Abstract
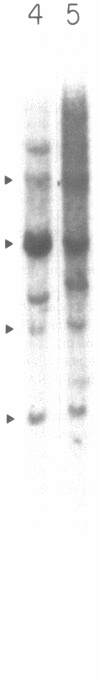
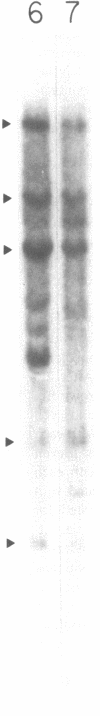
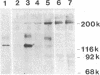
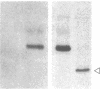
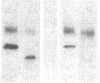
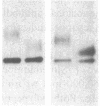
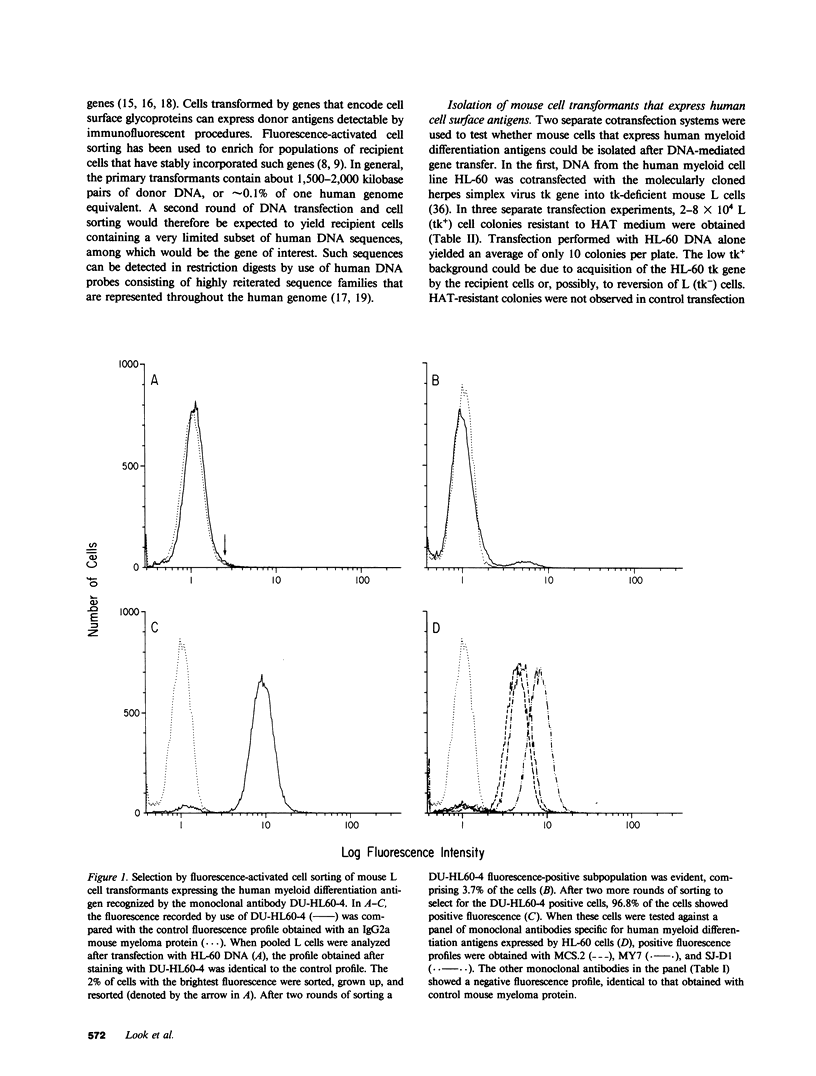
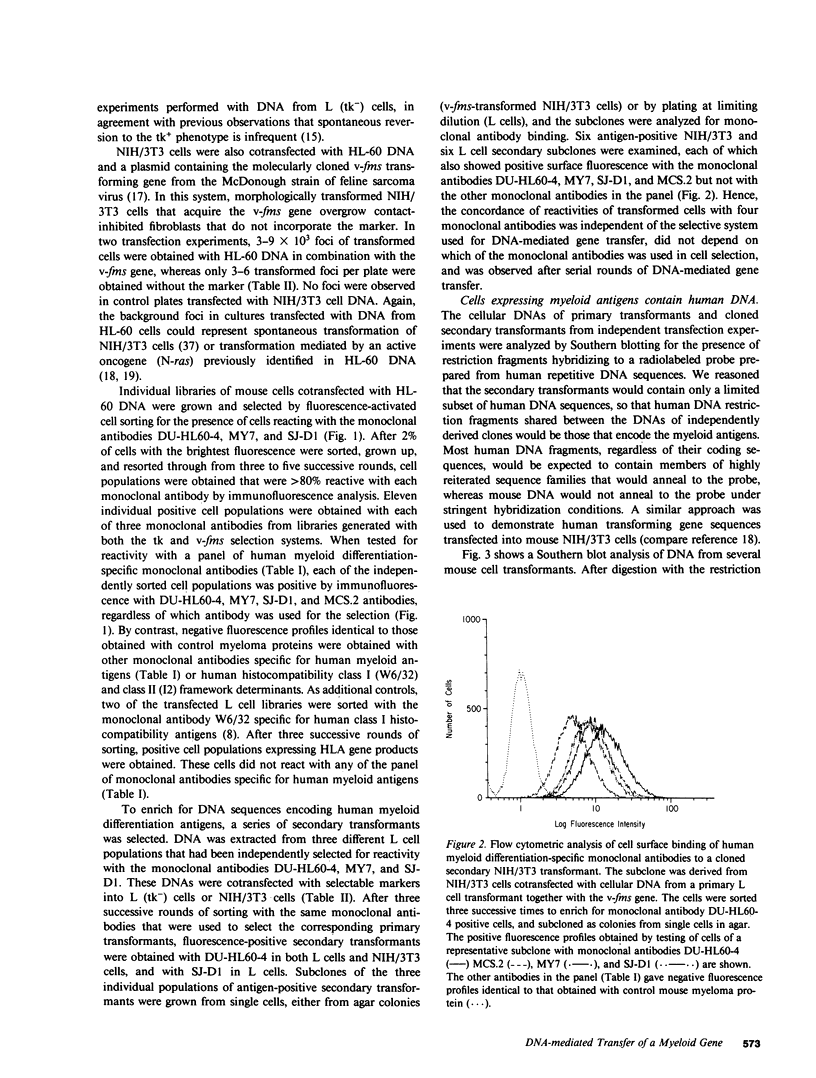
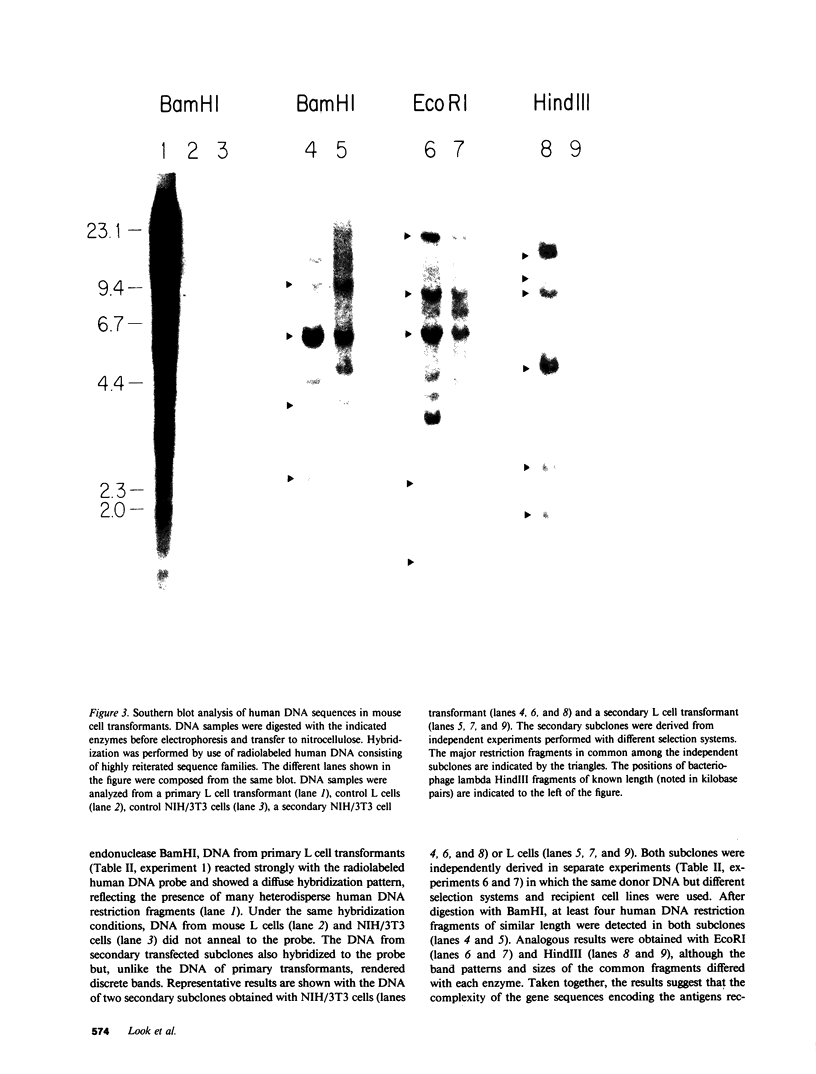
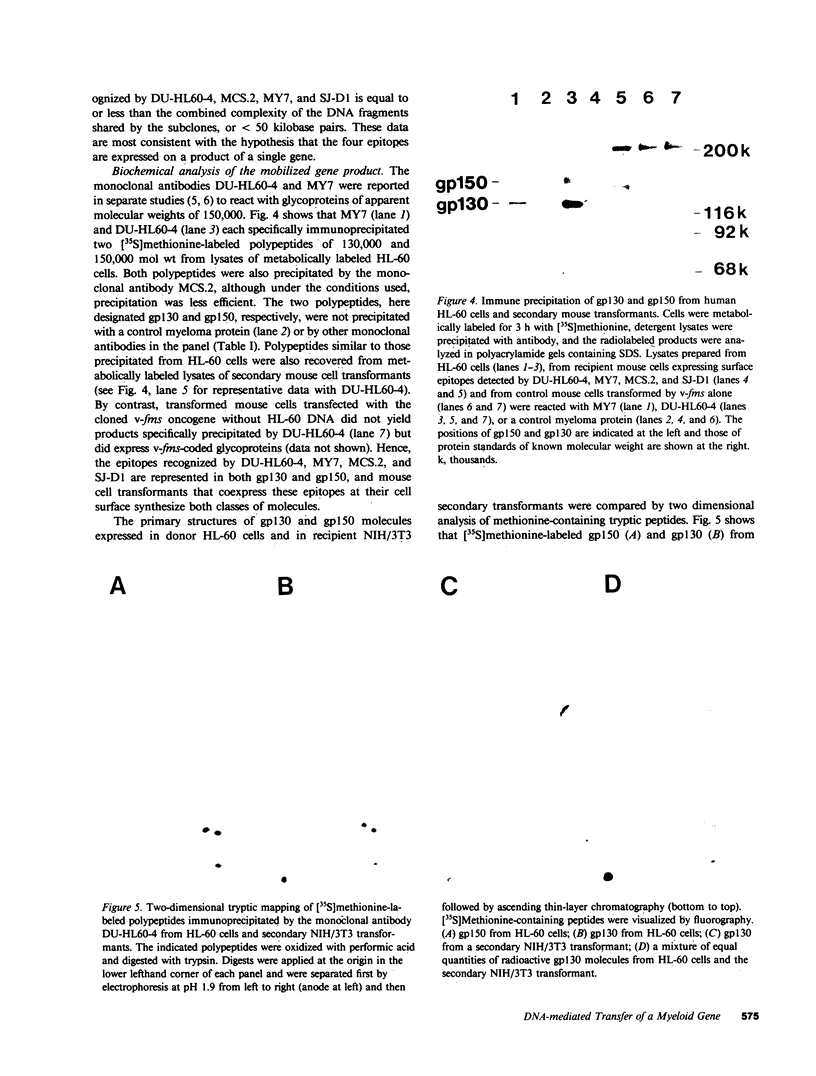
DNA from the human myeloid cell line HL-60 was cotransfected with the cloned thymidine kinase (tk) gene of herpes simplex virus into tk-deficient mouse L cells. tk-positive recipients expressing antigens detected on HL-60 cells were isolated with a fluorescence-activated cell sorter by use of a panel of monoclonal antibodies that detect epitopes on both normal and malignant myeloid cells. Independently sorted populations of transformed mouse cells showed concordant reactivities with four of the monoclonal antibodies in the panel (DU-HL60-4, MY7, MCS.2, and SJ-D1), which suggested that these antibodies reacted to products of a single human gene. A second round of DNA transfection and cell sorting was performed with donor DNA from primary transformants. Two different dominant selection systems were used to isolate secondary mouse L cell and NIH/3T3 cell transformants that coexpressed the same epitopes. Analysis of cellular DNA from secondary mouse cell subclones with a probe specific for human repetitive DNA sequences revealed a minimal human DNA complement containing a characteristic set of restriction fragments common to independently derived subclones. Two glycoproteins, of 130,000 (gp130) and 150,000 (gp150) mol wt, were specifically immunoprecipitated from metabolically labeled lysates of mouse cell transformants and were shown to contain [35S]methionine-labeled tryptic peptides identical to those of analogous glycoproteins expressed in the donor human myeloid cell line. Kinetic and biochemical analyses established that gp130 is a precursor that differs in its carbohydrate moiety from gp150, the mature form of the glycoprotein detected on the cell surface. The isolation of human gene sequences encoding gp150 in a mouse cell genetic background provides the possibility of molecularly cloning the gene and represents a general strategy for isolating human genes encoding differentiation-specific cell surface antigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. G., Torok-Storb B., Bernstein I. D. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983 Jul;62(1):124–132. [PubMed] [Google Scholar]
- Bernstein I. D., Andrews R. G., Cohen S. F., McMaster B. E. Normal and malignant human myelocytic and monocytic cells identified by monoclonal antibodies. J Immunol. 1982 Feb;128(2):876–881. [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Civin C. I., Mirro J., Banquerigo M. L. My-1, new myeloid-specific antigen identified by a mouse monoclonal antibody. Blood. 1981 May;57(5):842–845. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W., Burns G. F. Monoclonal antibody OKT-9 recognizes the receptor for transferrin on human acute lymphocytic leukemia cells. J Immunol. 1981 Sep;127(3):1256–1258. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Beveridge R. P., Lipton J. M., Daley J. F., Schlossman S. F. Expression of MY7 antigen on myeloid precursor cells. Int J Cell Cloning. 1983 Apr;1(1):33–48. doi: 10.1002/stem.5530010106. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Huang L. C., Civin C. I., Magnani J. L., Shaper J. H., Ginsburg V. My-1, the human myeloid-specific antigen detected by mouse monoclonal antibodies, is a sugar sequence found in lacto-N-fucopentaose III. Blood. 1983 May;61(5):1020–1023. [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKolanis J. R., Finn O. J., Metzgar R. S. Characterization of human myelomonocytic antigens using monoclonal antibodies. Prog Clin Biol Res. 1983;133:145–156. [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Newman R., Domingo D., Trotter J., Trowbridge I. Selection and properties of a mouse L-cell transformant expressing human transferrin receptor. Nature. 1983 Aug 18;304(5927):643–645. doi: 10.1038/304643a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Karess R. E., Anderson S. M., Hanafusa H. Tryptic peptide analysis of avian oncovirus gag and pol gene products. J Virol. 1979 Oct;32(1):102–113. doi: 10.1128/jvi.32.1.102-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Todaro G. J. Spontaneous release of type C viruses from clonal lines of spontaneously transformed Blab-3T3 cells. Nat New Biol. 1972 Nov 29;240(100):157–160. doi: 10.1038/newbio240157a0. [DOI] [PubMed] [Google Scholar]
- Urdal D. L., Brentnall T. A., Bernstein I. D., Hakomori S. I. A granulocyte reactive monoclonal antibody, 1G10, identifies the Gal beta 1-4 (Fuc alpha 1-3)GlcNAc (X determinant) expressed in HL-60 cells on both glycolipid and glycoprotein molecules. Blood. 1983 Nov;62(5):1022–1026. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]