (left) and cardiorespiratory unfit groups (right). These graphs were calculated using the values of alpha and beta in Table 3. Data are means ± SEM.
TABLE 3.
Carotid arterial elastic properties and stiffness
| Young | Middle-aged | ||||||
| Fit | Unfit | Fit | Unfit | Age effect | Fitness effect | Age × Fitness | |
| Dynamic arterial compliance (mm2/mmHg) | 0.126 ± 0.009 | 0.134 ± 0.008 | 0.099 ± 0.008 | 0.094 ± 0.008 | <0.001 | NS | NS |
| Beta-stiffness (arbitrary units) | 5.93 ± 0.31 | 5.73 ± 0.33 | 7.53 ± 0.44 | 8.39 ± 0.45 | <0.0001 | NS | NS |
| Alpha value (mmHg/mm) | 1.076 ± 0.058 | 1.160 ± 0.069 | 0.934 ± 0.055 | 0.871 ± 0.053 | <0.001 | NS | NS |
| Beta value (mm) | 1.572 ± 0.228 | 1.276 ± 0.303 | 2.232 ± 0.244 | 2.711 ± 0.214 | <0.0001 | NS | NS |
| Isobaric compliance (10−6 m/mmHg) | 11.68 ± 0.63 | 12.58 ± 0.74 | 10.14 ± 0.60 | 9.45 ± 0.58 | <0.001 | NS | NS |
| Effective compliance (10−6 m/mmHg) | 12.99 ± 0.84 | 13.40 ± 0.80 | 10.06 ± 0.70 | 9.17 ± 0.61 | <0.0001 | NS | NS |
| Isobaric stiffness (103 mmHg/m) | 83.4 ± 6.1 | 80.6 ± 4.9 | 113.4 ± 6.8 | 124.8 ± 7.2 | <0.0001 | NS | NS |
| Effective stiffness (103 mmHg/m) | 90.4 ± 5.3 | 86.0 ± 5.6 | 109.4 ± 6.0 | 119.7 ± 7.0 | <0.001 | NS | NS |
Data are means ± SEM. Alpha and beta values were defined each constant of equation ‘D = α + β•lnPelastic’. Isobaric compliance and stiffness were determined at pressure equal to 92.15 mmHg (average value of mean arterial pressure in all individuals). Effective compliance and stiffness were determined at mean arterial pressure in each individual.
Carotid elastic properties are summarized in Table 3. There were no significant interactions in carotid dynamic arterial compliance, effective compliance, isobaric compliance, effective stiffness or isobaric stiffness by ANOVA. These carotid arterial elastic properties were attenuated with advancing age but unaffected by cardiorespiratory fitness.
The wall viscosity index is shown in Fig. 4. In the unfit group, the wall viscosity index in the carotid artery was greater in middle-aged men than in young men, but no such difference was observed in the fit group. In the middle-aged men, the wall viscosity index in the carotid artery was markedly higher in the unfit group than in the fit peers, but not in the young men.
FIGURE 4.
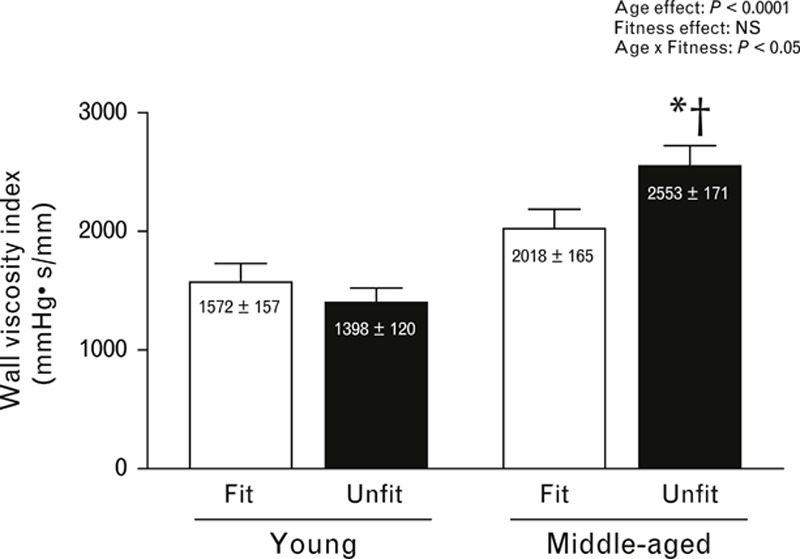
Bar graph showing wall viscosity index in each group. Data are means ± SEM. ∗P < 0.05 vs. young men in the same cardiorespiratory fitness level. †P < 0.05 vs. men of the same age in the cardiorespiratory fit group.
DISCUSSION
The most important findings in the present study were as follows. First, in the cardiorespiratory unfit group, the carotid wall viscosity index in middle-aged men was significantly greater than that in young men. Second, there was no such age-related difference in the fit group. Third, although the wall viscosity index in the middle-aged unfit men was significantly higher than that in the age-matched fit peers, there were no such differences in indices of elastic properties and stiffness in the central artery. To our knowledge, this is the first study to identify the influences of ageing and cardiorespiratory fitness on arterial wall viscosity, and the results suggest that the age-related increase in central arterial wall viscosity is attenuated in cardiorespiratory fit men.
Central elastic artery flexibly receives the pulsatile energy imposed by cardiac contractions to store energy, and the stored energy is effectively used for flowing blood into peripheral tissues. In this successive progression, wall viscosity reflects energy loss during conversion of mechanical energy from cardiac pulsation into elastic energy in the arterial wall. Thus, the results of the present study indicating greater wall viscosity of middle-aged men than young men suggest that advancing age results in inefficient mechanical transduction, and consequently, the efficiency of blood flow in elastic arteries may be impaired with advancing age. In addition, our results indicated that the age-related increase in wall viscosity was smaller in cardiorespiratory fit men than in unfit men, and there were no significant differences in wall viscosity or elasticity between young and middle-aged cardiorespiratory fit men. These findings suggest that the attenuation of age-associated reduction in cardiorespiratory fitness level prevents energy loss during mechanical transduction, leading to maintenance of effective flowing blood in elastic arteries.
Wall viscosity reflects not only energy dissipation during mechanical transduction but may also play a role in protection against pulsatile energy in the elastic arteries. Vessel wall structure deteriorates with advancing age (arteriosclerosis) because of alterations in the elastin-collagen ratio and fibrosis of vascular smooth muscle [27]. If pulsatile energy induced by heart contractions is unchanged with ageing [9], wall strain is increased, leading to an increased risk of vessel wall rupture, such as arterial dissection. Indeed, it has been reported that the isolated carotid artery indicated lower viscosity and greater wall strain in vitro, suggesting that reduction of wall viscosity caused by denervation is associated with greater wall strain [10]. However, middle-aged men in the present study showed a higher wall viscosity index than young men, suggesting that this increased wall viscosity with advancing age may attenuate excessive strain in the vessel wall. Therefore, the age-related increase in wall viscosity may be a compensatory adaptation to prevent the increase in wall strain. Furthermore, our results indicated that wall viscosity was not significantly different between young and middle-aged men in the cardiorespiratory fit group. Therefore, we speculated that because the deleterious alteration of wall structure with ageing may be attenuated in cardiorespiratory fit men [28], it may not be necessary to induce a compensatory increase in wall viscosity. On the contrary, the age-related increase in wall viscosity may mean that the heart must impose stronger contractions to maintain elastic energy in the vessel wall and provide constant blood flow to peripheral tissues, resulting in elevation of systolic pressure and left ventricular overload. This hypothesis was supported in part by a previous study indicating that advancing age is associated with systolic hypertension or left ventricular concentric hypertrophy [18,29]. Moreover, in the present study, the age-related increase in wall viscosity was smaller in the cardiorespiratory fit in middle-aged men than in their unfit peers, suggesting the prevention of age-related energy loss during mechanical transduction in cardiorespiratory fit men. This may contribute to mitigation of left ventricular load, leading to suppression of hypertension and left ventricular hypertrophy. Nevertheless, the relationships between wall viscosity and cardiorespiratory fitness level or left ventricular hypertrophy should be confirmed by further interventional or prospective studies.
In the present study focusing on untrained individuals, dynamic arterial compliance was lower and beta-stiffness was higher in middle-aged men than in young men, but no effect of cardiorespiratory fitness level was observed, consistent with the results of a previous study [7]. In addition to these existing elastic parameters, our study characterized the effects of ageing and cardiorespiratory fitness level on the intrinsic pressure-independent elastic properties of the carotid arterial wall. We showed compliance–pressure and stiffness–pressure relationships in each category group calculated by Eq. (2). In the unfit group, the compliance-pressure curve in middle-aged men was shifted downwards and stiffness–pressure linear regression was shifted upwards compared with young peers. However, although we were attempted to confirm these findings by comparison of effective and isobaric values of carotid compliance and stiffness between the two age groups in cardiorespiratory unfit individuals, ANOVA revealed no interaction of age and fitness. When we focused on the values of elastic parameters of the carotid artery, effective values of carotid compliance and stiffness (at the mean blood pressure in each individual) were reduced by 32 and 39%, respectively, in middle-aged men with respect to young men in the cardiorespiratory unfit group. Isobaric values of compliance and stiffness (at the average mean blood pressure in all individuals) were also reduced by 25 and 55%, respectively, in middle-aged men. Our results suggest that although blood pressure is increased with advancing age [30,31], age-related arterial stiffening in the unfit group is an intrinsic pressure-independent alteration of the elastic properties.
We can only speculate on the mechanisms responsible for the lack of age-associated increase in wall viscosity in the cardiorespiratory fit group. Wall viscosity is mainly affected by vascular smooth muscle. This view was confirmed by a previous study [10] indicating that the wall viscosity was substantially attenuated by approximately 50% under vascular smooth muscle null tonus conditions (inactivation in sympathetic nerve) using carotid arteries of brain-dead humans in vitro. This suggests a strong relationship between the wall viscosity and sympathetic-adrenergic tone of smooth muscle cells in arteries. However, even if vascular smooth muscle is inactive in sympathetic nerves, IMT or the substrate of the arteries is essentially unchanged. This exhibited incomplete null viscosity index [10]. Indeed, the wall viscosity was affected by the characteristics of wall morphology, in particular, IMT, as reported previously [11]. We also found that, in all individuals taken together, wall viscosity was related to IMT in the carotid artery (r = 0.371, P < 0.0001). Previous studies have demonstrated that both vascular smooth muscle tone and IMT increase with advancing age, and these age-related increases are attenuated by maintenance of cardiorespiratory fitness level [12,13], which may support the lower wall viscosity in cardiorespiratory fit men determined in the present study. On the contrary, as the effects of histological properties, such as elastin-collagen ratio or fibrotic smooth muscle in arteries on wall viscosity, remain unclear, further studies are needed to determine the structural mechanisms underlying the lack of age-related increase in wall viscosity in the middle-aged cardiorespiratory fit group.
Our results demonstrated that wall viscosity in the carotid artery increased with advancing age, which may be expanded to understand changes in autonomic regulation with ageing. Arterial wall viscosity is mainly related to vascular smooth muscle cells [1–4]. Thus, age-associated sympatho-excitation [32] may induce an increase in wall viscosity via smooth muscle contraction. On the contrary, increased wall viscosity leads to a reduction in wall strain [10]. Decreased wall strain may contribute to attenuation of baroreceptor sensitivity in response to blood pressure fluctuation. Therefore, age-related reductions in baroreflex sensitivity [33] may be associated with age-related increases in wall viscosity.
We emphasize two main points for assessment of wall viscosity. First, although there were no differences in dynamic, isobaric and effective arterial compliance or beta-stiffness between age-matched fit and unfit groups, the viscosity index in middle-aged men was lower in the fit group than in the unfit peers. Thus, wall viscosity may be a more sensitive parameter of mechanical characteristics for assessment of vascular health than existing mechanical parameters in the arteries, that is, dynamic arterial compliance or beta-stiffness. Second, arterial wall viscosity can be calculated noninvasively by using the diameter waveforms and blood pressure waveforms obtained by B-mode image sequence and applanation tonometry. A combination of B-mode images with applanation tonometry has been used to determine arterial compliance or beta-stiffness in many investigations [7,14,15,17,25,33,34]. Our procedure of hysteresis elimination, according to previous studies [11,16], based on characterization of the viscoelastic behaviour of the arterial wall, was used to determine the wall viscosity. In addition, this procedure was highly reproducible to determine wall viscosity, as the coefficient of variation was 1.6%. Therefore, this arterial viscoelasticity assessment can potentially be useful in both research and clinical application in the future.
The present study had a limitation. We found the significance of interaction (age vs. fitness) and age effects for viscosity index of the carotid artery. However, the statistical power was insufficient for interaction because of the small population size (111 individuals). Therefore, our results should be confirmed in larger populations and by interventional studies to negate the effects of heritable factors or dietary habits as much as possible.
In conclusion, we determined the associations among age, cardiorespiratory fitness and wall viscosity in a cross-sectional study design. The common carotid artery was noninvasively examined by tonometry and an automatic tracking system for B-mode images to obtain instantaneous pressure and diameter hysteresis loops, and we calculated the viscosity index. In conclusion, wall viscosity in the central artery increases with advancing age, and this age-related increase in wall viscosity may be attenuated in cardiorespiratory fit men.
ACKNOWLEDGEMENTS
We wish to thank Dr Motoyuki Iemitsu for supporting our study.
This study was supported by Grants-in-Aid for Scientific Research 13780041 (to M.M.), 23240089 (to M.M.), 21700706 (to K.Y.) and 21700707, 23700845 (to H.K.) from the Japan Society for the Promotion of Science, 25560376 (to H.K.) and the Global COE program ‘Sport Sciences for the Promotion of Active Life’ and by a Sasagawa Scientific Research Grant (to H.K.) from the Japan Science Society.
Conflicts of interest
The authors have no conflicts of interest to declare.
Reviewers’ Summary Evaluations
Reviewer 1
The strength of this study derives from a successful attempt to apply to humans advanced concepts from arterial dynamics, easier to assess in theory or in animals. Overall data provide a novel view of the beneficial effects of exercise on the cardiovascular system. Weakness is related to the fact that arterial dynamics still appears difficult to integrate in a comprehensive model of the circulation, particularly seen as an innervated system
Reviewer 2
This well designed study contributes significantly to the understanding of the associations among age, cardiorespiratory fitness, and carotid arterial wall viscosity. The results suggest that the age-related increase in central arterial wall viscosity is attenuated in cardiorespiratory fit men. Wall viscosity was determined using a method that assumes its constancy. However, literature shows that wall viscosity may not be constant and is strongly influenced by steady and pulsatile mechanical load but not merely by smooth muscle tone. Therefore, further refinement of the analysis method is required in order to better understand the association of wall viscosity with clinical and functional characteristics.
Footnotes
Correspondence to Hiroshi Kawano, Faculty of Letters, Kokushikan University, 4-28-1 Setagaya, Setagaya-ku, Tokyo 154-8515, Japan. Tel: +81 3 5451 8196; fax: +81 3 5451 8196; e-mail: hiroshi@kokushikan.ac.jp
Abbreviations: DEXA, dual-energy X-ray absorptiometry; IMT, intima–media thickness; VO2peak, peak oxygen uptake
Received 28 February, 2013
Revised 6 June, 2013
Accepted 3 July, 2013
REFERENCES
- 1.Bertram CD. Energy dissipation and pulse wave attenuation in the canine carotid artery. J Biomech 1980; 13:1061–1073. [DOI] [PubMed] [Google Scholar]
- 2.Bodley WE. Energy dissipation in mammalian arteries – an assessment of the distribution of energy dissipation between the blood and the vessel wall. J Biomech 1976; 9:489–494. [DOI] [PubMed] [Google Scholar]
- 3.Nichols W, O’Rourke M. McDonal's blood flow in arteries. London:Arnold; 1998. [Google Scholar]
- 4.Taylor MG. Wave transmission through an assembly of randomly branching elastic tubes. Biophys J 1966; 6:697–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Evans GW, Riley WA. Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 1994; 140:669–682. [DOI] [PubMed] [Google Scholar]
- 6.Parati G, Bernardi L. How to assess arterial compliance in humans. J Hypertens 2006; 24:1009–1012. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000; 102:1270–1275. [DOI] [PubMed] [Google Scholar]
- 8.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993; 88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 1998; 32:1221–1227. [DOI] [PubMed] [Google Scholar]
- 10.Armentano RL, Barra JG, Santana DB, Pessana FM, Graf S, Craiem D, et al. Smart damping modulation of carotid wall energetics in human hypertension: effects of angiotensin-converting enzyme inhibition. Hypertension 2006; 47:384–390. [DOI] [PubMed] [Google Scholar]
- 11.Armentano RL, Graf S, Barra JG, Velikovsky G, Baglivo H, Sanchez R, et al. Carotid wall viscosity increase is related to intima-media thickening in hypertensive patients. Hypertension 1998; 31:534–539. [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, et al. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol 2001; 534 (Pt 1):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 2009; 587 (Pt 9):2049–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, et al. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 2004; 110:2858–2863. [DOI] [PubMed] [Google Scholar]
- 15.Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens 2006; 24:1753–1759. [DOI] [PubMed] [Google Scholar]
- 16.Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 1995; 26:48–54. [DOI] [PubMed] [Google Scholar]
- 17.Kawano H, Tanimoto M, Yamamoto K, Sanada K, Gando Y, Tabata I, et al. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp Physiol 2008; 93:296–302. [DOI] [PubMed] [Google Scholar]
- 18.Gando Y, Kawano H, Yamamoto K, Sanada K, Tanimoto M, Oh T, et al. Age and cardiorespiratory fitness are associated with arterial stiffening and left ventricular remodelling. J Hum Hypertens 2010; 24:197–206. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14:377–381. [PubMed] [Google Scholar]
- 20.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol 2001; 90:2439–2444. [DOI] [PubMed] [Google Scholar]
- 21.Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol 1991; 11:331–341. [DOI] [PubMed] [Google Scholar]
- 22.Armentano R, Simon A, Levenson J, Chau NP, Megnien JL, Pichel R. Mechanical pressure versus intrinsic effects of hypertension on large arteries in humans. Hypertension 1991; 18:657–664. [DOI] [PubMed] [Google Scholar]
- 23.Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res 1993; 73:1040–1050. [DOI] [PubMed] [Google Scholar]
- 24.Bauer RD, Busse R, Schabert A, Summa Y, Wetterer E. Separate determination of the pulsatile elastic and viscous forces developed in the arterial wall in vivo. Pflugers Arch 1979; 380:221–226. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, et al. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 2003; 41:130–135. [DOI] [PubMed] [Google Scholar]
- 26.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 1989; 80:78–86. [DOI] [PubMed] [Google Scholar]
- 27.Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol A Biol Sci Med Sci 2007; 62:696–706. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, Nosaka T, Sato M, Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol 1993; 66:122–126. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 2005; 45:652–658. [DOI] [PubMed] [Google Scholar]
- 30.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315. [DOI] [PubMed] [Google Scholar]
- 31.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 2005; 46:454–462. [DOI] [PubMed] [Google Scholar]
- 32.Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 2004; 287:H1895–H1905. [DOI] [PubMed] [Google Scholar]
- 33.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation 2001; 104:1627–1632. [DOI] [PubMed] [Google Scholar]
- 34.Rakobowchuk M, McGowan CL, de Groot PC, Bruinsma D, Hartman JW, Phillips SM, et al. Effect of whole body resistance training on arterial compliance in young men. Exp Physiol 2005; 90:645–651. [DOI] [PubMed] [Google Scholar]


